Incidence and Clinical Implications of Anatomical Variations in the Pancreas and Its Ductal System: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Protocol
2.2. Electronic Search
2.3. Eligibility Criteria
2.4. Assessment of the Methodological Quality of the Included Studies
2.5. Data Collection Process
2.6. Statistical Methods
3. Results
3.1. Description of the Variants Studied
3.2. Characteristics Reported in the Articles
3.3. Prevalence and Risk of Bias
3.4. Clinical Considerations
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorczyca, J.; Tomaszewski, K.A.; Henry, B.M.; Pękala, P.A.; Pasternak, A.; Mizia, E.; Walocha, J.A. The Vascular Microarchitecture of the Human Fetal Pancreas. A Corrosion Casting and Scanning Electron Microscopy Study. Pancreas 2017, 46, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Toyama, H.; Tsugawa, D.; Kido, M.; Fukumoto, T. Two-in-one method: Novel pancreaticojejunostomy technique for the bifid pancreas. Ann. Gastroenterol. Surg. 2019, 4, 175–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourad, N.; Zhang, J.; Rath, A.; Chevrel, J. The venous drainage of the pancreas. Surg. Radiol. Anat. 1994, 16, 37–45. [Google Scholar] [CrossRef]
- Avisse, C.; Flament, J.-B.; Delattre, J.-F. AMPULLA OF VATER: Anatomic, Embryologic, and Surgical Aspects. Surg. Clin. N. Am. 2000, 80, 201–212. [Google Scholar] [CrossRef]
- Chey, W.Y.; Chang, T.-M. Neural hormonal regulation of exocrine pancreatic secretion. Pancreatology 2001, 1, 320–335. [Google Scholar] [CrossRef]
- Von Schönfeld, J.; Goebell, H.; Mütter, M.K. The islet-acinar axis of the pancreas. Int. J. Pancreatol. 1994, 16, 131–140. [Google Scholar] [CrossRef]
- Walkowska, J.; Zielinska, N.; Tubbs, R.S.; Podgórski, M.; Dłubek-Ruxer, J.; Olewnik, Ł. Diagnosis and Treatment of Acute Pancreatitis. Diagnostics 2022, 12, 1974. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Henry, B.M.; Tomaszewski, K.A.; Walocha, J.A. Methods of Evidence-Based Anatomy: A guide to conducting systematic reviews and meta-analysis of anatomical studies. Ann. Anat. 2016, 205, 16–21. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. Computer Software, Version 4.0. Available online: https://cran.r-project.org (accessed on 1 April 2021).
- Kanasker, N.; Bharambe, V. Study of annular pancreas—A rare finding. J. Anat. Soc. India 2016, 65, 12. [Google Scholar] [CrossRef]
- Qin, J.; Xu, J.; Xing, J. Portal annular pancreas: A case report. Asian J. Surg. 2019, 42, 708–710. [Google Scholar] [CrossRef]
- Yang, B.; He, F.; He, Q.; Wang, Z.; Fang, Q.; Zhong, W.; Wang, H. Diagnostic value of the acute angle between the prestenotic and poststenotic duodenum in neonatal annular pancreas. Eur. Radiol. 2019, 29, 2902–2909. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X. Investigation of annular pancreas through multiple detector spiral CT (MDCT) and MRI. J. Appl. Clin. Med. Phys. 2022, 23, e13487. [Google Scholar] [CrossRef]
- Gromski, M.A.; Lehman, G.A.; Zyromski, N.J.; Watkins, J.L.; El Hajj, I.I.; Tan, D.; McHenry, L.; Easler, J.J.; Tirkes, T.; Sherman, S.; et al. Annular pancreas: Endoscopic and pancreatographic findings from a tertiary referral ERCP center. Gastrointest. Endosc. 2019, 89, 322–328. [Google Scholar] [CrossRef]
- Adibelli, Z.H.; Adatepe, M.; Imamoglu, C.; Esen, O.S.; Erkan, N.; Yildirim, M. Anatomic variations of the pancreatic duct and their relevance with the Cambridge classification system: MRCP findings of 1158 consecutive patients. Radiol. Oncol. 2016, 50, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Jarrar, M.S.; Khenissi, A.; Ghrissi, R.; Hamila, F.; Letaief, R. Ansa pancreatica: An anatomic variation and a rare cause of acute pancreatitis. Surg. Radiol. Anat. 2013, 35, 745–748. [Google Scholar] [CrossRef]
- Malathi, K.; Prasad, K.; Chitra, R. A study of duct system of pancreas and its variations. J. Anat. Soc. India 2017, 66, S36. [Google Scholar] [CrossRef]
- Tajima, Y.; Adachi, T.; Kuroki, T.; Tsuneoka, N.; Mishima, T.; Kosaka, T.; Kanematsu, T. Intraductal papillary mucinous neoplasm of the pancreas with a bifid pancreatic duct. J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 865–868. [Google Scholar] [CrossRef] [Green Version]
