Simulated Microgravity-Induced Changes to Drug Response in Cancer Cells Quantified Using Fluorescence Morphometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
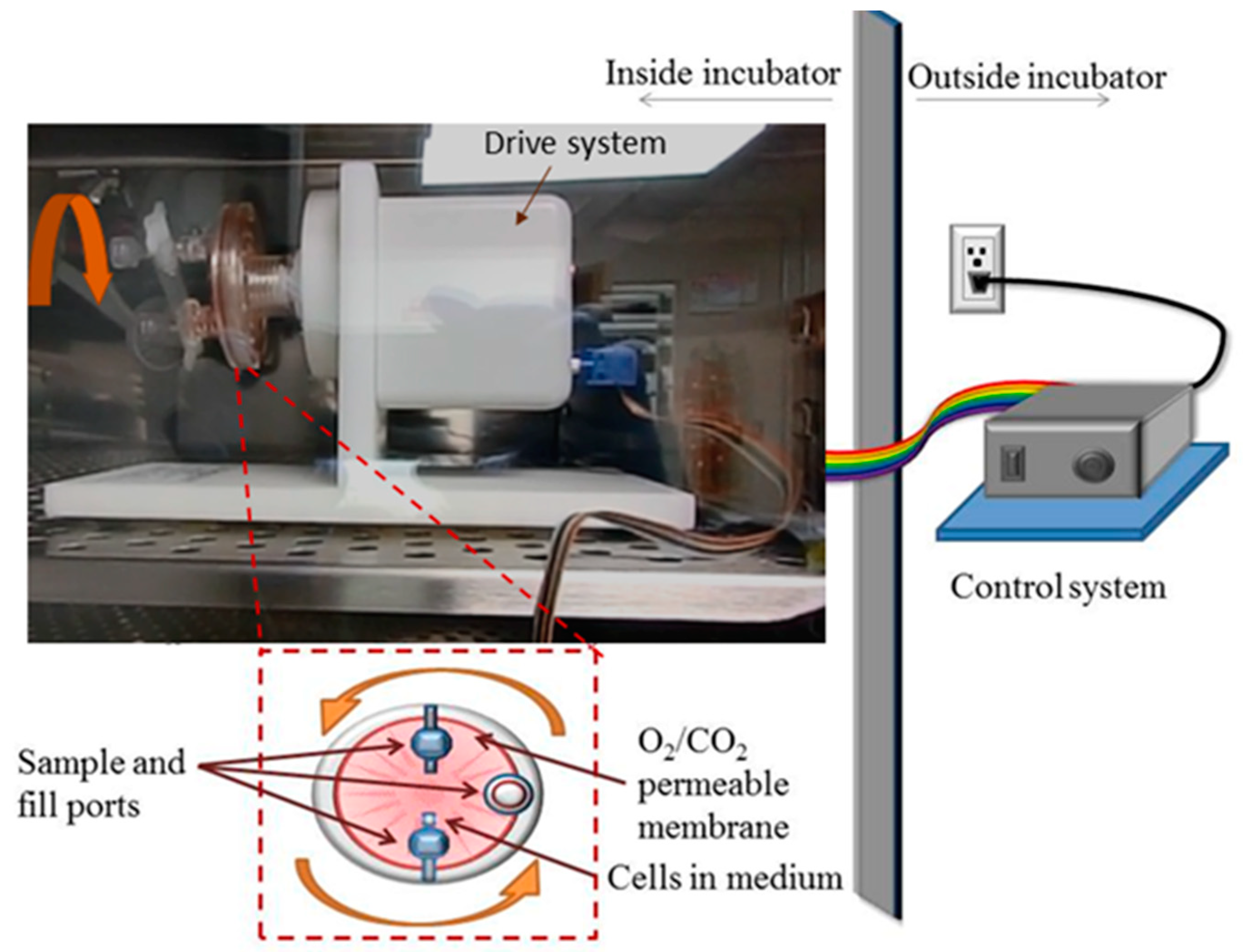
2.2. Rotary Cell Culture System
2.3. Pharmacological Interventions
2.4. Fluorescence Microscopy
2.5. Fluorescence-Guided Morphometry
- Lacunarity, Λ.
- 2.
- Circularity.
- 3.
- Nuclear to Cytoplasmic Ratio, N/C.
