The Possibility of Using Fruit-Bearing Plants of Temperate Climate in the Treatment and Prevention of Diabetes
Abstract
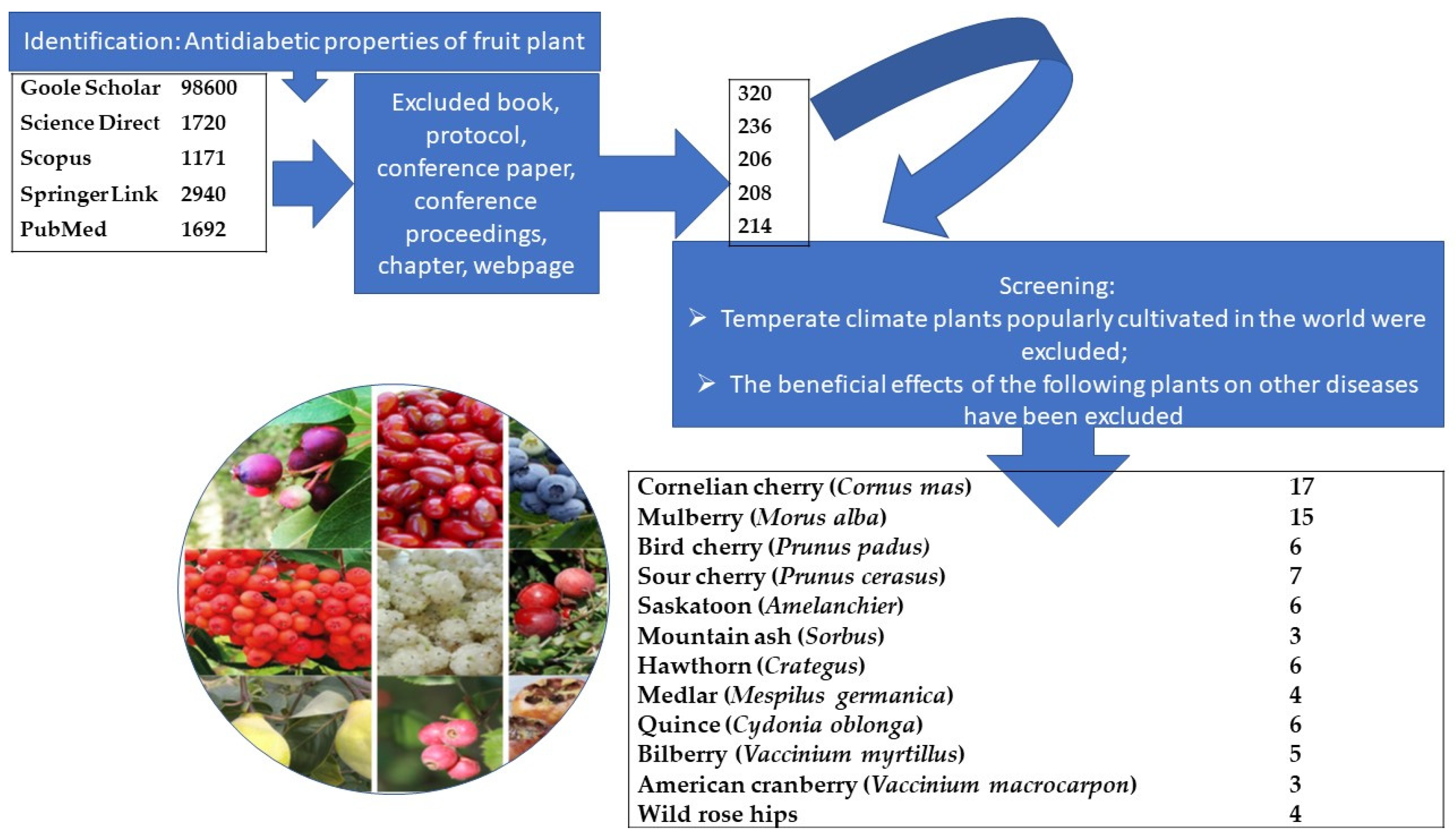
:1. Introduction
2. Methods
3. Cornelian Cherry (Cornus Mas)
3.1. Characteristics of the Species
3.2. Antidiabetic Properties of Fruit (Figure 2a)

3.3. Antidiabetic Properties of Leaves (Figure 2b)
3.4. Antidiabetic Properties of Flowers (Figure 2c)
4. Mulberry (Morus)
4.1. Characteristics of the Species
4.2. Antidiabetic Properties of Fruit (Figure 3a)

4.3. Antidiabetic Properties of Leaves (Figure 3b)
4.4. Antidiabetic Properties of Bark (Figure 3c)
5. Bird cherry (Prunus Padus)
5.1. Characteristics of the Species
5.2. Antidiabetic Properties of Fruit (Figure 4a)

5.3. Antidiabetic Properties of Bark (Figure 4b)
5.4. Antidiabetic Properties of Leaves (Figure 4c)
6. Sour Cherry (Prunus Ceresus)
6.1. Characteristics of the Species
6.2. Antidiabetic Properties of Fruit (Figure 5)

7. Plants of the Genera Amelanchier
7.1. Characteristics of the Species
7.2. Antidiabetic Properties of Fruit (Figure 6a)

7.3. Antidiabetic Properties of Leaves (Figure 6b)
8. Plants of Genera Sorbus
8.1. Characteristics of the Species
8.2. Antidiabetic Properties of Fruit (Figure 7a)

8.3. Antidiabetic Properties of Bark (Figure 7b)
9. Plants of Genera Crategus
9.1. Characteristics of the Species
9.2. Antidiabetic Properties of Fruit (Figure 8a)

9.3. Antidiabetic Properties of Leaves (Figure 8b)
10. Medlar (Mespilus Germanica)
10.1. Characteristics of the Species
10.2. Antidiabetic Properties of Fruit (Figure 9a)

10.3. Antidiabetic Properties of Leaves (Figure 9b)
11. Quince (Cydonia Oblonga)
11.1. Characteristics of the Species
11.2. Antidiabetic Properties of Fruit (Figure 10a)

11.3. Antidiabetic Properties of Leaves (Figure 10b)
11.4. Antidiabetic Properties of Bark (Figure 10c)
12. Plants of Genera Vaccinium
12.1. Bilberry
12.1.1. Characteristics of the Species
12.1.2. Antidiabetic Properties of Fruit (Figure 11a)

12.1.3. Antidiabetic Properties of Leaves (Figure 11b)
12.2. American Cranberry
12.2.1. Characteristics of the Species
12.2.2. Antidiabetic Properties of Fruit (Figure 12)

13. Wild Rose Hips—Plants of Genera Rosa
13.1. Characteristics of the Species
13.2. Antidiabetic Properties of Fruit (Figure 13a,b)

14. Conclusions
- -
- Inhibit the activity of the enzyme α-glucosidase and α-amylase;
- -
- Have a protective and regenerating effect on pancreatic β-cells in the islets of Langerhans, thus increasing insulin secretion;
- -
- Have a hypolipidemic effect by reducing lipid peroxidation and improve dysfunction of the adipocytes responsible for lipid metabolism;
- -
- Alleviate the effects of diabetes complications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García, A.B. Brief Update on Diabetes for General Practitioners. Rev. Esp. Sanid. Penit. 2017, 19, 57–65. [Google Scholar]
- Deepthi, B.; Sowjanya, K.; Lidiyo, B.; Bhargavi, R.S.; Babu, P.S. A Modern Review of Diabetes Mellitus: An Annihiulatory Metabolic Disorder. iMedPub J. 2017, 3, 1–5. [Google Scholar]
- Lorenzati, B.; Zucco, C.; Miglietta, S.; Lamberti, F.; Bruno, G. Oral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of ActionOral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of Action. Pharmaceuticals 2010, 3, 3005–3020. [Google Scholar] [CrossRef]
- Pandey, S.; Chaudhari, V. Impact of Public Education on Rational Use of Medicines. Int. J. Med. Sci. Public. Health 2017, 6, 1. [Google Scholar] [CrossRef]
- Ríos, J.; Francini, F.; Schinella, G. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Nasser Singab, A.; Youssef, F.S. Medicinal Plants with Potential Antidiabetic Activity and Their Assessment. Med. Aromat. Plants 2014, 3, 2167-0412. [Google Scholar] [CrossRef]
- Smith, L.G.; Somerset, S.M. Fruits of Temperate Climates|Commercial and Dietary Importance. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 2753–2761. ISBN 978-0-12-227055-0. [Google Scholar]
