Advances and Controversies in Acute Alcohol-Related Hepatitis: From Medical Therapy to Liver Transplantation
Abstract
:1. Introduction
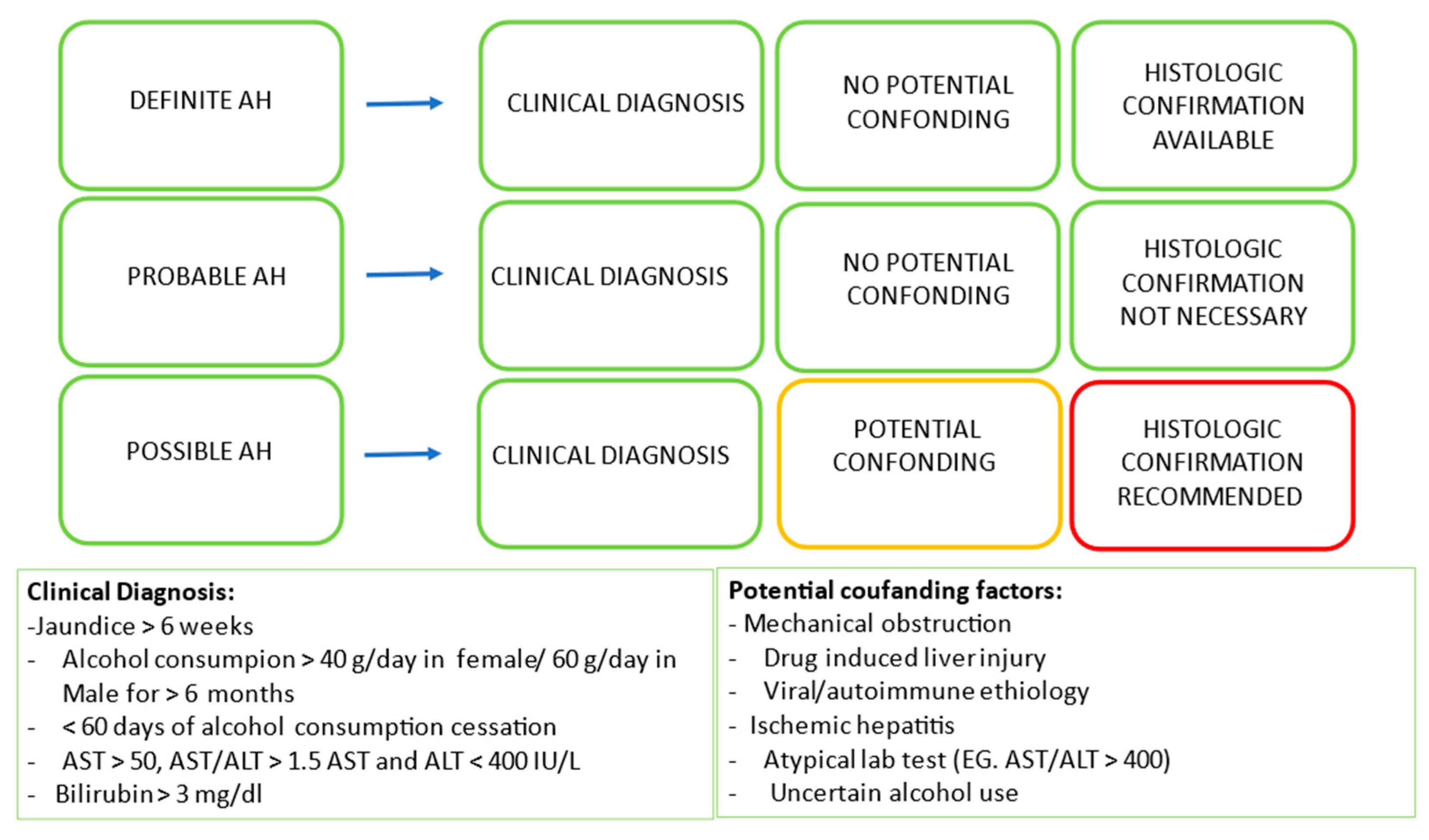
2. Definition and Prevalence
3. Pathogenesis and Predisposing Factors
The Link between ALD and MASLD
4. Long-Term Liver Complications of AH
5. Severity Assessment and Prognostic Models
6. Medical Management
6.1. Abstinence
6.2. Corticosteroid Therapy
6.3. Nutritional Therapy
6.4. Emerging Therapies for AH
7. Role of Liver Transplantation
8. Outcome after Early Liver Transplantation
8.1. Survival
8.2. Return to Alcohol Use after Liver Transplantation
8.3. Impact on LT Activity and Ethical Concern
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louvet, A.; Naveau, S.; Abdelnour, M.; Ramond, M.-J.; Diaz, E.; Fartoux, L.; Dharancy, S.; Texier, F.; Hollebecque, A.; Serfaty, L.; et al. The Lille model: A new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007, 45, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.C.; Kabacam, G.; Vibert, E.; Germani, G.; Petrowsky, H. Current status of liver transplantation in Europe. Int. J. Surg. 2020, 82, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lake, J.; Smith, J.; Skeans, M.; Schladt, D.; Edwards, E.; Harper, A.; Wainright, J.; Snyder, J.; Israni, A.; et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am. J. Transplant. 2017, 17, 174–251. [Google Scholar] [CrossRef]
- Crabb, D.W.; Bataller, R.; Chalasani, N.P.; Kamath, P.S.; Lucey, M.; Mathurin, P.; McClain, C.; McCullough, A.; Mitchell, M.C.; Morgan, T.R.; et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients with Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016, 150, 785–790. [Google Scholar] [CrossRef]
- Singal, A.K.; Mathurin, P. Diagnosis and Treatment of Alcohol-Associated Liver Disease. JAMA 2021, 326, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Arsalan, A.; Dunn, W.; Arab, J.P.; Wong, R.J.; Kuo, Y.; Kamath, P.S.; Shah, V.H. Alcohol-associated liver disease in the United States is associated with severe forms of disease among young, females and Hispanics. Aliment. Pharmacol. Ther. 2021, 54, 451–461. [Google Scholar] [CrossRef]
- Sehrawat, T.S.; Liu, M.; Shah, V.H. The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol. Hepatol. 2020, 5, 494–506. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef]
- Ribeiro, M.d.C.; Szabo, G. Role of the Inflammasome in Liver Disease. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 345–365. [Google Scholar] [CrossRef]
- Koskinas, J.; Kenna, J.; Bird, G.L.; Alexander, G.J.; Williams, R. Immunoglobulin a antibody to a 200-kilodalton cytosolic acetaldehyde adduct in alcoholic hepatitis. Gastroenterology 1992, 103, 1860–1867. [Google Scholar] [CrossRef]