2.6. Statistics and Error Analyses
3. Results
3.1. Generated 3D Tissue Spheroids Confirm Microgravity in RCCS
3.2. N/C Ratio of Untreated Cells Remains Unchanged following Microgravity
3.3. Hydroxyurea Treated Cells Have a Significantly Altered N/C Ratio in Normal G
3.4. Microgravity Eliminates the Reduction of the N/C Ratio in Hydroxyurea Treated Cells
3.5. Paclitaxel Treated Cells Show No Alterations in the N/C Ratio in Normal G and in Microgravity
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodkinson, P.D.; Anderton, R.A.; Posselt, B.N.; Fong, K.J. An Overview of Space Medicine. Br. J. Anaesth. 2017, 119, i143–i153. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.R.; Albrecht, M.H.; Collins, H.R.; Asemani, D.; Chatterjee, A.R.; Spampinato, M.V.; Zhu, X.; Chimowitz, M.I.; Antonucci, M.U. Effects of Spaceflight on Astronaut Brain Structure as Indicated on MRI. N. Engl. J. Med. 2017, 377, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Greener, M. Drug Discovery and Development: The Final Frontier. Prescriber 2020, 31, 18–22. [Google Scholar] [CrossRef][Green Version]
- Blue, R.S.; Bayuse, T.M.; Daniels, V.R.; Wotring, V.E.; Suresh, R.; Mulcahy, R.A.; Antonsen, E.L. Supplying a Pharmacy for NASA Exploration Spaceflight: Challenges and Current Understanding. NPJ Microgravity 2019, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Eyal, S.; Derendorf, H. Medications in Space: In Search of a Pharmacologist’s Guide to the Galaxy. Pharm. Res. 2019, 36, 148. [Google Scholar] [CrossRef]
- Pavez Loriè, E.; Baatout, S.; Choukér, A.; Buchheim, J.I.; Baselet, B.; Dello Russo, C.; Wotring, V.; Monici, M.; Morbidelli, L.; Gagliardi, D.; et al. The Future of Personalized Medicine in Space: From Observations to Countermeasures. Front. Bioeng. Biotechnol. 2021, 9, 1259. [Google Scholar] [CrossRef]
- Crucian, B.E.; Makedonas, G.; Sams, C.F.; Pierson, D.L.; Simpson, R.; Stowe, R.P.; Smith, S.M.; Zwart, S.R.; Krieger, S.S.; Rooney, B.; et al. Countermeasures-Based Improvements in Stress, Immune System Dysregulation and Latent Herpesvirus Reactivation Onboard the International Space Station—Relevance for Deep Space Missions and Terrestrial Medicine. Neurosci. Biobehav. Rev. 2020, 115, 68–76. [Google Scholar] [CrossRef]
- Lei, W.; Yuan, M.; Long, M.; Zhang, T.; Huang, Y.; Liu, H.; Jiang, W. ScDR: Predicting Drug Response at Single-Cell Resolution. Genes 2023, 14, 268. [Google Scholar] [CrossRef]
- Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A.; Sahana, J.; Corydon, T.J.; Hemmersbach, R.; Wise, P.M.; et al. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. Int. J. Mol. Sci. 2022, 23, 3073. [Google Scholar] [CrossRef]
- Jhala, D.V.; Kale, R.K.; Singh, R.P. Microgravity Alters Cancer Growth and Progression. Curr. Cancer Drug Targets 2014, 14, 394–406. [Google Scholar] [CrossRef]
- Prasanth, D.; Suresh, S.; Mimlitz, M.; Zetocha, N.; Ekpenyong, A.E. Microgravity Modulates Drug-Induced Enhancement of Cancer Cell Migration. Biophys. J. 2017, 112, 311a. [Google Scholar] [CrossRef][Green Version]
- Prasanth, D.; Suresh, S.; Prathivadhi-Bhayankaram, S.; Mimlitz, M.; Zetocha, N.; Lee, B.; Ekpenyong, A. Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration. Life 2020, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Rembiałkowska, N.; Baczyńska, D.; Dubińska-Magiera, M.; Choromańska, A.; Bieżuńska-Kusiak, K.; Gajewska-Naryniecka, A.; Novickij, V.; Saczko, J.; Przystupski, D.; Kulbacka, J. RCCS Bioreactor-Based Modeled Microgravity Affects Gastric Cancer Cells and Improves the Chemotherapeutic Effect. Membranes 2022, 12, 448. [Google Scholar] [CrossRef]