- Gondek, K.; Gondek, K.; Kopeć, M.; Mierzwa-Hersztek, M.; Jarosz, R.; Zaleski, T.; Bogdał, S.; Bieniasz, M.; Kaczmarczyk, E.; Knaga, J.; et al. Mineral Composition of Fruits and Leaves of San Andreas® Everbearing Strawberry in Soilless Cultivation. J. Elem. 2020, 25, 1333–1347. [Google Scholar] [CrossRef]
- Bieniek, A.; Piłat, B.; Szałkiewicz, M.; Markuszewski, B.; Gojło, E. Evaluation of Yield, Morphology and Quality of Fruits of Cherry Silverberry (Elaeagnus Multiflora Thunb.) Biotypes under Conditions of North-Eastern Poland. Pol. J. Nat. Sci. 2017, 32, 61–70. [Google Scholar]
- Błaszczyk, J.; Bieniasz, M.; Nawrocki, J.; Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Zaleski, T.; Knaga, J.; Bogdał, S. The Effect of Harvest Date and Storage Conditions on the Quality of Remontant Strawberry Cultivars Grown in a Gutter System under Covers. Agriculture 2022, 12, 1193. [Google Scholar] [CrossRef]
- Simeone, A.M.; Nota, P.; Ceccarelli, D.; Del Toro, A.; Piazza, G.; De Salvador, F.R.; Caboni, E.; Krupa, T. Anthocyanins in Blueberry Cultivars: Effect of the Growing Area. Acta Hortic. 2012, 926, 713–716. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D.; Łysiak, G.P. Effect of Storage Time on Contents of Polyphenolic Compounds in Selected Cultivars of Plum (Prunus domestica L.). Ecol. Chem. Eng. 2010, 17, 1197–1202. [Google Scholar]
- Lachowicz-Wiśniewska, S.; Kapusta, I.; Stinco, C.M.; Meléndez-Martínez, A.J.; Bieniek, A.; Ochmian, I.; Gil, Z. Distribution of Polyphenolic and Isoprenoid Compounds and Biological Activity Differences between in the Fruit Skin + Pulp, Seeds, and Leaves of New Biotypes of Elaeagnusmultiflora Thunb. Antioxidants 2021, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, K.P.; Kruczynska, D.E.; Zurawicz, E. Quality and Shelf Life of Strawberry Cultivars in Poland. Acta Hortic. 2006, 708, 329–332. [Google Scholar] [CrossRef]
- Bialasiewicz, P.; Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Glusac, J.; Nowak, P.; Markowski, J.; Rutkowski, K.P.; et al. Addition of Strawberries to the Usual Diet Decreases Resting Chemiluminescence of Fasting Blood in Healthy Subjects—Possible Health-Promoting Effect of These Fruits Consumption. J. Am. Coll. Nutr. 2014, 33, 274–287. [Google Scholar] [CrossRef]
- Sosna, I. Effect of Hand and Chemical Thinning on Yielding and Fruit Quality of Two Late—Ripening Plum Cultivars. Acta Sci. Polonorum. Hortorum Cultus 2012, 11, 41–51. [Google Scholar]
- Szpadzik, E.; Krupa, T.; Molska-Kawulok, K.; Przybyłko, S. Fruit Quality and Contents of Some Bioactive Compounds in Selected Czech Sweet Cherry (Prunus avium L.) Cultivars under Conditions of Central Poland. Agriculture 2022, 12, 1859. [Google Scholar] [CrossRef]
- Tomala, K. Orchard Factors Affecting Fruit Storage Quality and Prediction of Harvest Date of Apples. Acta Hortic. 1997, 448, 257–264. [Google Scholar] [CrossRef]
- Łata, B.; Łaźny, R.; Przybyłko, S.; Wrona, D. Malus Antioxidant Metabolism Following Bacterial–Fungal Inoculation in Organic Farming: From Root to Fruit. Appl. Sci. 2021, 11, 9466. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Grigsby, J.; Mbemi, A.; Nelson, D.; Mildort, B.; Latinwo, L.; Tchounwou, P.B. The Management of Diabetes Mellitus Using Medicinal Plants and Vitamins. IJMS 2023, 24, 9085. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Szot, I. The Use of Temperature Based Indices for Estimation of Fruit Production Conditions and Risks in Temperate Climates. Agriculture 2023, 13, 960. [Google Scholar] [CrossRef]
- Szot, I.; Szot, P.; Lipa, T.; Sosnowska, B.; Dobrzański, B. Changes in Physical and Chemical Properties of Cornelian Cherry (Cornus mas L.) Fruits in Dependence on Degree of Ripening and Ecotypes. Acta Sci. Pol. Hortorum Cultus 2019, 18, 251–262. [Google Scholar] [CrossRef]
- Yigit, D. Antimicrobial and Antioxidant Evaluation of Fruit Extract from Cornus mas L. Aksaray Univ. J. Sci. Eng. 2018, 2, 41–51. [Google Scholar] [CrossRef]