- Becker, U.; Deis, A.; Sorensen, T.I.; Gronbaek, M.; Borch-Johnsen, K.; Muller, C.F.; Schnohr, P.; Jensen, G. Prediction of risk of liver disease by alcohol intake, sex, and age: A prospective population study. Hepatology 1996, 23, 1025–1029. [Google Scholar] [CrossRef]
- Bizzaro, D.; Becchetti, C.; Trapani, S.; Lavezzo, B.; Zanetto, A.; D’Arcangelo, F.; Merli, M.; Lapenna, L.; Invernizzi, F.; Taliani, G.; et al. Influence of sex in alcohol-related liver disease: Pre-clinical and clinical settings. United Eur. Gastroenterol. J. 2023, 11, 218–227. [Google Scholar] [CrossRef]
- Li, T.-K.; Beard, J.D.; Orr, W.E.; Kwo, P.Y.; Ramchandani, V.A.; Thomasson, H.R. Variation in Ethanol Pharmacokinetics and Perceived Gender and Ethnic Differences in Alcohol Elimination. Alcohol. Clin. Exp. Res. 2000, 24, 415–416. [Google Scholar] [CrossRef]
- Eagon, P.K. Alcoholic liver injury: Influence of gender and hormones. World J. Gastroenterol. 2010, 16, 1377–1384. [Google Scholar] [CrossRef]
- Askgaard, G.; Grønbæk, M.; Kjær, M.S.; Tjønneland, A.; Tolstrup, J.S. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: A prospective cohort study. J. Hepatol. 2015, 62, 1061–1067. [Google Scholar] [CrossRef]
- Zeng, T.; Guo, F.-F.; Zhang, C.-L.; Song, F.-Y.; Zhao, X.-L.; Xie, K.-Q. Roles of Cytochrome P4502E1 Gene Polymorphisms and the Risks of Alcoholic Liver Disease: A Meta-Analysis. PLoS ONE 2013, 8, e54188. [Google Scholar] [CrossRef]
- Salameh, H.; Raff, E.; Erwin, A.; Seth, D.; Nischalke, H.D.; Falleti, E.; Burza, M.A.; Leathert, J.; Romeo, S.; Molinaro, A.; et al. PNPLA3 Gene Polymorphism Is Associated with Predisposition to and Severity of Alcoholic Liver Disease. Am. J. Gastroenterol. 2015, 110, 846–856. [Google Scholar] [CrossRef]
- Buch, S.; Stickel, F.; Trépo, E.; Way, M.; Herrmann, A.; Nischalke, H.D.; Brosch, M.; Rosendahl, J.; Berg, T.; Ridinger, M.; et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 2015, 47, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023. [Google Scholar] [CrossRef]
- Marcos, M.; Gómez-Munuera, M.; Pastor, I.; González-Sarmiento, R.; Laso, F.-J. Tumor Necrosis Factor Polymorphisms and Alcoholic Liver Disease: A HuGE Review and Meta-Analysis. Am. J. Epidemiol. 2009, 170, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Sumida, Y.; Umemura, A.; Matsuo, K.; Takahashi, M.; Takamura, T.; Yasui, K.; Saibara, T.; Hashimoto, E.; Kawanaka, M.; et al. Genetic Polymorphisms of the Human PNPLA3 Gene Are Strongly Associated with Severity of Non-Alcoholic Fatty Liver Disease in Japanese. PLoS ONE 2012, 7, e38322. [Google Scholar] [CrossRef]
