Occurrence of Microplastics in Most Consumed Fruits and Vegetables from Turkey and Public Risk Assessment for Consumers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Prevention of Contamination during the Laboratory Process
2.3. Extraction of Microplastics from Fruit and Vegetable Samples
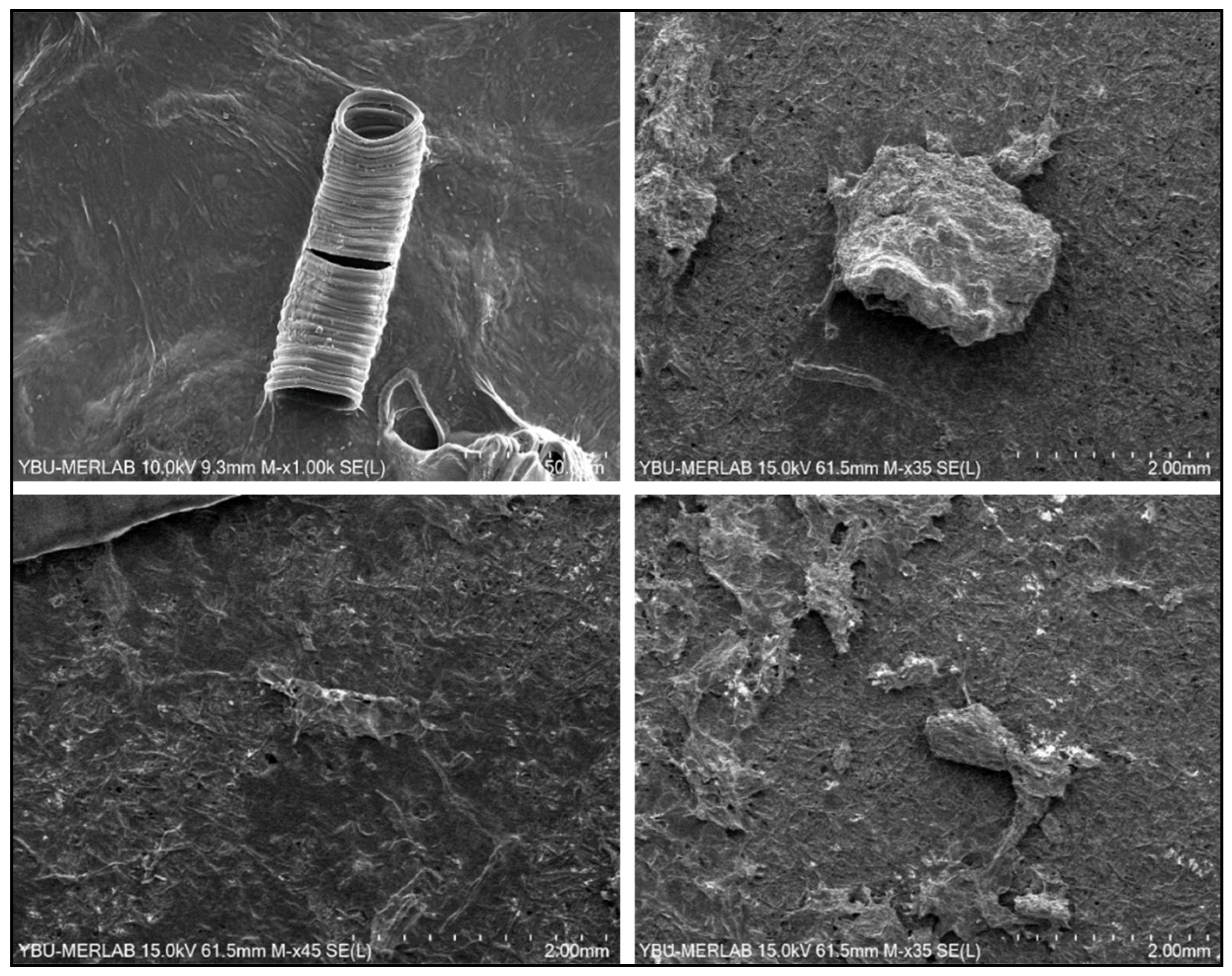
2.4. SEM Analysis
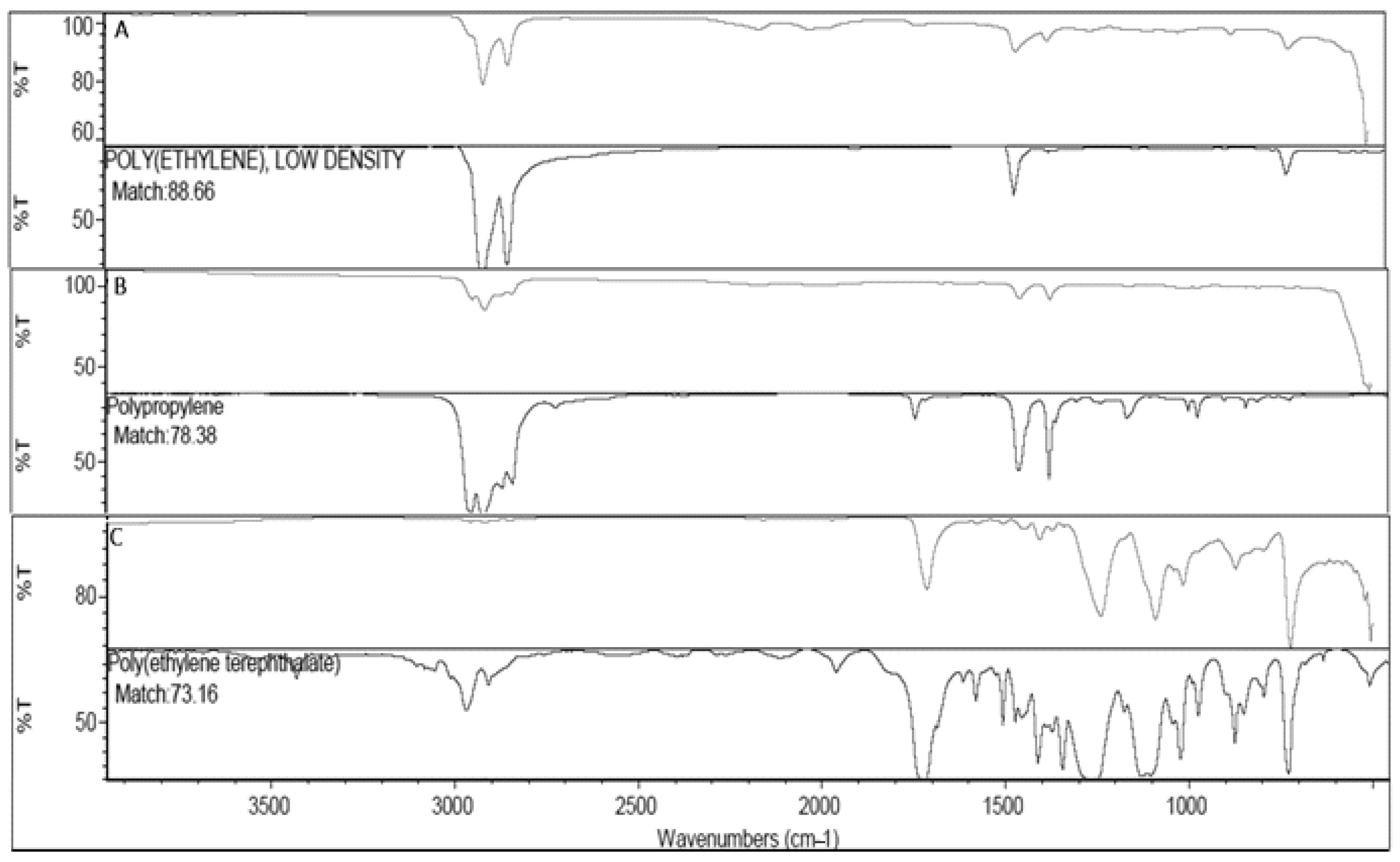
2.5. Polymers Characterization by ATR-FTIR
2.6. Risk Assessment
2.7. Statistical Analysis
3. Results
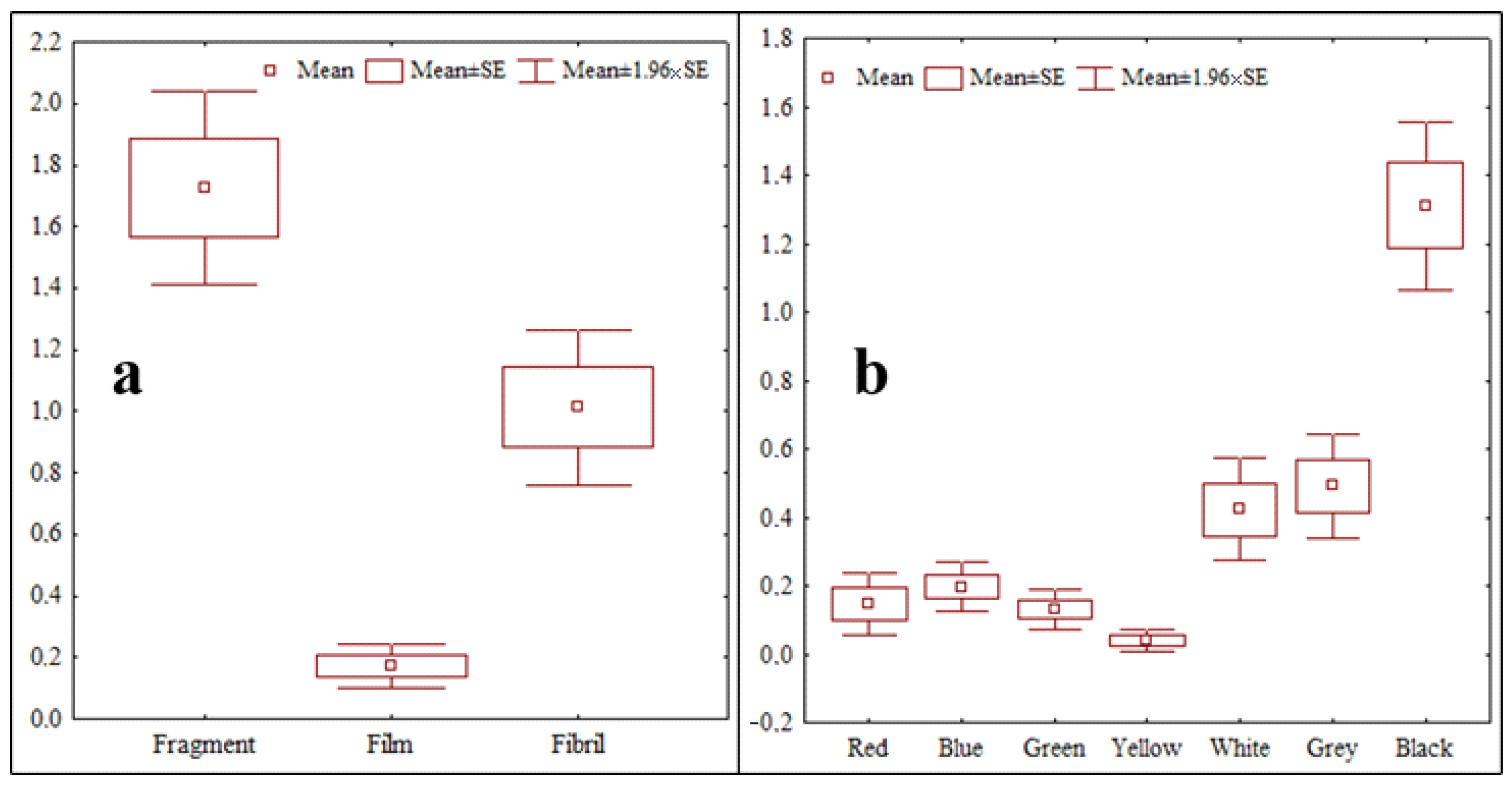
3.1. Classification of Microplastic in Terms of Abundance
3.2. Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PlasticsEurope. The Compelling Facts about Plastics 2007: An Analysis of Plastics Production, Demand and Recovery for 2007 in Europe. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2007-Compelling-facts.pdf (accessed on 25 March 2023).