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the Effects of Cornus mas. L. Fruit Extract on Glycemic Control and Insulin Level in Type 2 Diabetic Adult Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Evid. -Based Complement. Altern. Med. 2015, 2015, 740954. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Noh, J.S.; Kim, J.H.; Tanaka, T.; Zhao, Q.; Matsumoto, K.; Shibahara, N.; Yokozawa, T. Evaluation of Morroniside, Iridoid Glycoside from Corni Fructus, on Diabetes-Induced Alterations Such as Oxidative Stress, Inflammation, and Apoptosis in the Liver of Type 2 Diabetic Db/Db Mice. Biol. Pharm. Bull. 2011, 34, 1559–1565. [Google Scholar] [CrossRef]
- Shishehbor, F.; Azemi, M.E.; Zameni, D.; Saki, A. Inhibitory Effect of Hydroalcoholic Extracts of Barberry, Sour Cherry and Cornelian Cherry on α-Amylase and α-Glucosidase Activities. Int. J. Pharm. Res. Allied Sci. 2016, 5, 423–428. [Google Scholar]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic Effects of Extracts of Red and Yellow Fruits of Cornelian Cherries (Cornus mas. L.) on Rats with Streptozotocin-Induced Diabetes Mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef]
- Omelka, R.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Martiniakova, M. Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats. Animals 2020, 10, 2435. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin Secretion by Bioactive Anthocyanins and Anthocyanidins Present in Fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.-H.; Nair, M.G. Amelioration of Obesity and Glucose Intolerance in High-Fat-Fed C57BL/6 Mice by Anthocyanins and Ursolic Acid in Cornelian Cherry (Cornus mas.). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Narimani-Rad, M.; Zendahdel, M.; Abbasi, M.M.; Abdollahi, B.; Lofti, A. Cornelian Cherry (Cornus mas L.) Extract Affects Glycemic Status in Wistar Rats. Bull. Env. Pharmacol. Life Sci. 2013, 2, 48–50. [Google Scholar]
- Świerczewska, A.; Buchholz, T.; Melzig, M.F.; Czerwińska, M.E. In Vitro α-Amylase and Pancreatic Lipase Inhibitory Activity of Cornus mas L. and Cornus alba L. Fruit Extracts. J. Food Drug Anal. 2019, 27, 249–258. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kratz, M.; Damman, C.J.; Hullarg, M. Mechanisms Linking the Gut Microbiome and Glucose Metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 1445–1454. [Google Scholar] [CrossRef]
- Sip, S.; Szymanowska, D.; Chanaj-Kaczmarek, J.; Skalicka-Woźniak, K.; Budzyńska, B.; Wronikowska-Denysiuk, O.; Słowik, T.; Szulc, P.; Cielecka-Piontek, J. Potential for Prebiotic Stabilized Cornus mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats. Antioxidants 2022, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, A.; Muenz, H.; Akbulut, M.; Başer, K.H.C.; Durmuşkahya, C. Traditional Phytotherapy and Trans-Cultural Pharmacy among Turkish Migrants Living in Cologne, Germany. J. Ethnopharmacol. 2005, 102, 69–88. [Google Scholar] [CrossRef]
- Celep, E.; Aydın, A.; Kırmızıbekmez, H.; Yesilada, E. Appraisal of in Vitro and in Vivo Antioxidant Activity Potential of Cornelian Cherry Leaves. Food Chem. Toxicol. 2013, 62, 448–455. [Google Scholar] [CrossRef]
- Forman, V.; Šušaníková, I.; Kukurová, Ľ.; Švajdlenka, E.; Nagy, M.; Mučaji, P. Flower Infusions From Cornus mas. and Cornus kousa Inhibit Aldose Reductase Enzyme, Without Any Effects on Lipotoxicity. Nat. Product. Commun. 2020, 15, 1934578X2091286. [Google Scholar] [CrossRef]
- Datwyler, S.L.; Weiblen, G.D. On the Origin of the Fig: Phylogenetic Relationships of Moraceae from Ndh F Sequences. Am. J. Bot. 2004, 91, 767–777. [Google Scholar] [CrossRef]
- Lim, S.H.; Yu, J.S.; Lee, H.S.; Choi, C.-I.; Kim, K.H. Antidiabetic Flavonoids from Fruits of Morus Alba Promoting Insulin-Stimulated Glucose Uptake via Akt and AMP-Activated Protein Kinase Activation in 3T3-L1 Adipocytes. Pharmaceutics 2021, 13, 526. [Google Scholar] [CrossRef]