- Kitamoto, T.; Kitamoto, A.; Yoneda, M.; Hyogo, H.; Ochi, H.; Nakamura, T.; Teranishi, H.; Mizusawa, S.; Ueno, T.; Chayama, K.; et al. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Hum. Genet. 2013, 132, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Israelsen, M.; Torp, N.; Johansen, S.; Thiele, M.; Krag, A. MetALD: New opportunities to understand the role of alcohol in steatotic liver disease. Lancet Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-M.; Yu, C.-H.; Wang, Y.; Xu, C. T1864 Early Mortality of Alcoholic Hepatitis: A Review of Data from Placebo-Controlled Clinical Trials. Gastroenterology 2009, 5, A-853. [Google Scholar] [CrossRef]
- Bird, G.; Williams, R. Factors determining cirrhosis in alcoholic liver disease. Mol. Asp. Med. 1988, 10, 97–105. [Google Scholar] [CrossRef]
- Alexander, J.F.; Lischner, M.W.; Galambos, J.T. Natural history of alcoholic hepatitis. II. The long-term prognosis. Am. J. Gastroenterol. 1971, 56, 515–525. [Google Scholar]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Nischalke, H.D.; Berger, C.; Luda, C.; Berg, T.; Müller, T.; Grünhage, F.; Lammert, F.; Coenen, M.; Krämer, B.; Körner, C.; et al. The PNPLA3 rs738409 148M/M Genotype Is a Risk Factor for Liver Cancer in Alcoholic Cirrhosis but Shows No or Weak Association in Hepatitis C Cirrhosis. PLoS ONE 2011, 6, e27087. [Google Scholar] [CrossRef]
- Guyot, E.; Sutton, A.; Rufat, P.; Laguillier, C.; Mansouri, A.; Moreau, R.; Ganne-Carrié, N.; Beaugrand, M.; Charnaux, N.; Trinchet, J.-C.; et al. PNPLA3 rs738409, hepatocellular carcinoma occurrence and risk model prediction in patients with cirrhosis. J. Hepatol. 2013, 58, 312–318. [Google Scholar] [CrossRef]
- Carithers, R.L.; Herlong, H.F.; Diehl, A.M.; Shaw, E.W.; Combes, B.; Fallon, H.J.; Maddrey, W.C. Methylprednisolone Therapy in Patients with Severe Alcoholic Hepatitis. Ann. Intern. Med. 1989, 110, 685–690. [Google Scholar] [CrossRef]
- Crabb, D.W.; Im, G.Y.; Szabo, G.; Mellinger, J.L.; Lucey, M.R. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 2020, 71, 306–333. [Google Scholar] [CrossRef] [PubMed]
- Forrest, E.H.; Evans, C.D.J.; Stewart, S.; Phillips, M.; Oo, Y.H.; McAvoy, N.C.; Fisher, N.C.; Singhal, S.; Brind, A.; Haydon, G.; et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005, 54, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Srikureja, W.; Kyulo, N.L.; Runyon, B.A.; Hu, K.-Q. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J. Hepatol. 2005, 42, 700–706. [Google Scholar] [CrossRef]
- Morales-Arráez, D.; Ventura-Cots, M.; Altamirano, J.; Abraldes, J.; Cruz-Lemini, M.; Thursz, M.; Atkinson, S.; Sarin, S.; Kim, W.; Chavez-Araujo, R.; et al. The MELD Score Is Superior to the Maddrey Discriminant Function Score to Predict Short-Term Mortality in Alcohol-Associated Hepatitis: A Global Study. Am. J. Gastroenterol. 2021, 117, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Díaz, L.A.; Baeza, N.; Idalsoaga, F.; Fuentes-López, E.; Arnold, J.; Ramírez, C.A.; Morales-Arraez, D.; Ventura-Cots, M.; Alvarado-Tapias, E.; et al. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: A worldwide study. J. Hepatol. 2021, 75, 1026–1033. [Google Scholar] [CrossRef]