- Przystupski, D.; Górska, A.; Szewczyk, A.; Drąg-Zalesińska, M.; Kulbacka, J. 3D Clinorotation Affects Drug Sensitivity of Human Ovarian Cancer Cells. Microgravity Sci. Technol. 2021, 33, 3. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Andersen, J.K.; Miletic, H.; Hossain, J.A. Tumor-Associated Macrophages in Gliomas—Basic Insights and Treatment Opportunities. Cancers 2022, 14, 1319. [Google Scholar] [CrossRef]
- Tauber, S.; Lauber, B.A.; Paulsen, K.; Layer, L.E.; Lehmann, M.; Hauschild, S.; Shepherd, N.R.; Polzer, J.; Segerer, J.; Thiel, C.S.; et al. Cytoskeletal Stability and Metabolic Alterations in Primary Human Macrophages in Long-Term Microgravity. PLoS ONE 2017, 12, e0175599. [Google Scholar] [CrossRef]
- Thiel, C.S.; de Zélicourt, D.; Tauber, S.; Adrian, A.; Franz, M.; Simmet, D.M.; Schoppmann, K.; Hauschild, S.; Krammer, S.; Christen, M.; et al. Rapid Adaptation to Microgravity in Mammalian Macrophage Cells. Sci. Rep. 2017, 7, 43. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Gathings, W.E.; McDonald, J.M. Modeled Microgravity Inhibits Osteogenic Differentiation of Human Mesenchymal Stem Cells and Increases Adipogenesis. Endocrinology 2004, 145, 2421–2432. [Google Scholar] [CrossRef]
- Mitsuhara, T.; Takeda, M.; Yamaguchi, S.; Manabe, T.; Matsumoto, M.; Kawahara, Y.; Yuge, L.; Kurisu, K. Simulated Microgravity Facilitates Cell Migration and Neuroprotection after Bone Marrow Stromal Cell Transplantation in Spinal Cord Injury. Stem Cell Res. Ther. 2013, 4, 35. [Google Scholar] [CrossRef]
- Costantini, D.; Overi, D.; Casadei, L.; Cardinale, V.; Nevi, L.; Carpino, G.; Di Matteo, S.; Safarikia, S.; Valerio, M.; Melandro, F.; et al. Simulated Microgravity Promotes the Formation of Tridimensional Cultures and Stimulates Pluripotency and a Glycolytic Metabolism in Human Hepatic and Biliary Tree Stem/Progenitor Cells. Sci. Rep. 2019, 9, 5559. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, S.; Peng, G.; Liu, T.; Li, Y.; Xiang, D.; Wassler, M.J.; Shelat, H.S.; Geng, Y. Rotating Microgravity-Bioreactor Cultivation Enhances the Hepatic Differentiation of Mouse Embryonic Stem Cells on Biodegradable Polymer Scaffolds. Tissue Eng. Part A 2012, 18, 2376–2385. [Google Scholar] [CrossRef]
- Ran, F.; An, L.; Fan, Y.; Hang, H.; Wang, S. Simulated Microgravity Potentiates Generation of Reactive Oxygen Species in Cells. Biophys. Rep. 2016, 2, 100–105. [Google Scholar] [CrossRef]
- Gambacurta, A.; Merlini, G.; Ruggiero, C.; Diedenhofen, G.; Battista, N.; Bari, M.; Balsamo, M.; Piccirillo, S.; Valentini, G.; Mascetti, G.; et al. Human Osteogenic Differentiation in Space: Proteomic and Epigenetic Clues to Better Understand Osteoporosis. Sci. Rep. 2019, 9, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Wehland, M.; Corydon, T.J.; Richter, P.; Prasad, B.; Bauer, J.; Egli, M.; Kopp, S.; Lebert, M.; Krüger, M. The Effects of Microgravity on Differentiation and Cell Growth in Stem Cells and Cancer Stem Cells. Stem Cells Transl. Med. 2020, 9, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.; Sharma, A.; Chen, H.; Wu, H.; Shao, N.Y.; Sayed, N.; Liu, C.; Countryman, S.; Stodieck, L.S.; Rubins, K.H.; et al. Effects of Spaceflight on Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Structure and Function. Stem Cell Rep. 2019, 13, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.A.; Aanei, C. Revisiting the Hallmarks of Cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Stein, G.H. T98G: An Anchorage-independent Human Tumor Cell Line That Exhibits Stationary Phase G1 Arrest in Vitro. J. Cell. Physiol. 