- Sarikaphuti, A.; Nararatwanchai, T.; Hashiguchi, T.; Ito, T.; Thaworanunta, S.; Kikuchi, K.; Oyama, Y.; Maruyama, I.; Tancharoen, S. Preventive Effects of Morus alba L. Anthocyanins on Diabetes in Zucker Diabetic Fatty Rats. Exp. Ther. Med. 2013, 6, 689–695. [Google Scholar] [CrossRef]
- Swathi, P.; Gana Manjusha, K.; Vivekanand, M.; Ramkishan, A.; Bhavani, B. Effect of Morus Alba against Hyperglycemic and Hyperlipidemic Activities in Streptozotocin Induced Diabetic Nephropathy. Biosci. Biotech. Res. Asia 2017, 14, 1441–1447. [Google Scholar] [CrossRef]
- Hwang, S.H.; Li, H.M.; Lim, S.S.; Wang, Z.; Hong, J.-S.; Huang, B. Evaluation of a Standardized Extract from Morus Alba against α-Glucosidase Inhibitory Effect and Postprandial Antihyperglycemic in Patients with Impaired Glucose Tolerance: A Randomized Double-Blind Clinical Trial. Evid. Based Complement. Altern. Med. 2016, 2016, 8983232. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Bong, H.Y.; Jeong, H.I.; Kim, Y.K.; Kim, J.Y.; Kwon, O. Postprandial Hypoglycemic Effect of Mulberry Leaf in Goto-Kakizaki Rats and Counterpart Control Wistar Rats. Nutr. Res. Pract. 2009, 3, 272. [Google Scholar] [CrossRef]
- Chen, F.; Nakashima, N.; Kimura, I.; Kimura, M. Hypoglycemic Activity and Mechanisms of Extracts from Mulberry Leaves (Folium Mori) and Cortex Mori Radicis in Streptozotocin-Induced Diabetic Mice. Yakugaku Zasshi 1995, 115, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Martins, A.; Hsieh, T.-J.; Seres, A.; Zupkó, I. Chlorogenic Acid and Rutin Play a Major Role in the In Vivo Anti-Diabetic Activity of Morus Alba Leaf Extract on Type II Diabetic Rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef] [PubMed]
- Andallu, B.; Suryakantham, V.; Lakshmi Srikanthi, B.; Kesava Reddy, G. Effect of Mulberry (Morus indica L.) Therapy on Plasma and Erythrocyte Membrane Lipids in Patients with Type 2 Diabetes. Clin. Chim. Acta 2001, 314, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.D.; Islam, S. Effects of White Mulberry (Morus alba) Leaf Tea Investigated in a Type 2 Diabetes Model of Rats. Drug Res. 2015, 72, 153–160. [Google Scholar]
- Mohammadi, J.; Naik, P.R. Evaluation of Hypoglycemic Effect of Morus alba in an Animal Model. Indian J. Pharmacol. 2008, 40, 15. [Google Scholar] [CrossRef]
- Zeni, A.L.B.; Dall’Molin, M. Hypotriglyceridemic Effect of Morus alba L., Moraceae, Leaves in Hyperlipidemic Rats. Rev. Bras. Farm. 2010, 20, 130–133. [Google Scholar] [CrossRef]
- Oh, K.-S.; Ryu, S.Y.; Lee, S.; Seo, H.W.; Oh, B.K.; Kim, Y.S.; Lee, B.H. Melanin-Concentrating Hormone-1 Receptor Antagonism and Anti-Obesity Effects of Ethanolic Extract from Morus alba Leaves in Diet-Induced Obese Mice. J. Ethnopharmacol. 2009, 122, 216–220. [Google Scholar] [CrossRef]
- Hu, X.; Wu, J.-W.; Zhang, X.-D.; Zhao, Q.-S.; Huang, J.-M.; Wang, H.-Y.; Hou, A.-J. Isoprenylated Flavonoids and Adipogenesis-Promoting Constituents from Morus Nigra. J. Nat. Prod. 2011, 74, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, T.; Xiao, J.; Wang, X. α-Glucosidase Inhibitors and Antioxidants from Root Bark of Morus Alba. Chin. Herbal. Med. 2018, 10, 331–335. [Google Scholar] [CrossRef]
- Singab, A.N.B.; El-Beshbishy, H.A.; Yonekawa, M.; Nomura, T.; Fukai, T. Hypoglycemic Effect of Egyptian Morus Alba Root Bark Extract: Effect on Diabetes and Lipid Peroxidation of Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-P.; Kim, J.-K.; Lim, Y.-H. Antihyperlipidemic Effects of Stilbenoids Isolated from Morus Alba in Rats Fed a High-Cholesterol Diet. Food Chem. Toxicol. 2014, 65, 213–218. [Google Scholar] [CrossRef]