- PlasticEurope. Plastics—The Facts 2022: An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2023/03/PE-PLASTICS-THE-FACTS_FINAL_DIGITAL-5.pdf (accessed on 25 March 2023).
- Kısacık, H. Depozıto and plastic bag fee applications: Accountıng process. Bus. Manag. Stud. Int. J. 2019, 7, 183. [Google Scholar]
- de Wit, W.; Hamilton, A.; Scheer, R.; Stakes, T.; Allan, S. Solving plastic pollution through accountability. WWF Plast. Rep. 2019, 25, 6–46. [Google Scholar]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Galgani, F.; Fleet, D.; Van Franeker, J.A.; Katsanevakis, S.; Maes, T.; Mouat, J.; Janssen, C. Marine Strategy Framework Directive-Task Group 10 Report Marine Litter; Office for Official Publications of the European Communities: Luxembourg, 2010. [Google Scholar]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L.; Obbard, J.P. Prevalence of microplastics in Singapore’s coastal marine environment. Mar. Pollut. Bull. 2006, 52, 761–767. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Efimova, I.; Bagaeva, M.; Bagaev, A.; Kileso, A.; Chubarenko, I.P. Secondary microplastics generation in the sea swash zone with coarse bottom sediments: Laboratory experiments. Front. Mar. Sci. 2018, 5, 313. [Google Scholar] [CrossRef]
- Kazour, M.; Jemaa, S.; Issa, C.; Khalaf, G.; Amara, R. Microplastics pollution along the Lebanese coast (Eastern Mediterranean Basin): Occurrence in surface water, sediments and biota samples. Sci. Total Environ. 2019, 696, 133933. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.E.; Bamford, H.A. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris; NOAA Technical Memorandum NOS-OR & R-30; University of Washington Tacoma: Tacoma, WA, USA, 2008. [Google Scholar]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- GESAMP. Sources, fate and effects of microplastics in the marine environment: A global assessment. In Reports and Studies-IMO/FAO/Unesco-IOC/WMO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP) Eng No. 93; Kershaw, P.J., Ed.; International Maritime Organization: London, UK, 2015; Volume 90, 96p. [Google Scholar]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C.; Herring, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Technical Memorandum NOS-OR&R-48; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015; 31p. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Statement on the presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, 4501. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017; Volume 615, 147p. [Google Scholar]
- Lebreton, L.; Egger, M.; Slat, B. A global mass budget for positively buoyant macroplastic debris in the ocean. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene spherules in coastal waters. Science 1972, 17, 749–750. Available online: http://links.jstor.org/sici?sici=0036-8075%2819721117%293%3A178%3A4062%3C749%3APSICW%3E2.0.CO%3B2-E (accessed on 14 June 2023). [CrossRef] [PubMed]
- Gregory, M.R. Plastic pellets on New Zealand beaches. Mar. Pollut. Bull. 1977, 8, 82–84. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Rochman, C.M. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Horton, A.A.; Barnes, D.K. Microplastic pollution in a rapidly changing world: Implications for remote and vulnerable marine ecosystems. Sci. Total Environ. 2020, 738, 140349. [Google Scholar] [CrossRef]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Hu, D.; Shen, M.; Zhang, Y.; Li, H.; Zeng, G. Microplastics and nanoplastics: Would they affect global biodiversity change? Environ. Sci. Pollut. Res. 2019, 26, 19997–20002. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Takada, H. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef]
- Mammo, F.K.; Amoah, I.D.; Gani, K.M.; Pillay, M.; Ratha, S.K.; Bux, F.; Kumari, S. Microplastics in the environment: Interactions with microbes and chemical contaminants. Sci. Total Environ. 2020, 743, 140518. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef]
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Cai, Y. Focus topics on microplastics in soil: Analytical methods, occurrence, transport, and ecological risks. Environ. Pollut. 2020, 257, 113570. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Souza Machado, A.A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. 2009, 364, 2097–2113. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalona, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed]