- Thursz, M.; Gual, A.; Lackner, C.; Mathurin, P.; Moreno, C.; Spahr, L.; Sterneck, M.; Cortez-Pinto, H. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar] [CrossRef]
- Garcia-Saenz-De-Sicilia, M.; Duvoor, C.; Altamirano, J.; Chavez-Araujo, R.; Prado, V.; Candolo-Martinelli, A.d.L.; Holanda-Almeida, P.; Becerra-Martins-De-Oliveira, B.; Fernandez-De-Almeida, S.; Bataller, R.; et al. A Day-4 Lille Model Predicts Response to Corticosteroids and Mortality in Severe Alcoholic Hepatitis. Am. J. Gastroenterol. 2017, 112, 306–315. [Google Scholar] [CrossRef]
- Louvet, A.; Labreuche, J.; Artru, F.; Boursier, J.; Kim, D.J.; O’grady, J.; Trépo, E.; Nahon, P.; Ganne-Carrié, N.; Naveau, S.; et al. Combining Data from Liver Disease Scoring Systems Better Predicts Outcomes of Patients with Alcoholic Hepatitis. Gastroenterology 2015, 149, 398–406.e8. [Google Scholar] [CrossRef]
- Mathurin, P.; Duchatelle, V.; Ramond, M.; Degott, C.; Bedossa, P.; Erlinger, S.; Benhamou, J.; Chaput, J.; Rueff, B.; Poynard, T. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology 1996, 110, 1847–1853. [Google Scholar] [CrossRef]
- Imperiale, T.F.; McCullough, A.J. Do Corticosteroids Reduce Mortality from Alcoholic Hepatitis? Ann. Intern. Med. 1990, 113, 299–307. [Google Scholar] [CrossRef]
- Christensen, E.; Gluud, C. Glucocorticoids are ineffective in alcoholic hepatitis: A meta-analysis adjusting for confounding variables. Gut 1995, 37, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Gluud, C. Glucocorticosteroids Are Not Effective in Alcoholic Hepatitis. Am. J. Gastroenterol. 1999, 94, 3065–3066. [Google Scholar] [CrossRef] [PubMed]
- Rambaldi, A.; Saconato, H.H.; Christensen, E.; Thorlund, K.; Wetterslev, J.; Gluud, C. Systematic review: Glucocorticosteroids for alcoholic hepatitis—A Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment. Pharmacol. Ther. 2008, 27, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Thursz, M.R.; Richardson, P.; Allison, M.; Austin, A.; Bowers, M.; Day, C.P.; Downs, N.; Gleeson, D.; Macgilchrist, A.; Grant, A.; et al. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N. Engl. J. Med. 2015, 372, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, C.L.; Moritz, T.E.; Roselle, G.A.; Morgan, T.R.; Nemchausky, B.A.; Tamburro, C.H.; Schiff, E.R.; Mcclain, C.J.; Marsano, L.S.; Allen, J.I.; et al. Protein Energy Malnutrition in Severe Alcoholic Hepatitis: Diagnosis and Response to Treatment. J. Parenter. Enter. Nutr. 1995, 19, 258–265. [Google Scholar] [CrossRef]