- Nestby, R.D.J. The Status of Prunus padus L. (Bird Cherry) in Forest Communities throughout Europe and Asia. Forests 2020, 11, 497. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Cielecka-Piontek, J.; Sip, S.; Stuper-Szablewska, K.; Szulc, P. Prunus padus L. as a Source of Functional Compounds—Antioxidant Activity and Antidiabetic Effect. Emir. J. Food Agric. 2022, 34, 135–143. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, M.; Mai, X. Optimisation of Ultrasonic-Assisted Extraction and Biological Activity of Total Flavonoids from Leaves of Murrayae exotica Using Response Surface Methodology. Folia Hortic. 2023, 35, 135–148. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of Gallic Acid on α-Amylase and α-Glucosidase Inhibitory Properties of Acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Szulc, P.; Wilk, R.; Szwajgier, D.; Szymanowska, D. Prunus padus L. Bark as a Functional Promoting Component in Functional Herbal Infusions—Cyclooxygenase-2 Inhibitory, Antioxidant, and Antimicrobial Effects. Open Chem. 2021, 19, 1052–1061. [Google Scholar] [CrossRef]
- Kharyal, S.; Puri, R. Analysis of Antioxidant, Antidiabetic Potential, and Corosolic Acid Content in Prunus Padus Leaves. Def. Life. Sc. J. 2022, 7, 282–287. [Google Scholar] [CrossRef]
- Hyun, T.; Kim, H.; Kim, J. In Vitro Screening for Antioxidant, Antimicrobial, and Antidiabetic Properties of Some Korean Native Plants on Mt. Halla, Jeju Island. Indian J. Pharm. Sci. 2015, 77, 668. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Cherries (Prunus Cerasus)—Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas; Springer International Publishing: Cham, Switzerland, 2021; pp. 63–75. ISBN 978-3-030-75501-0. [Google Scholar]
- Łysiak, G.; Kurlus, R.; Zydlik, Z.; Walkowiak-Tomczak, D. Apple Skin Colour Changes During Harvest as an Indicator of Maturity. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–83. [Google Scholar]
- Ataie-Jafari, A.; Hosseini, S.; Karimi, F.; Pajouhi, M. Effects of Sour Cherry Juice on Blood Glucose and Some Cardiovascular Risk Factors Improvements in Diabetic Women: A Pilot Study. Nutr. Food Sci. 2008, 38, 355–360. [Google Scholar] [CrossRef]
- Papp, N.; Blázovics, A.; Fébel, H.; Salido, S.; Altarejos, J.; Fehér, E.; Kocsis, I.; Szentmihályi, K.; Abrankó, L.; Hegedűs, A.; et al. Antihyperlipidemic Effects of Sour Cherries Characterized by Different In Vitro Antioxidant Power and Polyphenolic Composition. Plant Foods Hum. Nutr. 2015, 70, 408–413. [Google Scholar] [CrossRef]
- Van Der Werf, R.; Walter, C.; Bietiger, W.; Seyfritz, E.; Mura, C.; Peronet, C.; Legrandois, J.; Werner, D.; Ennahar, S.; Digel, F.; et al. Beneficial Effects of Cherry Consumption as a Dietary Intervention for Metabolic, Hepatic and Vascular Complications in Type 2 Diabetic Rats. Cardiovasc. Diabetol. 2018, 17, 104. [Google Scholar] [CrossRef]
- Šarić, A.; Sobočanec, S.; Balog, T.; Kušić, B.; Šverko, V.; Dragović-Uzelac, V.; Levaj, B.; Čosić, Z.; Mačak Šafranko, Ž.; Marotti, T. Improved Antioxidant and Anti-Inflammatory Potential in Mice Consuming Sour Cherry Juice (Prunus Cerasus Cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of Sour Cherry (Prunus cerasus L.) Fruits for Their Polyphenol Content, Antioxidant Properties, and Nutritional Components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of Ripeness and Postharvest Storage on the Evolution of Colour and Anthocyanins in Cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant Properties of Sour Cherries (Prunus cerasus L.): Role of Colorless Phytochemicals from the Methanolic Extract of Ripe Fruits. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef]
- Cruise, J.E. Studies of Natural Hybrids in Amelanchier. Can. J. Bot. 1964, 42, 651–663. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, F.; Hui, A.L.; Shen, G.X. Bioactive Components and Health Benefits of Saskatoon Berry. J. Diabetes Res. 2020, 2020, 3901636. [Google Scholar] [CrossRef] [PubMed]
- Juríková, T.; Balla, S.; Sochor, J.; Pohanka, M.; Mlcek, J.; Baron, M. Flavonoid Profile of Saskatoon Berries (Amelanchier Alnifolia Nutt.) and Their Health Promoting Effects. Molecules 2013, 18, 12571–12586. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.F.B.; Dey, M.; Rogers, R.B.; Ribnicky, D.M.; Gipp, D.M.; Cefalu, W.T.; Raskin, I.; Lila, M.A. Phytochemical Composition and Metabolic Performance-Enhancing Activity of Dietary Berries Traditionally Used by Native North Americans. J. Agric. Food Chem. 2008, 56, 654–660. [Google Scholar] [CrossRef]