- Akoueson, F.; Sheldon, L.M.; Danopoulos, E.; Morris, S.; Hotten, J.; Chapman, E.; Li, J.; Rotchell, J.M. A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environ. Pollut. 2020, 263, 114452. [Google Scholar] [CrossRef]
- Rashid, C.P.; Jyothibabu, R.; Arunpandi, N.; Abhijith, V.T.; Josna, M.P.; Vidhya, V.; Gupta, G.V.M.; Ramanamurty, M.V. Microplastics in zooplankton in the eastern Arabian Sea: The threats they pose to fish and corals favoured by coastal currents. Mar. Pollut. Bull. 2021, 173, 113042. [Google Scholar] [CrossRef] [PubMed]
- Yozukmaz, A. Investigation of microplastics in edible wild mussels from İzmir Bay (Aegean Sea, Western Turkey): A risk assessment for the consumers. Mar. Pollut. Bull. 2021, 171, 112733. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. 2014, 31, 1574–1578. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential health impact of environmentally released micro-and nanoplastics in the human food production chain: Experiences from nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Know, J.H.; Kim, J.W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.H.; Lee, S.H.; Kang, D.Y.; Kim, J.Y.; Kim, S.B.; et al. Microplastics in food: A review on analytical methods and challenges. Int. J. Environ. Health Res. 2020, 17, 6710. [Google Scholar]
- Mei, T.; Wang, J.; Xiao, X.; Lv, J.; Li, Q.; Dai, H.; Liu, X.; Pi, F. Identification and Evaluation of Microplastics from Tea Filter Bags Based on Raman Imaging. Foods 2022, 11, 2871. [Google Scholar] [CrossRef]
- Conti, G.O.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro-and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Uptake of microplastics by carrots in presence of As (III): Combined toxic effects. J. Hazard. Mater. 2021, 411, 125055. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Rajendiran, R.; Pasupathi, M.S.; Ahamed, S.B.N.; Kalyanasundaram, P.; Velu, R.K. Authentication of Microplastic Accumulation in Customary Fruits and Vegetables. Preprint 2022, 1–12. [Google Scholar] [CrossRef]
- Torre, M.; Digka, N.; Anastasopoulou, A.; Tsangaris, C.; Mytilineou, C. Anthropogenic microfibres pollution in marine biota. A new and simple methodology to minimize airborne contamination. Mar. Pollut. Bull. 2016, 113, 55–61. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic collection techniques. In Microplastic Pollutants, 1st ed.; Elsevier Limited: Amsterdam, The Netherland, 2017; pp. 179–202. [Google Scholar] [CrossRef]
- Lusher, A.L.; Burke, A.; O’Connor, I.; Officer, R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017, 36, 947–951. [Google Scholar] [CrossRef] [PubMed]
- La Daana, K.K.; Officer, R.; Lyashevska, O.; Thompson, R.C.; O’Connor, I. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 2017, 115, 307–314. [Google Scholar]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Lots, F.A.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A large-scale investigation of microplastic contamination: Abundance and characteristics of microplastics in European beach sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef]
- Avio, G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef]
- Garcia, A.G.; Suárez, D.C.; Li, J.; Rotchell, J.M. A comparison of microplastic contamination in freshwater fish from natural and farmed sources. Environ. Sci. Pollut. Res. 2021, 28, 14488–14497. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Qu, L.; Zheng, Y.; He, C. Detection of microplastics in local marine organisms using a multi-technology system. Anal. Methods 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Anuar, S.T.; Abdullah, N.S.; Yahya, N.K.E.; Chin, T.T.; Yusoff, K.M.K.K.; Mohamad, Y.; Azmi, A.A.; Jaafar, M.; Mohammad, N.; Khalik, W.M.A.W.M.; et al. A multidimensional approach for microplastics monitoring in two major tropical river basins, Malaysia. Environ. Res. 2023, 227, 115717. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Guidance For Assessing Chemical Contamination Data for Use in Fish Advisories: Volume II Risk Assessment and Fish Consumption Limits, 3rd ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 2000; 383p.
- Lin, Q.; Zhao, S.; Pang, L.; Sun, C.; Chen, L.; Li, F. Potential risk of microplastics in processed foods: Preliminary risk assessment concerning polymer types, abundance, and human exposure of microplastics. Ecotoxicol. Environ. Saf. 2022, 247, 114260. [Google Scholar] [CrossRef]
- TURKSTAT. Address Based Population Registration System Results; Turkish Statistical Institute: Ankara, Turkey, 2020.
