Quercetin and Ferroptosis
Abstract
:1. Introduction
2. Ferroptosis
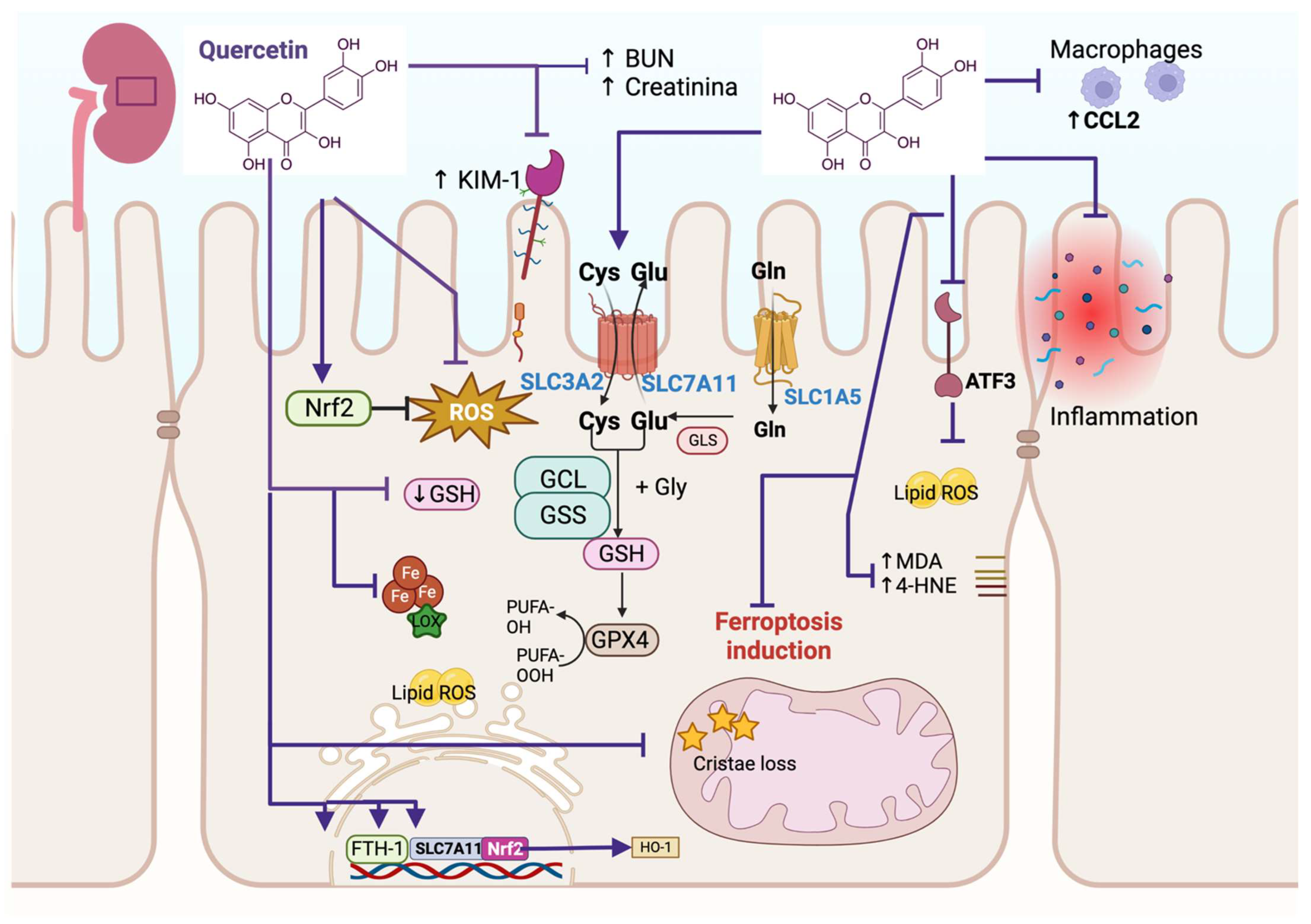
3. Quercetin Alleviates Ferroptosis in Renal Injury
4. Quercetin Avoids Ferroptosis in Liver Injury
5. Quercetin Avoids Ferroptosis in Central Nervous System Injury
6. Quercetin Prevents Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) Injury
7. Quercetin Alleviates Ferroptosis in Pancreatic β Cells Injury in Type 2 Diabetes (T2DM)
8. Quercetin Alleviates Ferroptosis during Inflammation
9. Quercetin Promotes Ferroptosis as Anticancer Activity
10. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, D.O.; Wightman, E.L. Herbal Extracts and Phytochemicals: Plant Secondary Metabolites and the Enhancement of Human Brain Function1. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, Y.; Qiao, Y.; Zheng, Y.; Yu, X.; Liu, F.; Wang, H.; Zheng, B.; Pan, S.; Ren, K.; et al. Quercetin Ameliorates Diabetic Kidney Injury by Inhibiting Ferroptosis via Activating Nrf2/HO-1 Signaling Pathway. Am. J. Chin. Med. 2023, 51, 997–1018. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin Alleviates Acute Kidney Injury by Inhibiting Ferroptosis. J. Adv. Res. 2020, 28, 231–243. [Google Scholar] [CrossRef]
- Willebrords, J.; Pereira, I.V.A.; Maes, M.; Crespo Yanguas, S.; Colle, I.; Van Den Bossche, B.; Da Silva, T.C.; de Oliveira, C.P.M.S.; Andraus, W.; Alves, V.A.; et al. Strategies, Models and Biomarkers in Experimental Non-Alcoholic Fatty Liver Disease Research. Prog. Lipid Res. 2015, 59, 106–125. [Google Scholar] [CrossRef] [Green Version]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.-J.; Zhang, G.-F.; Zheng, J.-Y.; Sun, J.-H.; Ding, S.-B. Targeting Mitochondrial ROS-Mediated Ferroptosis by Quercetin Alleviates High-Fat Diet-Induced Hepatic Lipotoxicity. Front. Pharmacol. 2022, 13, 876550. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, K.; Wang, J.; He, K.; Zhou, X.; Nie, S. Quercetin Alleviates Acrylamide-Induced Liver Injury by Inhibiting Autophagy-Dependent Ferroptosis. J. Agric. Food Chem. 2023, 71, 7427–7439. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, G.; Alimujiang, A.; Xie, H.; Yang, W.; Yin, F.; Huang, D. Protective Effects of Querectin against MPP+-Induced Dopaminergic Neurons Injury via the Nrf2 Signaling Pathway. Front. Biosci. 2023, 28, 42. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, W.; Lowe, S.; Bentley, R.; Hu, G.; Mei, H.; Jiang, X.; Sun, C.; Wu, Y.; Liu, Y. Quercetin Alleviates Kainic Acid-Induced Seizure by Inhibiting the Nrf2-Mediated Ferroptosis Pathway. Free Radic. Biol. Med. 2022, 191, 212–226. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Wang, M.; Chen, H.; Li, Y.; Wei, W.; Liu, X.; Wu, Y.; Luo, S.; Liu, X.; et al. Quercetin Prevents the Ferroptosis of OPCs by Inhibiting the Id2/Transferrin Pathway. Chem. Biol. Interact. 2023, 381, 110556. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Yang, Y.-H.; Chen, J.-W.; Jiang, L.-S. Isolated Osteoblasts from Spinal Cord-Injured Rats Respond Less to Mechanical Loading as Compared with Those from Hindlimb-Immobilized Rats. J. Spinal Cord Med. 2013, 36, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Lean, J.M.; Jagger, C.J.; Kirstein, B.; Fuller, K.; Chambers, T.J. Hydrogen Peroxide Is Essential for Estrogen-Deficiency Bone Loss and Osteoclast Formation. Endocrinology 2005, 146, 728–735. [Google Scholar] [CrossRef]
- Lan, D.; Qi, S.; Yao, C.; Li, X.; Liu, H.; Wang, D.; Wang, Y. Quercetin Protects Rat BMSCs from Oxidative Stress via Ferroptosis. J. Mol. Endocrinol. 2022, 69, 401–413. [Google Scholar] [CrossRef]
- Li, X.; Zeng, J.; Liu, Y.; Liang, M.; Liu, Q.; Li, Z.; Zhao, X.; Chen, D. Inhibitory Effect and Mechanism of Action of Quercetin and Quercetin Diels-Alder Anti-Dimer on Erastin-Induced Ferroptosis in Bone Marrow-Derived Mesenchymal Stem Cells. Antioxidants 2020, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, R.; Peng, W.; Zhao, X.; Bai, W.; Hu, C. Quercetin Alleviates Ferroptosis Accompanied by Reducing M1 Macrophage Polarization during Neutrophilic Airway Inflammation. Eur. J. Pharmacol. 2023, 938, 175407. [Google Scholar] [CrossRef]
- An, S.; Hu, M. Quercetin Promotes TFEB Nuclear Translocation and Activates Lysosomal Degradation of Ferritin to Induce Ferroptosis in Breast Cancer Cells. Comput. Intell. Neurosci. 2022, 2022, 5299218. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Q.; Ma, M.; Guo, H. Quercetin Inhibits the Progression of Endometrial HEC-1-A Cells by Regulating Ferroptosis-a Preliminary Study. Eur. J. Med. Res. 2022, 27, 292. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Ma, J.; Li, X.-Y.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.-M.; Lu, G.-D.; Zhou, J. Quercetin Induces P53-Independent Cancer Cell Death through Lysosome Activation by the Transcription Factor EB and Reactive Oxygen Species-Dependent Ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef]