- Lim, T.K. Amelanchier Alnifolia. In Edible Medicinal And Non-Medicinal Plants; Fruits; Springer: Dordrecht, The Netherlands, 2012; Volume 4, pp. 358–363. ISBN 978-94-007-4052-5. [Google Scholar]
- Adhikari, D.P.; Francis, J.A.; Schutzki, R.E.; Chandra, A.; Nair, M.G. Quantification and Characterisation of Cyclo-Oxygenase and Lipid Peroxidation Inhibitory Anthocyanins in Fruits OfAmelanchier. Phytochem. Anal. 2005, 16, 175–180. [Google Scholar] [CrossRef]
- Zhang, A.J.; Rimando, A.M.; Fish, W.; Mentreddy, S.R.; Mathews, S.T. Serviceberry [Amelanchier Alnifolia (Nutt.) Nutt. Ex. M. Roem (Rosaceae)] Leaf Extract Inhibits Mammalian α-Glucosidase Activity and Suppresses Postprandial Glycemic Response in a Mouse Model of Diet-Induced Obesity and Hyperglycemia. J. Ethnopharmacol. 2012, 143, 481–487. [Google Scholar] [CrossRef]
- Wierusz-Wysocka, B. Diabetic Vascular Complication and Oxidative Stress. Diabetol. Prakt. 2001, 2, 11–15. [Google Scholar]
- Meczarska, K.; Cyboran-Mikolajczyk, S.; Wloch, A.; Bonarska-Kujawa, D.; Oszmianski, J.; Kleszczynska, H. Polyphenol Content and Bioactivity of Saskatoon (Amelanchier Alnifolia Nutt.) Leaves and Berries. Acta Pol. Pharm. 2017, 74, 660–669. [Google Scholar] [PubMed]
- Aldasoro, J.J.; Aedo, C.; Navarro, C.; Garmendia, F.M. The Genus Sorbus (Maloideae, Rosaceae) in Europe and in North Africa: Morphological Analysis and Systematics. Syst. Bot. 1998, 23, 189. [Google Scholar] [CrossRef]
- Hasbal, G.; Yilmaz Ozden, T.; Can, A. In Vitro Antidiabetic Activities of Two Sorbus Species. Eur. J. Biol. 2017, 76, 57–60. [Google Scholar] [CrossRef]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit α-Amylase in Vitro: Identifying Active Components in Rowanberry and Raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef]
- Vianna, R.; Brault, A.; Martineau, L.C.; Couture, R.; Arnason, J.T.; Haddad, P.S. In Vivo Anti-Diabetic Activity of the Ethanolic Crude Extract of Sorbus Decora C.K.Schneid. (Rosacea): A Medicinal Plant Used by Canadian James Bay Cree Nations to Treat Symptoms Related to Diabetes. Evid. -Based Complement. Altern. Med. 2011, 2011, 237941. [Google Scholar] [CrossRef] [PubMed]
- Attard, E.; Attard, H. Hawthorn: Crataegus Oxyacantha, Crataegus Monogyna and Related Species. Nonvitamin Nonmineral Nutr. Suppl. 2019, 289–293. [Google Scholar] [CrossRef]
- Pirmoghani, A.; Salehi, I.; Moradkhani, S.; Karimi, S.A.; Salehi, S. Effect of Crataegus Extract Supplementation on Diabetes Induced Memory Deficits and Serum Biochemical Parameters in Male Rats. IBRO Rep. 2019, 7, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zarrinkalam, E.; Ranjbar, K.; Salehi, I.; Kheiripour, N.; Komaki, A. Resistance Training and Hawthorn Extract Ameliorate Cognitive Deficits in Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 2018, 97, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xie, M.; Wang, N.; Chen, L.; Huang, X. Effects of Combination Treatment of Metformin and Hawthorn in Patients with Prediabetes Complicated by Nonalcoholic Fatty Liver Disease. Int. J. Clin. Exp. Med. 2019, 12, 1979–1984. [Google Scholar]
- Qin, C.; Xia, T.; Li, G.; Zou, Y.; Cheng, Z.; Wang, Q. Hawthorne Leaf Flavonoids Prevent Oxidative Stress Injury of Renal Tissues in Rats with Diabetic Kidney Disease by Regulating the P38 MAPK Signaling Pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 3440–3446. [Google Scholar]
- Li, Z.; Xu, J.; Zheng, P.; Xing, L.; Shen, H.; Yang, L.; Zhang, L.; Ji, G. Hawthorn Leaf Flavonoids Alleviate Nonalcoholic Fatty Liver Disease by Enhancing the Adiponectin/AMPK Pathway. Int. J. Clin. Exp. Med. 2015, 8, 17295–17307. [Google Scholar]