- Copat, C.; Vinceti, M.; D’Agati, M.G.; Arena, G.; Mauceri, V.; Grasso, A.; Fallico, R.; Sciacca, S.; Ferrante, M. Mercury and selenium intake by seafood from the Ionian Sea: A risk evaluation. Ecotoxicol. Environ. Saf. 2014, 100, 87–92. [Google Scholar] [CrossRef]
- Wakkaf, T.; El Zrelli, R.; Kedzierski, M.; Balti, R.; Shaiek, M.; Mansour, L.; Tlig-Zouari, S.; Bruzaud, S.; Rabaoui, L. Microplastics in edible mussels from a southern Mediterranean lagoon: Preliminary results on seawater-mussel transfer and implications for environmental protection and seafood safety. Mar. Pollut. Bull. 2020, 158, 111355. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Dobaradaran, S.; Nabipour, I.; Tajbakhsh, S.; Darabi, A.H.; Spitz, J. Abundance, composition, and potential intake of microplastics in canned fish. Mar. Pollut. Bull. 2020, 160, 111633. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series, No. 916; WHO: Geneva, Switzerland, 2003; 149p. [Google Scholar]
- Ng, E.L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total Environ. 2022, 805, 150431. [Google Scholar] [CrossRef]
- Rilling, M.C.; Ingraffia, R.; de Souza Machado, A.A. Microplastic incorporation into soil in agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef] [PubMed]
- O’Conner, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Zou, M.; Jia, Z.; Zhou, S.; Li, Y. Microplastics in soils: A review of methods, occurrence, fate, transport, ecological and environmental risks. Sci. Total Environ. 2020, 748, 141368. [Google Scholar] [CrossRef] [PubMed]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and accumulation of nano/microplastics in plants: A critical review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Gan, Q.; Cui, J.; Jin, B. Environmental microplastics: Classification, sources, fates, and effects on plants. Chemosphere 2022, 313, 137559. [Google Scholar] [CrossRef]
- Sharma, U.; Sharma, S.; Rana, V.S.; Rana, N.; Kumar, V.; Sharma, S.; Qadri, H.; Kumar, V.; Bhat, S.A. Assessment of microplastics pollution on soil health and eco-toxicological risk in horticulture. Soil Syst. 2023, 7, 7. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Q.; Yin, N.; Tu, C.; Luo, Y. Uptake and accumulation of microplastics in an edible plant. Sci. Bull. 2019, 64, 928–934. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Li, R.; Zhou, J.; Wang, G. The distribution and impact of polystyrene nanoplastics on cucumber plants. Environ. Sci. Pollut. Res. 2021, 28, 16042–16053. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef]
- Lv, L.; Yan, X.; Feng, L.; Jiang, S.; Lu, Z.; Xie, H.; Sun, S.; Chen, J.; Li, C. Challenge for the detection of microplastics in the environment. Water Environ. Res. 2021, 93, 5–15. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Guo, T.; Li, J.; Wang, F. Optimize lettuce washing methods to reduce the risk of microplastics ingestion: The evidence from microplastics residues on the surface of lettuce leaves and in the lettuce washing wastewater. Sci. Total Environ. 2023, 868, 161726. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.C.; Mazor, M.H.; Keoleian, G.A. Plastics in the US: Toward a material flow characterization of production, markets and end of life. Environ. Res. Lett. 2020, 15, 094034. [Google Scholar] [CrossRef]
- White, A.; Lockyer, S. Removing plastic packaging from fresh produce–what’s the impact? Nutr. Bull. 2020, 45, 35–50. [Google Scholar] [CrossRef]
- Terry, L.A.; Mena, C.; Williams, A.; Jenney, N.; Whitehead, P. Fruit and Vegetable Resource Maps: Mapping Fruit and Vegetable Waste through the Wholesale Supply Chain; Final Report RSC008; Waste & Resources Action Programme (WRAP): Banbury, UK, 2011; 95p. [Google Scholar]
- Waste & Resources Action Programme. Evidence Review: Plastic Packaging and Fresh Produce. Available online: http://www.wrap.org.uk/content/evidence-review-plastic-packaging-and-fresh-produce (accessed on 6 November 2019).