- Halpert, R.D.; Shabot, J.M.; Heare, B.R.; Rogers, R.E. The bifid pancreas: A rare anatomical variation. Gastrointest. Endosc. 1990, 36, 60–61. [Google Scholar] [CrossRef]
- Addeo, P.; Locicero, A.; Bachellier, P. Circumportal pancreas. J. Visc. Surg. 2019, 156, 467–468. [Google Scholar] [CrossRef]
- Gonoi, W.; Akahane, M.; Akai, H.; Hagiwara, K.; Kiryu, S.; Hayashi, N.; Ohtomo, K. Retroportal main pancreatic duct with circumportal pancreas: Radiographic visualization. Clin. Imaging 2011, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Mori, Y.; Ishigami, K.; Fujimoto, T.; Miyasaka, Y.; Nakata, K.; Ohuchida, K.; Nagai, E.; Oda, Y.; Shimizu, S.; et al. Clinical significance of circumportal pancreas, a rare congenital anomaly, in pancreatectomy. Am. J. Surg. 2016, 214, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Celik, A. Circumportal pancreas: Prevalence, subtypes and vascular variations of 55 patients. Surg. Radiol. Anat. 2018, 40, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.M.; Braumann, C.; Herzog, T.; Janot, M.; Uhl, W.; Chromik, A.M. Circumportal Pancreas—A Must Know Pancreatic Anomaly for the Pancreatic Surgeon. J. Gastrointest. Surg. 2017, 21, 344–351. [Google Scholar] [CrossRef]
- Kiuchi, R.; Mizuno, T.; Okamura, Y.; Sugiura, T.; Kanemoto, H.; Uesaka, K. Circumportal pancreas—A hazardous anomaly in pancreatic surgery. HPB 2018, 20, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Suh, J.H.; Park, B.K.; Park, S.W.; Song, S.Y.; Chung, J.B. The Relationship of Anatomic Variation of Pancreatic Ductal System and Pancreaticobiliary Diseases. Yonsei Med. J. 2006, 47, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Delhaye, M.; Engelholm, L.; Cremer, M. Pancreas divisum: Congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology 1985, 89, 951–958. [Google Scholar] [CrossRef]
- Coruh, A.G.; Gulpinar, B.; Bas, H.; Erden, A. Frequency of bile duct confluence variations in subjects with pancreas divisum: An analysis of MRCP findings. Diagn. Interv. Radiol. 2018, 24, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Myung, S.J.; Lee, S.S.; Lee, S.K.; Kim, M.H. Classification and Nomenclature of Gallstones Revisited. Yonsei Med. J. 2003, 44, 561–570. [Google Scholar] [CrossRef]
- Montagnani, M.; Cazzato, S.; Mutignani, M.; Cevenini, M.; Guidetti, E.; Ben Zvi, I.; Aldini, R.; Saraceni, G.; Cavoli, C.; Garagnani, P.; et al. A Patient with Pancreas Divisum, Recurrent Acute Pancreatitis, and Homozygosity for the Cystic Fibrosis Transmembrane Regulator–Associated Protein 5T Allele. Clin. Gastroenterol. Hepatol. 2013, 11, 579–581. [Google Scholar] [CrossRef]
- Morgan, K.A.; Romagnuolo, J.; Adams, D.B. Transduodenal Sphincteroplasty in the Management of Sphincter of Oddi Dysfunction and Pancreas Divisum in the Modern Era. J. Am. Coll. Surg. 2008, 206, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Nahmod, M.; Alle, L.; Ferraina, P. Pancreas divisum pancreatitis: Surgical sphincteroplasty, a case report. Pancreatology 2017, 17, S43. [Google Scholar] [CrossRef]
- Pina, L.N.; Tejedor, M.P.; Carles, G.; Alle, L.; Sarotto, L. ¿Tiene el páncreas divisum un rol determinante en la pancreatitis aguda? Rev. Chil. Cir. 2017, 69, 459–466. [Google Scholar] [CrossRef]
- Sanada, Y.; Yoshizawa, Y.; Chiba, M.; Nemoto, H.; Midorikawa, T.; Kumada, K. Ventral pancreatitis in a patient with pancreas divisum. J. Pediatr. Surg. 1995, 30, 665–667. [Google Scholar] [CrossRef]
- Sugawa, C.; Walt, A.J. Endoscopic retrograde pancreatography in the surgery of pancreatic pseudocysts. Surgery 1979, 86, 639–647. [Google Scholar]
- White, J.J.; Roberts, Z.N.; Gest, T.R.; Beale, E.G. Pancreas divisum: A common developmental variant that deserves attention in preclinical medical education. Clin. Anat. 2014, 27, 1038–1045. [Google Scholar] [CrossRef]
- Kumar, N.; Aithal, A.P.; Guru, A. Unusual duplication and vulnerable intrapancreatic course of the left gastroepiploic artery: A rare anatomical variation. Surg. Radiol. Anat. 2019, 41, 351–353. [Google Scholar] [CrossRef]
- Ross, B.A.; Jeffrey, R.B.; Mindelzun, R.E.; Ross, R.B.J.B.A.; Yu, J.; Turner, M.A.; Fulcher, A.S.; Halvorsen, R.A.; Martin, L.C.; Merkle, E.M.; et al. Normal variations in the lateral contour of the head and neck of the pancreas mimicking neoplasm: Evaluation with dual-phase helical CT. Am. J. Roentgenol. 1996, 166, 799–801. [Google Scholar] [CrossRef]