- Pollmann, B.; Jacomet, S. First Evidence of Mespilus germanica L. (Medlar) in Roman Switzerland. Veget Hist. Archaeobot 2012, 21, 61–68. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Ciałek, S.; Styczyńska, M.; Oziembłowski, M. Functional Properties of Fruits of Common Medlar (Mespilus germanica L.) Extract. Appl. Sci. 2021, 11, 7528. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Mićanović, N.; Grozdanić, N.; Kostić, A.Ž.; Gašić, U.; Stanojković, T.; Popović-Djordjević, J.B. Polyphenolic Profile, Antioxidant and Antidiabetic Potential of Medlar (Mespilus germanica L.), Blackthorn (Prunus spinosa L.) and Common Hawthorn (Crataegus Monogyna Jacq.) Fruit Extracts from Serbia. Horticulturae 2022, 8, 1053. [Google Scholar] [CrossRef]
- Shafiee, F.; Khoshvishkaie, E.; Davoodi, A.; Dashti Kalantar, A.; Bakhshi Jouybari, H.; Ataee, R. The Determination of Blood Glucose Lowering and Metabolic Effects of Mespilus germanica L. Hydroacetonic Extract on Streptozocin-Induced Diabetic Balb/c Mice. Medicines 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, C.; Mutaf, F.; Demirtaş, İ.; Öztürk, G.; Pektaş, M.; Ergül, A. Characterization of Anatolian Traditional Quince Cultivars, Based on Microsatellite Markers. Genet. Mol. Res. 2013, 12, 5880–5888. [Google Scholar] [CrossRef] [PubMed]
- Marwat, S.K.; Khan, M.A.; Ahmad, M.; Zafer, M.; Rehman, F.; Sultana, S. (Fruit Plant Species Mentioned in the Holy Quran and Ahadith and Their Ethnomedicinal Importance. Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 284–295. [Google Scholar]
- Lee, H.S.; Jeon, Y.E.; Jung, J.I.; Kim, S.M.; Hong, S.H.; Lee, J.; Hwang, J.S.; Hwang, M.O.; Kwon, K.; Kim, E.J. Anti-Obesity Effect of Cydonia Oblonga Miller Extract in High-Fat Diet-Induced Obese C57BL/6 Mice. J. Funct. Foods 2022, 89, 104945. [Google Scholar] [CrossRef]
- Khalil, R.R.; Mohammed, E.T.; Musafa, Y.F. Evaluation of In Vitro Antioxidant and Antidiabetic Properties of Cydonia Oblonga Seeds’ Extracts. J. Med. Chem. Sci. 2022, 5, 1048–1058. [Google Scholar]
- Mirmohammadlu, M.; Hosseini, S.H.; Kamalinejad, M.; Gavgani, M.E.; Noubarani, M.; Eskandari, M.R. Hypolipidemic, Hepatoprotective and Renoprotective Effects of Cydonia Oblonga Mill. Fruit in Streptozotocin-Induced Diabetic Rats. Iran. J. Pharm. Res. 2015, 14, 1207–1214. [Google Scholar]
- Ferreira, D.M.; De Oliveira, N.M.; Lopes, L.; Machado, J.; Oliveira, M.B. Potential Therapeutic Properties of the Leaf of Cydonia Oblonga Mill. Based on Mineral and Organic Profiles. Plants 2022, 11, 2638. [Google Scholar] [CrossRef]
- Akyurt, B.; BaşyiĞiT, B.; Çam, M. Phenolic Compounds Content, Antioxidant and Antidiabetic Potentials of Seven Edible Leaves. Gıda 2018, 43, 876–885. [Google Scholar] [CrossRef]
- Abed, S.N.; Bibi, S.; Jan, M.; Talha, M.; Islam, N.U.; Zahoor, M.; Al-Joufi, F.A. Phytochemical Composition, Antibacterial, Antioxidant and Antidiabetic Potentials of Cydonia Oblonga Bark. Molecules 2022, 27, 6360. [Google Scholar] [CrossRef]
- Gailīte, A.; Gaile, A.; Ruņģis, D. Genetic Diversity and Structure of Wild Vaccinium Populations—V. Myrtillus, V. Vitis-Idaea and V. Uliginosum in the Baltic States. Silva Fenn. 2020, 54, 10396. [Google Scholar] [CrossRef]
- De Mello, V.D.; Lankinen, M.A.; Lindström, J.; Puupponen-Pimiä, R.; Laaksonen, D.E.; Pihlajamäki, J.; Lehtonen, M.; Uusitupa, M.; Tuomilehto, J.; Kolehmainen, M.; et al. Fasting Serum Hippuric Acid Is Elevated after Bilberry (Vaccinium Myrtillus) Consumption and Associates with Improvement of Fasting Glucose Levels and Insulin Secretion in Persons at High Risk of Developing Type 2 Diabetes. Mol. Nutr. Food Res. 2017, 61, 1700019. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.A.-L.; Persson, K.; Andersson, R.G.G. Effect of Vaccinium Myrtillus and Its Polyphenols on Angiotensin-Converting Enzyme Activity in Human Endothelial Cells. J. Agric. Food Chem. 2009, 57, 4626–4629. [Google Scholar] [CrossRef] [PubMed]
- Bljajić, K.; Petlevski, R.; Vujić, L.; Čačić, A.; Šoštarić, N.; Jablan, J.; Saraiva De Carvalho, I.; Zovko Končić, M. Chemical Composition, Antioxidant and α-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium Myrtillus Leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef] [PubMed]