- Fadare, O.O.; Wan, B.; Guo, L.H.; Zhao, L. Microplastics from consumer plastic food containers: Are we consuming it? Chemosphere 2020, 253, 126787. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Aquaculture Newsletter; FAO: Rome, Italy, 2017; Volume 57, 64p. [Google Scholar]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Conti, G.O. Exposure to microplastics (<10 μm) associated to plastic bottles mineral water consumption: The first quantitative study. Water Res. 2019, 157, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208–222. [Google Scholar] [CrossRef]
- Renzella, J.; Townsend, N.; Jewell, J.; Breda, J.; Roberts, N.; Rayner, M.; Wickramasinghe, K. What National and Subnational Interventions and Policies Based on Mediterranean and Nordic Diets are Recommended or Implemented in the WHO European Region and Is There Evidence of Effectiveness in Reducing Noncommunicable Diseases, 1st ed.; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2018; 58p. [Google Scholar]


| Purchase Site | Product | ||||||
|---|---|---|---|---|---|---|---|
| Pear | Tomato | Apple | Potatoes | Cucumber | Onion | Mean | |
| M1 | 3.7 ± 0.6 | 3.3 ± 2.1 | 3.5 ± 0.7 | 0.5 ± 0.5 | 3.5 ± 3.3 | 3.5 ± 2.3 | 3.0 ± 2 |
| M2 | 3.0 ± 0.7 | 3.7 ± 0.5 | 2.2 ± 0.7 | 1.0 ± 0.8 | 4.1 ± 0.8 | 2.8 ± 0.6 | 2.8 ± 1.2 |
| F1 | 3.6 ± 0.5 | 4.9 ± 0.8 | 3.5 ± 2.2 | 1.0 ± 0.7 | 2.1 ± 1.1 | 2.5 ± 1.7 | 2.9 ± 1.7 |
| F2 | 2.2 ± 2.4 | 2.5 ± 0.8 | 3 ± 0.9 | 3.3 ± 2.1 | 4.6 ± 0.9 | 1.7 ± 0.8 | 2.9 ± 1.6 |
| Mean | 3.1 ± 1.3 | 3.6 ± 1.4 | 3.1 ± 1.2 | 1.5 ± 1.6 | 3.6 ± 1.8 | 2.6 ± 1.5 | 2.9 ± 1.6 |
| EAI (Particles Individual−1 Year−1) | EDI (Particles kg−1 Day−1) | ||
|---|---|---|---|
| Children | Adult | ||
| Pear | 16,127.6 | 2.76 | 0.63 |
| Apple | 96,617.4 | 16.54 | 3.78 |
| Tomato | 398,520.0 | 68.24 | 15.60 |
| Onion | 54,860.0 | 9.39 | 2.15 |
| Potatoes | 76,950.0 | 13.18 | 3.01 |
| Cucumber | 66,600.0 | 11.40 | 2.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydın, R.B.; Yozukmaz, A.; Şener, İ.; Temiz, F.; Giannetto, D. Occurrence of Microplastics in Most Consumed Fruits and Vegetables from Turkey and Public Risk Assessment for Consumers. Life 2023, 13, 1686. https://doi.org/10.3390/life13081686
Aydın RB, Yozukmaz A, Şener İ, Temiz F, Giannetto D. Occurrence of Microplastics in Most Consumed Fruits and Vegetables from Turkey and Public Risk Assessment for Consumers. Life. 2023; 13(8):1686. https://doi.org/10.3390/life13081686
Chicago/Turabian StyleAydın, Rana Berfin, Aykut Yozukmaz, İdris Şener, Funda Temiz, and Daniela Giannetto. 2023. "Occurrence of Microplastics in Most Consumed Fruits and Vegetables from Turkey and Public Risk Assessment for Consumers" Life 13, no. 8: 1686. https://doi.org/10.3390/life13081686