| Disease or Disorder | Induction of Ferroptosis | Effect of Quercetin in Ferroptosis | Reference |
|---|---|---|---|
| Cell injury of human kidney proximal tubular epithelial (HK-2) via HG treatment | ↓ GSH ↑ iron, MDA, and 4-HNE content ↓ mitochondrial crista and even disappearance and outer membrane rupture in HK-2 cells. ↓ GPX4, FTH-1, SLC7A11 mRNA levels | ↑ cell viability ↓ Kim-1 and ROS production ↓ iron, MDA, and 4-HNE content ↑ mitochondrial crista ↑ Nrf2 and HO-1 ↓ ferroptosis | [7] |
| Ferroptosis induced via erastin in renal tubular cell lines HK2 and NRK-52E and ferroptosis induced via I/R and FA during AKI | Recruits macrophages via CCL2 chemokine | ↑ cell viability and GSH ↓ MDA and lipid ROS in HK2 cells In IR and FA mice models, quercetin ↓ BUN and creatinine markers of kidney injury ↑ GPX4, SLC7A11 and SLC3A2 ↓ TNF-α, IL-1α, IL-6, and ATF3 expression, and the recruitment of macrophages, avoiding inflammation, and AKI ↓ ferroptosis | [8] |
| NAFLD is characterized by ferroptosis | ↑ Oxidative stress and lipid peroxidation | ↓ Total triglycerides and cholesterol in serum and liver, MtROS, lipid peroxidation, and liver iron content ↑ GPX4 and GSH/GSSG ratio ↓ ferroptosis | [11] |
| Hepatotoxicity via ACR ↑ ALT, AST, and ferroptosis | ↑ ROS and lipid peroxidation | ↓ ROS, MDA, ferroptosis-promoting genes such as ACSL6 ↑ GPX4, GSH levels, and ferroptosis-inhibiting genes such as SLC7A11. Quercetin reacts with autophagic cargo receptor NCOA4, ↓ iron degradation storage in FTH1 ↓ ferroptosis | [12] |
| Ferroptosis induced via MPP+ in a neuroblastoma cell line | ↑ OS and lipid peroxidation | ↓ MDA, iron content, and NCOA4 levels ↑ mitochondrial membrane potential, GPX4, SLC7A11 and Nrf2 pathway ↓ ferroptosis | [13] |
| Seizure-induced neuronal death via ferroptosis in a kainic acid-induced epileptic mouse model HT22 neuronal cell death induced via glutamate | ↑ ferroptosis | ↑ Nrf2 and GSH ↓ MDA and 4HNE ↓ lipid ROS ↑ SIRT1/Nrf2/SLC7A11/GPX4 pathway ↓ ferroptosis | [14] |
| The loos of OPCs results in poor SCI recovery | ↑ Iron concentration, ROS generation, and PTGS2 protein levels ↓ cell viability, GSH, and GPX4 protein levels ↑ shrunken mitochondria, membrane density ↓ decreased mitochondrial cristae | ↓ DNA binding 2 (Id2)/transferrin pathway, iron concentration, ROS production, and PTGS2 levels ↑ cell viability, GSH, and GPx4 levels ↓ ferroptosis ↑ transferrin and Id2 reversed the protective effect against ferroptosis and axonal and myelin loss induced via quercetin, inducing ↑ iron concentration, ROS production, and abnormally structured mitochondria, decreasing GSH and cell viability | [15] |