- Ştefănescu (Braic), R.; Vari, C.; Imre, S.; Huţanu, A.; Fogarasi, E.; Todea, T.; Groşan, A.; Eşianu, S.; Laczkó-Zöld, E.; Dogaru, M. Vaccinium Extracts as Modulators in Experimental Type 1 Diabetes. J. Med. Food 2018, 21, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Varut, R.M.; Gîrd, C.E.; Rotaru, L.T.; Varut, M.C.; Pisoschi, C.G. Evaluation of Polyphenol and Flavonoid Profiles and the Antioxidant Effect of Carduus Acanthoides Hydroalcoholic Extract Compared with Vaccinium Myrtillus in an Animal Model of Diabetes Mellitus. Pharm. Chem. J. 2018, 51, 1088–1095. [Google Scholar] [CrossRef]
- Sultana, N.; Menzel, G.; Seibt, K.M.; Garcia, S.; Weber, B.; Serçe, S.; Heitkam, T. Genome-Wide Analysis of Long Terminal Repeat Retrotransposons from the Cranberry Vaccinium Macrocarpon. J. Berry Res. 2022, 12, 165–185. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin Glucosides Inhibit Glucose Uptake into Brush-Border-Membrane Vesicles of Porcine Jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef]
- Strobel, P.; Allard, C.; Perez-Acle, T.; Calderon, R.; Aldunate, R.; Leighton, F. Myricetin, Quercetin and Catechin-Gallate Inhibit Glucose Uptake in Isolated Rat Adipocytes. Biochem. J. 2005, 386, 471–478. [Google Scholar] [CrossRef]
- Schell, J.; Betts, N.M.; Foster, M.; Scofield, R.H.; Basu, A. Cranberries Improve Postprandial Glucose Excursions in Type 2 Diabetes. Food Funct. 2017, 8, 3083–3090. [Google Scholar] [CrossRef]
- Rocha, D.M.U.P.; Caldas, A.P.S.; Da Silva, B.P.; Hermsdorff, H.H.M.; Alfenas, R.D.C.G. Effects of Blueberry and Cranberry Consumption on Type 2 Diabetes Glycemic Control: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1816–1828. [Google Scholar] [CrossRef]
- Liska, D.J.; Kern, H.J.; Maki, K.C. Cranberries and Urinary Tract Infections: How Can the Same Evidence Lead to Conflicting Advice? Adv. Nutr. 2016, 7, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, L.; Chupakhina, G.; Feduraev, P.; Chupakhina, N.; Maslennikov, P. Evaluation of the Rose Hips of Rosa canina L. and Rosa Rugosa Thunb. as a Valuable Source of Biological Active Compounds and Antioxidants on the Baltic Sea Coast. Pol. J. Nat. Sci. 2019, 34, 395–413. [Google Scholar]
- Ninomiya, K.; Matsuda, H.; Kubo, M.; Morikawa, T.; Nishida, N.; Yoshikawa, M. Potent Anti-Obese Principle from Rosa Canina: Structural Requirements and Mode of Action of Trans-Tiliroside. Bioorg. Med. Chem. Lett. 2007, 17, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Henriksson, E.; Ström, K.; Alenfall, J.; Göransson, O.; Holm, C. Rose Hip Exerts Antidiabetic Effects via a Mechanism Involving Downregulation of the Hepatic Lipogenic Program. Am. J. Physiol. -Endocrinol. Metab. 2011, 300, E111–E121. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Berger, K.; Högberg, A.; Landin-Olsson, M.; Holm, C. Effects of Rose Hip Intake on Risk Markers of Type 2 Diabetes and Cardiovascular Disease: A Randomized, Double-Blind, Cross-over Investigation in Obese Persons. Eur. J. Clin. Nutr. 2012, 66, 585–590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysiak, G.P.; Szot, I. The Possibility of Using Fruit-Bearing Plants of Temperate Climate in the Treatment and Prevention of Diabetes. Life 2023, 13, 1795. https://doi.org/10.3390/life13091795
Łysiak GP, Szot I. The Possibility of Using Fruit-Bearing Plants of Temperate Climate in the Treatment and Prevention of Diabetes. Life. 2023; 13(9):1795. https://doi.org/10.3390/life13091795
Chicago/Turabian StyleŁysiak, Grzegorz P., and Iwona Szot. 2023. "The Possibility of Using Fruit-Bearing Plants of Temperate Climate in the Treatment and Prevention of Diabetes" Life 13, no. 9: 1795. https://doi.org/10.3390/life13091795
APA StyleŁysiak, G. P., & Szot, I. (2023). The Possibility of Using Fruit-Bearing Plants of Temperate Climate in the Treatment and Prevention of Diabetes. Life, 13(9), 1795. https://doi.org/10.3390/life13091795