1979, 99, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Fuster, E.; Candela, H.; Estévez, J.; Vilanova, E.; Sogorb, M.A. A Transcriptomic Analysis of T98G Human Glioblastoma Cells after Exposure to Cadmium-Selenium Quantum Dots Mainly Reveals Alterations in Neuroinflammation Processes and Hypothalamus Regulation. Int. J. Mol. Sci. 2022, 23, 2267. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Ben-Bassat, H.; Neumann, H.; Ralph, P.; Zeuthen, J.; Polliack, A.; Vánky, F. Properties of the K562 Cell Line, Derived from a Patient with Chronic Myeloid Leukemia. Int. J. Cancer 1976, 18, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ho, S.S.; Greer, S.U.; Zhu, X.; Bell, J.M.; Arthur, J.G.; Spies, N.; Zhang, X.; Byeon, S.; Pattni, R.; et al. Comprehensive, Integrated, and Phased Whole-Genome Analysis of the Primary ENCODE Cell Line K562. Genome Res. 2019, 29, 472–484. [Google Scholar] [CrossRef]
- Cui, Y.; Yin, Y.; Zou, Y.; Zhao, Y.; Han, J.; Xu, B.; Chen, B.; Xiao, Z.; Song, H.; Shi, Y.; et al. The Rotary Cell Culture System Increases NTRK3 Expression and Promotes Neuronal Differentiation and Migratory Ability of Neural Stem Cells Cultured on Collagen Sponge. Stem Cell Res. Ther. 2021, 12, 298. [Google Scholar] [CrossRef]
- Yi, Z.-C.; Xia, B.; Xue, M.; Zhang, G.-Y.; Wang, H.; Zhou, H.-M.; Sun, Y.; Zhuang, F.-Y. Simulated Microgravity Inhibits the Proliferation of K562 Erythroleukemia Cells but Does Not Result in Apoptosis. Adv. Space Res. 2009, 44, 233–244. [Google Scholar] [CrossRef]
- Morabito, C.; Steimberg, N.; Mazzoleni, G.; Guarnieri, S.; Fanò-Illic, G.; Mariggiò, M.A. RCCS Bioreactor-Based Modelled Microgravity Induces Significant Changes on In Vitro 3D Neuroglial Cell Cultures. BioMed Res. Int. 2015, 2015, 754283. [Google Scholar] [CrossRef]
- Mylabathula, P.L.; Li, L.; Bigley, A.B.; Markofski, M.M.; Crucian, B.E.; Mehta, S.K.; Pierson, D.L.; Laughlin, M.S.; Rezvani, K.; Simpson, R.J. Simulated Microgravity Disarms Human NK-Cells and Inhibits Anti-Tumor Cytotoxicity in Vitro. Acta Astronaut. 2020, 174, 32–40. [Google Scholar] [CrossRef]
- Madaan, K.; Kaushik, D.; Verma, T. Hydroxyurea: A Key Player in Cancer Chemotherapy. Expert Rev. Anticancer Ther. 2012, 12, 19–29. [Google Scholar] [CrossRef]
- Merrick, M.; Mimlitz, M.J.; Weeder, C.; Akhter, H.; Bray, A.; Walther, A.; Nwakama, C.; Bamesberger, J.; Djam, H.; Abid, K.; et al. In Vitro Radiotherapy and Chemotherapy Alter Migration of Brain Cancer Cells before Cell Death. Biochem. Biophys. Rep. 2021, 27, 101071. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in Research on Paclitaxel and Tumor Immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Prathivadhi-Bhayankaram, S.V.; Ning, J.; Mimlitz, M.; Taylor, C.; Gross, E.; Nichols, M.; Guck, J.; Ekpenyong, A.E. Chemotherapy Impedes in Vitro Microcirculation and Promotes Migration of Leukemic Cells with Impact on Metastasis. Biochem. Biophys. Res. Commun. 2016, 479, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Walter, Y.; Hubbard, A.; Benoit, A.; Jank, E.; Salas, O.; Jordan, D.; Ekpenyong, A. Development of In Vitro Assays for Advancing Radioimmunotherapy against Brain Tumors. Biomedicines 2022, 10, 1796. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Fulford, M.; Lewis, M.L. Effects of Microgravity on Osteoblast Growth Activation. Exp. Cell Res. 1996, 224, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, C.W. The ABCG2 Transporter Is an Efficient Hoechst 33342 Efflux Pump and Is Preferentially Expressed by Immature Human Hematopoietic Progenitors. Blood 2002, 99, 507–512. [Google Scholar] [CrossRef]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. Calcein-Acetyoxymethyl Cytotoxicity Assay: Standardization of a Method Allowing Additional Analyses on Recovered Effector Cells and Supernatants. Clin. Diagn. Lab. Immunol. 2001, 8, 1131–1135. [Google Scholar] [CrossRef]