- Kubota, Y.; Yamaguchi, T.; Tani, K.; Takaoka, M.; Fujimura, K.; Ogura, M.; Yamamoto, S.; Mizuno, T.; Inoue, K. Anatomical variation of pancreatobiliary ducts in biliary stone diseases. Abdom. Imaging 1993, 18, 145–149. [Google Scholar] [CrossRef]
- Sherifi, F.; Bexheti, S.; Gashi, Z.; Bajraktari, I.; Shatri, J.; Lahu, A. Anatomic Variations of Pancreaticobiliary Union. Open Access Maced. J. Med. Sci. 2018, 6, 988–991. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.J.; Aggarwal, A.; Kochhar, R.K.; Yadav, T.D.; Gupta, T.; Sahni, D. Closed loop of main duct of pancreas: A rare variant configuration. Surg. Radiol. Anat. 2017, 39, 1405–1407. [Google Scholar] [CrossRef]
- Moffatt, D.C.; Coté, G.A.; Avula, H.; Watkins, J.L.; McHenry, L.; Sherman, S.; Lehman, G.A.; Fogel, E.L. Risk factors for ERCP-related complications in patients with pancreas divisum: A retrospective study. Gastrointest. Endosc. 2011, 73, 963–970. [Google Scholar] [CrossRef]
- Adike, A.; El Kurdi, B.I.; Gaddam, S.; Kosiorek, H.E.; Fukami, N.; Faigel, D.O.; Collins, J.M.; Ramirez, F.C. Pancreatitis in Patients with Pancreas Divisum. Pancreas 2017, 46, e80–e81. [Google Scholar] [CrossRef] [PubMed]
- Alazmi, W.M.; Mosler, P.; Watkins, J.L.; McHenry, L.; Fogel, E.L.; Sherman, S.; Lehman, G.A. Predicting Pancreas Divisum by Inspection of the Minor Papilla: A Prospective Study. J. Clin. Gastroenterol. 2007, 41, 422–426. [Google Scholar] [CrossRef]
- Brenner, P.; Duncombe, V.; Ham, J.M. Pancreatitis and Pancreas Divisum: Aetiological and Surgical Considerations. ANZ J. Surg. 1990, 60, 899–903. [Google Scholar] [CrossRef]
- Chacko, L.N.; Chen, Y.K.; Shah, R.J. Clinical outcomes and nonendoscopic interventions after minor papilla endotherapy in patients with symptomatic pancreas divisum. Gastrointest. Endosc. 2008, 68, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Tu, Y.; Egawa, N.; Tsuruta, K.; Okamoto, A.; Matsukawa, M. Pancreas divisum in pancreaticobiliary maljunction. Hepato-Gastroenterol. 2008, 55, 249–253. [Google Scholar]
- Kin, T.; Shapiro, A.M.J.; Lakey, J.R. Pancreas Divisum: A Study of the Cadaveric Donor Pancreas for Islet Isolation. Pancreas 2005, 30, 325–327. [Google Scholar] [CrossRef]
- Meng, Q.-Q.; Zhao, S.-B.; Wang, Z.-J.; Shen, Z.; Xia, T.; Wang, S.-L.; Gu, L.; Pan, P.; Li, Z.-S.; Yao, J.; et al. Incidence and risk factors for post-ERCP pancreatitis in pancreas divisum patients without chronic pancreatitis. Scand. J. Gastroenterol. 2020, 55, 732–736. [Google Scholar] [CrossRef]
- Pappas, S.G.; Pilgrim, C.H.C.; Keim, R.; Harris, R.; Wilson, S.; Turaga, K.; Tsai, S.; Dua, K.; Khan, A.; Oh, Y.; et al. The Frey Procedure for Chronic Pancreatitis Secondary to Pancreas Divisum. JAMA Surg. 2013, 148, 1057–1062. [Google Scholar] [CrossRef]
- Rustagi, T.; Golioto, M. Diagnosis and therapy of pancreas divisum by ERCP: A single center experience. J. Dig. Dis. 2013, 14, 93–99. [Google Scholar] [CrossRef]
- Sugawa, C.; Walt, A.J.; Nunez, D.C.; Masuyama, H. Pancreas divisum: Is it a normal anatomic variant? Am. J. Surg. 1987, 153, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Kamisawa, T.; Tabata, T.; Egawa, N.; Igarashi, Y. Pancreatic Diseases Associated with Pancreas Divisum. Dig. Surg. 2010, 27, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-B. Pancreatitis in patients with pancreas divisum: Imaging features at MRI and MRCP. World J. Gastroenterol. 2013, 19, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Marfil-Garza, B.A.; Shapiro, A.M.J.; Kin, T. Circumportal pancreas accompanied with pancreas divisum in a deceased donor for islet transplantation. Surg. Radiol. Anat. 2018, 40, 1323–1325. [Google Scholar] [CrossRef]
- Mosler, P.; Akisik, F.; Sandrasegaran, K.; Fogel, E.; Watkins, J.; Alazmi, W.; Sherman, S.; Lehman, G.; Imperiale, T.; McHenry, L. Accuracy of Magnetic Resonance Cholangiopancreatography in the Diagnosis of Pancreas Divisum. Dig. Dis. Sci. 2012, 57, 170–174. [Google Scholar] [CrossRef]
- Bret, P.M.; Reinhold, C.; Taourel, P.; Guibaud, L.; Atri, M.; Barkun, A.N. Pancreas divisum: Evaluation with MR cholangiopancreatography. Radiology 1996, 199, 99–103. [Google Scholar] [CrossRef]