| Oxidative stress leads to cell death via ferroptosis in osteoporosis | ↑ ROS production ↓ BMSCs viability | ↑ BMSCs proliferation, GPX4, SLC7A11, and Nrf2 ↓ ROS production ↓ ferroptosis | [18] |
| Iron deposition has been found in PBC, inducing ferroptosis and dysfunction of PBC, during T2DM. | ↓ GPX levels, SOD activity, and GSH ↑ ROS production and MDA content Smaller, fewer, and shrunken mitochondria with increased membrane density, accompanied by progressive loss of cristae. | ↑ GPX4 levels, SOD activity, and GSH levels ↓ ROS levels and MDA ↓ Mitochondria dysfunction ↓ ferroptosis | [20] |
| Ferroptosis is induced in LPS/OVA neutrophilic asthma mouse model | ↑ 4HNE, MDA, and mitochondria distorted and enlarged ↓ GPX4 and SLC7A11 levels | ↓ 4HNE, MDA, and mitochondria dysfunction ↑ GPX4 and SLC7A11 levels ↓ ferroptosis | [21] |
| ↓ Cell viability of MCF-7, MDA-MB-231 breast cancer | ↑ Iron concentration, MDA, carbonyl protein levels, TFEB, and LAMP proteins ↑ ferroptosis | [22] | |
| ↓ Proliferation and cell migration of HEC-1-A endometrial carcinoma cells. | ↑ ROS production and transferrin receptor ↓ mitochondria membrane potential, aconitase 1, GPX4, and SLC7A11 levels ↑ ferroptosis | [23] | |
| ↓ Cell viability of human hepatocellular carcinoma cells HepG2 and Hep3B, colorectal cancer cells HCT116, and cervical cancer cells HeLa | ↑ TFEB activation, activating LAMP1, V-ATPase, ferritin degradation, iron release, ROS production, and lipid peroxidation increase ↑ ferroptosis ↑ Bid, caspase 9, and PARP cleavage promoting apoptosis, suggesting that ferroptosis may act upstream of apoptosis | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Gregorio, A.; Aranda-Rivera, A.K. Quercetin and Ferroptosis. Life 2023, 13, 1730. https://doi.org/10.3390/life13081730
Cruz-Gregorio A, Aranda-Rivera AK. Quercetin and Ferroptosis. Life. 2023; 13(8):1730. https://doi.org/10.3390/life13081730
Chicago/Turabian StyleCruz-Gregorio, Alfredo, and Ana Karina Aranda-Rivera. 2023. "Quercetin and Ferroptosis" Life 13, no. 8: 1730. https://doi.org/10.3390/life13081730
APA StyleCruz-Gregorio, A., & Aranda-Rivera, A. K. (2023). Quercetin and Ferroptosis. Life, 13(8), 1730. https://doi.org/10.3390/life13081730