- Cascione, M.; De Matteis, V.; Mandriota, G.; Leporatti, S.; Rinaldi, R. Acute Cytotoxic Effects on Morphology and Mechanical Behavior in Mcf-7 Induced by Tio2nps Exposure. Int. J. Mol. Sci. 2019, 20, 3594. [Google Scholar] [CrossRef]
- Lai, F.; Shen, Z.; Wen, H.; Chen, J.; Zhang, X.; Lin, P.; Yin, D.; Cui, H.; Chen, X. A Morphological Identification Cell Cytotoxicity Assay Using Cytoplasm-Localized Fluorescent Probe (CLFP) to Distinguish Living and Dead Cells. Biochem. Biophys. Res. Commun. 2017, 482, 257–263. [Google Scholar] [CrossRef]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-Time Deformability Cytometry: On-the-Fly Cell Mechanical Phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef]
- Asuquo, M.I.; Effa, E.; Gbotosho, O.; Otu, A.; Toepfner, N.; Ameh, S.; Bhayankaram, S.-P.; Zetocha, N.; Nwakama, C.; Egbe, W.; et al. Microfluidic Microcirculation Mimetic as a Tool for the Study of Rheological Characteristics of Red Blood Cells in Patients with Sickle Cell Anemia. Appl. Sci. 2022, 12, 4394. [Google Scholar] [CrossRef]
- Moore, M.J.; Sebastian, J.A.; Kolios, M.C. Determination of Cell Nucleus-to-Cytoplasmic Ratio Using Imaging Flow Cytometry and a Combined Ultrasound and Photoacoustic Technique: A Comparison Study. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Lele, T.P. Nuclear Morphological Abnormalities in Cancer: A Search for Unifying Mechanisms. In Results and Problems in Cell Differentiation; Springer: Cham, Switzerland, 2022; Volume 70, pp. 443–467. [Google Scholar]
- Musiałek, M.W.; Rybaczek, D. Hydroxyurea—The Good, the Bad and the Ugly. Genes 2021, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Koç, A.; Wheeler, L.J.; Mathews, C.K.; Merrill, G.F. Hydroxyurea Arrests DNA Replication by a Mechanism That Preserves Basal DNTP Pools. J. Biol. Chem. 2004, 279, 223–230. [Google Scholar] [CrossRef]
- Nichols, H.L.; Zhang, N.; Wen, X. Proteomics and Genomics of Microgravity. Physiol. Genom. 2006, 26, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Vidyasekar, P.; Shyamsunder, P.; Arun, R.; Santhakumar, R.; Kapadia, N.K.; Kumar, R.; Verma, R.S.; Schatten, H.; Lewis, M.; Chakrabarti, A.; et al. Genome Wide Expression Profiling of Cancer Cell Lines Cultured in Microgravity Reveals Significant Dysregulation of Cell Cycle and MicroRNA Gene Networks. PLoS ONE 2015, 10, e0135958. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKinley, S.; Taylor, A.; Peeples, C.; Jacob, M.; Khaparde, G.; Walter, Y.; Ekpenyong, A. Simulated Microgravity-Induced Changes to Drug Response in Cancer Cells Quantified Using Fluorescence Morphometry. Life 2023, 13, 1683. https://doi.org/10.3390/life13081683
McKinley S, Taylor A, Peeples C, Jacob M, Khaparde G, Walter Y, Ekpenyong A. Simulated Microgravity-Induced Changes to Drug Response in Cancer Cells Quantified Using Fluorescence Morphometry. Life. 2023; 13(8):1683. https://doi.org/10.3390/life13081683
Chicago/Turabian StyleMcKinley, Spencer, Adam Taylor, Conner Peeples, Megha Jacob, Gargee Khaparde, Yohan Walter, and Andrew Ekpenyong. 2023. "Simulated Microgravity-Induced Changes to Drug Response in Cancer Cells Quantified Using Fluorescence Morphometry" Life 13, no. 8: 1683. https://doi.org/10.3390/life13081683
APA StyleMcKinley, S., Taylor, A., Peeples, C., Jacob, M., Khaparde, G., Walter, Y., & Ekpenyong, A. (2023). Simulated Microgravity-Induced Changes to Drug Response in Cancer Cells Quantified Using Fluorescence Morphometry. Life, 13(8), 1683. https://doi.org/10.3390/life13081683