- Malathi, S.; Nandhakumar, P.; Pandiyan, V.; Webster, T.J.; Balasubramanian, S. Novel PLGA-based nanoparticles for the oral delivery of insulin. Int. J. Nanomed. 2015, 10, 2207–2218. [Google Scholar] [CrossRef] [Green Version]
- Tappouni, R.; Perumpillichira, J.; Sekala, M.; Hosseinzadeh, K.; Clark, C.; Leyendecker, J. Circumportal pancreas: Imaging findings in 40 patients. Abdom. Imaging 2015, 40, 521–530. [Google Scholar] [CrossRef]
- Taj, M.A. ’ Qureshi, S.’ Ghazanfar, S.’ Siddiqui, A.R.; Niaz, S.K.; Quraishy, M.S.; Shahid, M. Pancreas Divisum. J. Coll. Physicians Surg. Pak. 2016, 26, 96–99. [Google Scholar]
- Warshaw, A.L.; Richter, J.M.; Schapiro, R.H. The Cause and Treatment of Pancreatitis Associated with Pancreas Divisum. Ann. Surg. 1983, 198, 443–452. [Google Scholar] [CrossRef]
- Cubilla, A.L.; Fitzgerald, P.J. (Eds.) Gross anatomy. In Tumors of the Exocrine Pancreas; 2nd series, Fascicle 19; Armed Forces Institute of Pathology: Washington, DC, USA, 1984; pp. 31–52. [Google Scholar]
- Asayama, Y.; Fang, W.; Stolpen, A.; Kuehn, D. Detectability of pancreas divisum in patients with acute pancreatitis on multi-detector row computed tomography. Emerg. Radiol. 2012, 19, 121–125. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Harper, S.J.F. Anatomical Variation and Its Management in Transplantation. Am. J. Transplant. 2015, 15, 1459–1471. [Google Scholar] [CrossRef]
- Yamauchi, S.; Koga, A.; Matsumoto, S.; Tanaka, M.; Nakayama, F. Anomalous junction of pancreaticobiliary duct without congenital choledochal cyst: A possible risk factor for gallbladder cancer. Am. J. Gastroenterol. 1987, 82, 20–24. [Google Scholar] [PubMed]
- Garber, A.; Frakes, C.; Arora, Z.; Chahal, P. Mechanisms and Management of Acute Pancreatitis. Gastroenterol. Res. Pract. 2018, 2018, 6218798. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Raju, R.S.; Vyas, F.L.; Eapen, A.; Sitaram, V. Portal annular pancreas. A rare variant and a new classification. JOP 2010, 11, 453–455. [Google Scholar] [PubMed]
- Dimitriou, I.; Katsourakis, A.; Nikolaidou, E.; Noussios, G. The Main Anatomical Variations of the Pancreatic Duct System: Review of the Literature and Its Importance in Surgical Practice. J. Clin. Med. Res. 2018, 10, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Bertin, C.; Pelletier, A.-L.; Vullierme, M.P.; Bienvenu, T.; Rebours, V.; Hentic, O.; Maire, F.; Hammel, P.; Vilgrain, V.; Ruszniewski, P.; et al. Pancreas Divisum Is Not a Cause of Pancreatitis by Itself But Acts as a Partner of Genetic Mutations. Am. J. Gastroenterol. 2012, 107, 311–317. [Google Scholar] [CrossRef]
- Spaziani, E.; Trentino, P.; Picchio, M.; Di Filippo, A.; Briganti, M.; Pietricola, G.; Elisei, W.; Ceci, F.; Coda, S.; Pattaro, G.; et al. Endoscopic sphincterotomy of the major duodenal papilla in acute relapsing pancreatitis associated with pancreas divisum: A case report. G. Chir. 2010, 31, 233–235. [Google Scholar]
- Manso, V.; Pou, J.; Iturralde, A. Páncreas anular: Presentación de un caso. Rev. Cuba. Cir. 1987, 26, 85–88. [Google Scholar]
- Skandalakis, L.J.; Rowe, J.S.; Gray, S.W.; Skandalakis, J.E. Surgical Embryology and Anatomy of the Pancreas. Surg. Clin. N. Am. 1993, 73, 661–697. [Google Scholar] [CrossRef] [PubMed]


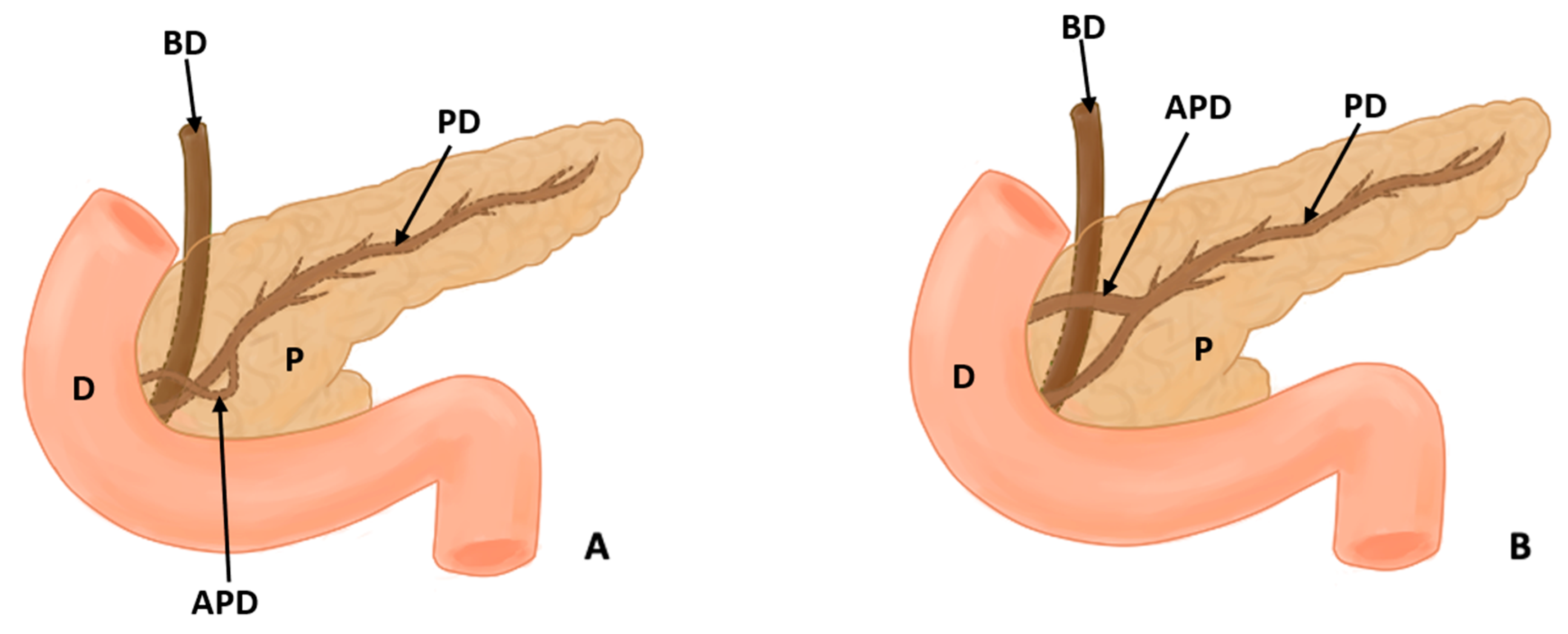
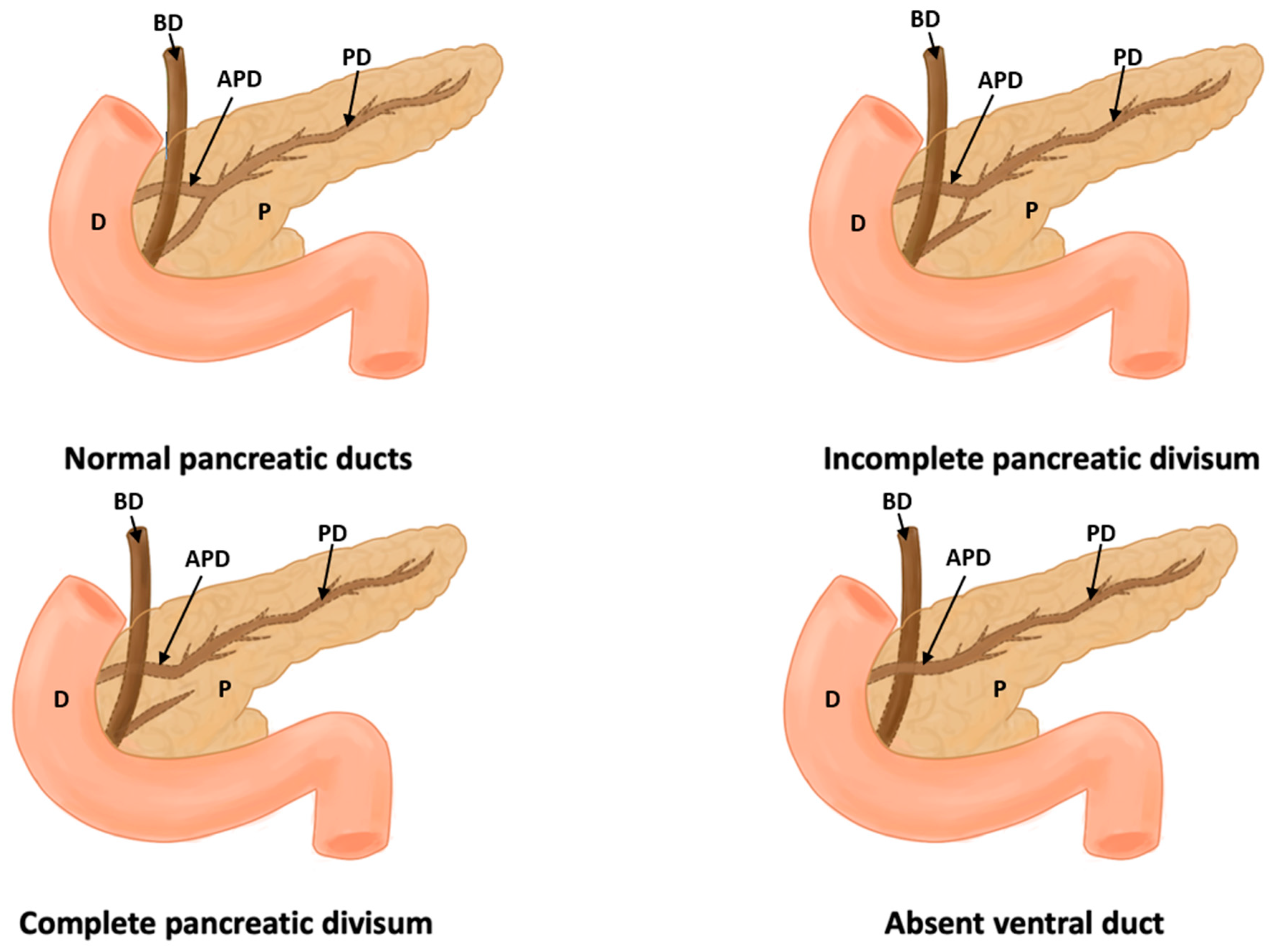


| Author(s), Year | Type of Study and Number of Participants (N) | Incidence | Statistical Values | Geographic Region | Sex/Gender |
|---|---|---|---|---|---|
| Addeo et al., 2019 [21] | Case study, 1 patient | 100% circumportal pancreas | Not presented | France | Female |
| Adibelli et al., 2016 [16] | Retrospective study, 1158 patients | The anatomical variation in the pancreatic duct was 55%; variations in the course of the pancreatic duct and ansa pancreatica was 37.5%; pancreas divisum of 4.7%; and 2.8% was without variants. | Female–male ratio was 1.36. The proportions of type II configuration showed a value of 0.03 (IS), while the ratio of the vertical course was 0.0048 (IS). | Turkey | 490 males (42.3%) and 668 females (57.7%) |
| Bang et al., 2006 [27] | Retrospective study, 582 patients | The anatomical variation of the pancreatic ductal system was 56.4%. | The rates of hyperamylasemia in types Cyd were significantly higher than in types A and B (p = 0.018). | Republic of Korea | 325 males (55.8%) and 257 females (44.2%) |
| Delhaye et al., 1985 [28] | Case series, 5357 patients | The pancreas divisum was present in 5.7% of patients. | Significant correlation between PD with chronic pancreatitis (p < 0.001) and acute pancreatitis (p < 0.05). | Belgium | Not specified |
| Qin et al., 2019 [12] | Case study, 1 patient | 100% incidence for portal annular pancreas. | Not presented | China | Female |
| Yang et al., 2019 [13] | Retrospective study, 60 patients with ERCP | 28 patients with AP. | 2 of 28 patients presented duodenal obstruction. | China | Not specified |
| Zhou et al., 2022 [14] | Retrospective study, 24 patients with ERCP | 13 patients with AP. | 2 patients with pancreatitis. | China | 10 males and 14 females |
| Gromski et al., 2019 [15] | Prospective study, 49 patients with ERCP | 1 patients with AP. | Does not present | Poland | Not specified |
| Halpert et al., 1990 [20] | Case study, 1 patient | 100% incidence of bifid pancreas. | Not presented | USA | Female |
| Ishida et al., 2019 [2] | Case study, 1 patient | 100% incidence of bifid pancreas. | Not presented | Japan | Female |
| Jarrar et al., 2013 [17] | Case study, 1 patient | 100% incidence of pancreatic ansa. | Not presented | France | Male |
| Kanasker and Bharambe, 2016 [11] | Congress summary, 50 corpses | Incidence of 4% complete annular pancreas and 4% incomplete annular pancreas. | Not presented | India | Not mentioned |
| Gonoi et al., 2011 [22] | Retrospective study, 22,628 patients | The incidence of circumportal pancreas was 2 patients (0.009). | Not reported | Japan | Not reported |
| Ohtsuka et al., 2016 [23] | Retrospective study, 508 patients | The incidence was 9 patients with circumportal pancreas, which is equivalent to 1.8%. | There were no significant differences in age, sex, and ASA. Patients with circumportal pancreas have a more frequent diagnosis of bile duct cancer (p = 0.03) and a higher frequency of pancreatic fistula compared with a normal pancreas (p = 0.03). | Japan | 293 males (57.7%) and 215 females (42.3%) |
| Yilmaz and Celik, 2018 [24] | Retrospective study | 0.8% incidence for circumportal pancreas. | Does not present statistical values | Turkey | Does not fully report the sex of those studied |
| Luu et al., 2017 [25] | Retrospective study, 1102 patients through surgical analysis | 6 patients with CP. | Does not present | Germany | 368 males and 734 females |
| Kumar et al., 2019 [38] | Case study, 1 cadaver | 100% incidence of duplication of the left gastroepiploic (gastro-omental) artery. | Not presented | France | Male |
| Delhaye et al., 1985 [28] | Retrospective study, 5347 cholangiopancreatography retrograde | 304 patients with PD. | Does not present | Belgium | 147 males and 157 females |
| Montagnani et al., 2013 [30] | Case study, 1 patient | 100% incidence for PD. | Not presented | Italy | Male |
| Morgan et al., 2008 [32] | Retrospective study, 68 patients | 100% incidence for PD. | There was no significance in the patients with PD and their response to surgery (p = 0.5). | USA | 14 males (20.6%) and 54 females (79.4%) |
| Nahmod et al., 2017 [33] | Case study, 1 patient | 100% incidence for PD. | Not presented | Argentina | Male |
| Pina et al., 2017 [34] | Case series study, 100 cadavers | Incidence of 1 pancreas divisum with 1%. | Not presented | Argentina | Not specified |
| Sanada et al., 1995 [35] | Case study | 100% incidence for pancreas divisum. | Not presented | Japan | Male |
| Sugawa et al., 1979 [36] | Retrospective study, 1529 patients | 2.7% incidence for PD. | Not presented | USA | Not registered |
| White et al., 2014 [37] | Observational study; 8 patients | The incidence for pancreas divisum was 25%. | Does not present statistical values | USA | 4 males (50%) and 4 females (50%) |
| Ross et al., 1996 [39] | Retrospective study, 119 patients | 35% incidence regarding variations in the contour of the head and neck of the pancreas. | Not presented | USA | 69 males (58%) and 50 females (42%) |
| Kubota et al., 1993 [40] | Prospective study, 310 patients | Percentages for common duct length: 7.7 ± 2.1 mm 48 (15.5%); 7.8 ± 3.1 mm 20 (6.5%); 8 ± 2.7 mm 77 (24.8%). | Patients with choledocholithiasfrequently present separate drainage of both ducts in the duodenum (p < 0.01). | Japan | 138 males (44.5%) and 172 females (55.5%) |
| Sherifi et al., 2018 [41] | Observational study; 63 patients studied | The incidences were: 31.7% of the BP type, 30.2% had a pathology that deforms the PB junction, 28.6% of the duodenal type, 7.9% of the PB type, and 1.6% presented artifacts. | Without statistical significance into the size of P–B according to sex (p = 0.633). No correlation was found between age and the size of the P–B angle (p = 0.792). | Kosovo | 32 males (50.8%) and 31 females (49.2%) |
| Singh et al., 2017 [42] | 1 case study | 100% incidence in the presence of a closed loop of the main pancreatic duct. | Not reported | India | Male |
| Moffatt et al., 2011 [43] | Retrospective study, 2753 patients with ERCP | 1476 patients with PD. | Does not present | Germany | Not specified |
| Adike et al., 2010 [44] | Retrospective study, 3456 patients | 284 patients with pancreas divisum. | 82 without pancreatitis and 202 with pancreatitis p = 0.008. | USA | 108 males and 176 females |
| Alazmi et al., 2007 [45] | Case series, 80 patients evaluated with ERCP | 6 patients with PD. | Does not present | USA | Not present |
| Tajima et al., 2009 [19] | Case study | 100% incidence in relation to bifid pancreatic duct. | Not presented | Japan | Female |
| Brenner et al., 1990 [46] | Retrospective study, 441 pancreatography | 23 patients with PD. | Does not present | France | Not specified |
| Chacko et al. 2008 [47] | Retrospective study, 114 patients | 47 patients with PD. | Does not present | USA | Not specified |
| Kamisawa et al., 2008 [48] | Case series, 84 patients | 8 patients with PD. | 1 patient with PD was associated with gallbladder cancer. | China | Not specified |
| Kin et al., 2005 [49] | Case series, 127 patients | 28 patients with PD. | Does not present | Canada | 67 males and 60 females |
| Meng et al., 2020 [50] | Retrospective study, 187 patients | 136 patients with pancreas divisum. | 15.7% without pancreas divisum presented pancreatitis vs. 5.6% with pancreas divisum presented pancreatitis, p = 0.005) | China | 107 males (57%) and 80 females (43%) |
| Pappas et al., 2012 [51] | Retrospective study, 14 patients | 6 patients with PD. | Does not present | USA | 5 males and 9 females |
| Rustagi et al., 2013 [52] | Prospective study, 4121 patients | 45 patients with pancreas divisum. | Does not present | USA | 23 males and 25 females |
| Sugawa et al., 1987 [53] | Retrospective study, 1529 pancreatography | 41 pancreatography presented PD. | 17 of 41 PD presented pancreatitis. | USA | Not specified |
| Takuma et al., 2010 [54] | Case series, 3246 patients | 54 patients with complete PD; 50 patients with incomplete PD. | 12 of 54 patients with PD presented pancreatitis p = 0.01. | Japan | Not specified |
| Wang et al., 2013 [55] | Retrospective study, 1439 patients | 38 patients with PD. | Does not present | China | 698 males (49%) and 741 females (51%) |
| Kim et al., 2018 [56] | Case study, 1 patient | The reported incidence was 100% circumportal pancreas accompanied by PD. | Not presented | France | Female |
| Mosler et al., 2012 [57] | Case series, 146 patients | 28 patients with PD. | 8 of 28 patients with PD presented pancreatitis. | USA | Not specified |
| Bret et al., 1996 [58] | Retrospective study, 310 pancreatography | 25 patients with PD. | Does not present | Canada | Not specified |
| Malathi et al., 2017 [59] | Congress summary, 19 corpses | The incidence was 13 adult patients with ansa pancreatica (68.4%), and 2 embryonic type patients (10.5%). | Not presented | India | Not specified |
| Tappouni et al., 2015 [60] | 44 case studies through CT scan | 37 patients with CP. | 2 patients with pancreatitis. | USA | 13 males and 31 females |
| Taj et al., 2016 [61] | Retrospective study, 3600 patients | 17 pancreas divisum (0.47%). | Does not present statistical values | Saudi Arabia | report the sex of those studied |
| Warshaw et al., 1983 [62] | Case series, 140 patients | 40 patients with PD. | 4 patients with PD presented pancreatitis p = 0.01. | USA | 13 males and 27 females |
| References | Study | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | ||
| Addeo et al., 2019 [21] | Retrospective study | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Adibelli et al., 2016 [16] | Retrospective study | U | Y | U | N | Y | Y | U | Y | Y | U | Y | N | N | N | N | N | Y | N | N | N | Y | Y | Y | NA | Y |
| Bang et al., 2006 [27] | Cadaveric study | N | Y | U | N | Y | Y | N | Y | Y | Y | N | N | N | Y | Y | N | N | Y | N | N | Y | Y | Y | NA | Y |
| Delhaye et al., 1985 [28] | Cadaveric study | Y | N | N | N | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | N | NA | Y |
| Qin et al., 2019 [12] | Cadaveric study | Y | Y | N | N | Y | N | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | N | N | Y | Y | Y | NA | Y |
| Yang et al., 2019 [13] | Cadaveric study | Y | N | N | Y | N | Y | Y | Y | Y | Y | N | N | Y | N | Y | N | Y | Y | N | Y | Y | Y | N | NA | Y |
| Zhou et al., 2022 [14] | Retrospective study | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | N | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | NA | Y |
| Gromski et al., 2019 [15] | Retrospective study | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | NA | Y |
| Halpert et al., 1990 [20] | Cadaveric study | Y | N | Y | N | Y | N | N | Y | N | Y | Y | N | Y | N | Y | N | Y | N | N | Y | Y | Y | Y | NA | Y |
| Ishida et al., 2019 [2] | Cadaveric study | U | Y | N | Y | N | Y | Y | N | N | Y | Y | N | N | N | Y | N | N | Y | N | Y | Y | Y | Y | NA | N |
| Jarrar et al., 2013 [17] | Cadaveric study | U | Y | N | N | Y | Y | N | Y | N | Y | N | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | NA | Y |
| Kanasker and Bharambe, 2016 [11] | Cadaveric study | Y | N | Y | N | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | N | N | Y | N | Y | N | Y | Y | NA | Y |
| Gonoi et al., 2011 [22] | Cadaveric study | Y | N | N | Y | N | Y | N | Y | Y | N | Y | N | Y | N | Y | Y | N | N | Y | N | Y | Y | Y | NA | Y |
| Ohtsuka et al., 2016 [23] | Cadaveric study | N | Y | Y | Y | N | Y | N | Y | N | N | Y | Y | N | N | Y | N | N | Y | N | Y | Y | Y | Y | NA | Y |
| Yilmaz and Celik, 2018 [24] | Cadaveric study | Y | Y | Y | Y | N | Y | N | N | N | N | Y | Y | N | N | Y | N | N | Y | Y | Y | Y | Y | Y | NA | Y |
| Luu et al., 2017 [25] | Cadaveric study | Y | N | N | Y | Y | Y | Y | Y | N | N | Y | N | N | N | Y | N | N | Y | N | Y | Y | Y | Y | NA | Y |
| Kumar et al., 2019 [38] | Retrospective study | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | NA | N |
| Delhaye et al., 1985 [28] | Retrospective study | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | NA | N |
| Montagnani et al., 2013 [30] | Retrospective study | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | NA | N |
| Morgan et al., 2008 [31] | Retrospective study | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | NA | N |
| Nahmod et al., 2017 [33] | Cadaveric study | Y | Y | Y | N | Y | N | Y | N | Y | N | N | Y | Y | Y | N | Y | N | Y | Y | N | Y | Y | Y | NA | Y |
| Pina et al., 2017 [34] | Retrospective study | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| Sanada et al., 1995 [35] | Retrospective study | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | NA | N |
| Sugawa et al., 1979 [36] | Cadaveric study | U | Y | N | N | Y | Y | N | Y | N | Y | N | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | NA | Y |
| White et al., 2014 [37] | Cadaveric study | Y | N | Y | N | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | N | N | Y | N | Y | N | Y | Y | NA | Y |
| Ross et al., 1996 [39] | Cadaveric study | Y | Y | Y | N | Y | N | Y | N | Y | N | N | Y | Y | Y | N | Y | N | Y | Y | N | Y | Y | Y | NA | Y |
| Kubota et al., 1993 [40] | Cadaveric study | Y | N | N | Y | N | Y | N | Y | Y | N | Y | N | Y | N | Y | Y | N | N | Y | N | Y | Y | Y | NA | Y |
| Sherifi et al., 2018 [41] | Retrospective study | Y | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N | Y | Y | Y | Y | NA | B |
| Singh et al., 2017 [42] | Retrospective study | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | N | Y | Y | NA | Y |
| Moffatt et al., 2011 [43] | Prospective study | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | NA | N |
| Adike et al., 2010 [44] | Retrospective study | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | NA | Y |
| Alazmi et al., 2007 [45] | Case series | Y | N | Y | Y | N | N | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | N | NA | Y |
| Case series | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | N | NA | Y | |
| Tajima et al., 2009 [19] | Prospective study | Y | N | Y | Y | N | N | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | N | NA | Y |
| Brenner et al., 1990 [46] | Case series | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | N | NA | Y |
| Chacko et al. 2008 [47] | Retrospective study | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| Kamisawa et al., 2008 [48] | Case series | Y | Y | Y | N | Y | Y | Y | Y | N | N | Y | N | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | NA | N |
| Kin et al., 2005 [49] | Case series | Y | Y | Y | Y | N | Y | Y | Y | N | N | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | NA | N |
| Meng et al., 2020 [50] | Retrospective study | Y | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N | Y | Y | Y | Y | NA | B |
| Pappas et al., 2012 [51] | Retrospective study | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | N | Y | Y | NA | Y |
| Rustagi et al., 2013 [52] | Retrospective study | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | NA | N |
| Sugawa et al., 1987 [53] | Retrospective study | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | NA | Y |
| Takuma et al., 2010 [54] | Retrospective study | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | NA | N |
| Wang et al., 2013 [55] | Case series | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | NA | N |
| Kim et al., 2018 [56] | Retrospective study | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | N | Y | Y | NA | Y |
| Mosler et al., 2012 [57] | Retrospective study | Y | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N | Y | Y | Y | Y | NA | B |
| Bret et al., 1996 [58] | Prospective study | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | N | Y | Y | NA | Y |
| Malathi et al., 2017 [59] | Retrospective study | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | NA | N |
| Tappouni et al., 2015 [60] | Retrospective study | Y | Y | Y | N | Y | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | NA | Y |
| Taj et al., 2016 [61] | Retrospective study | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | N | Y | Y | NA | Y |
| Warshaw et al., 1983 [62] | Case series | Y | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | NA | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orellana-Donoso, M.; Milos-Brandenberg, D.; Benavente-Urtubia, A.; Guerra-Loyola, J.; Bruna-Mejias, A.; Nova-Baeza, P.; Becerra-Farfán, Á.; Sepulveda-Loyola, W.; Luque-Bernal, R.M.; Valenzuela-Fuenzalida, J.J. Incidence and Clinical Implications of Anatomical Variations in the Pancreas and Its Ductal System: A Systematic Review and Meta-Analysis. Life 2023, 13, 1710. https://doi.org/10.3390/life13081710
Orellana-Donoso M, Milos-Brandenberg D, Benavente-Urtubia A, Guerra-Loyola J, Bruna-Mejias A, Nova-Baeza P, Becerra-Farfán Á, Sepulveda-Loyola W, Luque-Bernal RM, Valenzuela-Fuenzalida JJ. Incidence and Clinical Implications of Anatomical Variations in the Pancreas and Its Ductal System: A Systematic Review and Meta-Analysis. Life. 2023; 13(8):1710. https://doi.org/10.3390/life13081710
Chicago/Turabian StyleOrellana-Donoso, Mathias, Daniel Milos-Brandenberg, Andoni Benavente-Urtubia, Javier Guerra-Loyola, Alejandro Bruna-Mejias, Pablo Nova-Baeza, Álvaro Becerra-Farfán, Walter Sepulveda-Loyola, Ricardo Miguel Luque-Bernal, and Juan José Valenzuela-Fuenzalida. 2023. "Incidence and Clinical Implications of Anatomical Variations in the Pancreas and Its Ductal System: A Systematic Review and Meta-Analysis" Life 13, no. 8: 1710. https://doi.org/10.3390/life13081710
APA StyleOrellana-Donoso, M., Milos-Brandenberg, D., Benavente-Urtubia, A., Guerra-Loyola, J., Bruna-Mejias, A., Nova-Baeza, P., Becerra-Farfán, Á., Sepulveda-Loyola, W., Luque-Bernal, R. M., & Valenzuela-Fuenzalida, J. J. (2023). Incidence and Clinical Implications of Anatomical Variations in the Pancreas and Its Ductal System: A Systematic Review and Meta-Analysis. Life, 13(8), 1710. https://doi.org/10.3390/life13081710