- Moreno, C.; Deltenre, P.; Senterre, C.; Louvet, A.; Gustot, T.; Bastens, B.; Hittelet, A.; Piquet, M.-A.; Laleman, W.; Orlent, H.; et al. Intensive Enteral Nutrition Is Ineffective for Patients with Severe Alcoholic Hepatitis Treated with Corticosteroids. Gastroenterology 2016, 150, 903–910.e8. [Google Scholar] [CrossRef]
- Yoshida, T.; Muto, Y.; Moriwaki, H.; Yamato, M. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol. Jpn. 1989, 24, 692–698. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef]
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Fanelli, F.R.; Abbiati, R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology 2003, 124, 1792–1801. [Google Scholar] [CrossRef]
- Matsuoka, S.; Tamura, A.; Nakagawara, H.; Moriyama, M. Improvement in the nutritional status and clinical conditions of patients with liver failure using a liver diet combined with a branched chain amino acids-enriched elemental diet. Hepatogastroenterology 2014, 61, 1308–1312. [Google Scholar]
- Van Vugt, J.L.A.; Levolger, S.; De Bruin, R.W.F.; van Rosmalen, J.; Metselaar, H.J.; Ijzermans, J.N.M. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am. J. Transplant. 2016, 16, 2277–2292. [Google Scholar] [CrossRef] [PubMed]
- Boetticher, N.C.; Peine, C.J.; Kwo, P.; Abrams, G.A.; Patel, T.; Aqel, B.; Boardman, L.; Gores, G.J.; Harmsen, W.S.; McClain, C.J.; et al. A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial of Etanercept in the Treatment of Alcoholic Hepatitis. Gastroenterology 2008, 135, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Naveau, S.; Chollet-Martin, S.; Dharancy, S.; Mathurin, P.; Jouet, P.; Piquet, M.-A.; Davion, T.; Oberti, F.; Broët, P.; Emilie, D.; et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004, 39, 1390–1397. [Google Scholar] [CrossRef]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.-Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Khac, E.; Thevenot, T.; Piquet, M.-A.; Benferhat, S.; Goria, O.; Chatelain, D.; Tramier, B.; Dewaele, F.; Ghrib, S.; Rudler, M.; et al. Glucocorticoids plusN-Acetylcysteine in Severe Alcoholic Hepatitis. N. Engl. J. Med. 2011, 365, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Murad, M.H.; Chandar, A.K.; Bongiorno, C.M.; Singal, A.K.; Atkinson, S.R.; Thursz, M.R.; Loomba, R.; Shah, V.H. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology 2015, 149, 958–970.e12. [Google Scholar] [CrossRef]
- la Tijera, F.H.-D. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J. Gastroenterol. 2015, 21, 4975–4985. [Google Scholar] [CrossRef]
- Philips, C.A.; Pande, A.; Shasthry, S.M.; Jamwal, K.D.; Khillan, V.; Chandel, S.S.; Kumar, G.; Sharma, M.K.; Maiwall, R.; Jindal, A.; et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin. Gastroenterol. Hepatol. 2016, 15, 600–602. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Gómez-Bravo, M.; Sánchez-Antolín, G.; De la Rosa, G.; Bilbao, I.; Colmenero, J. Expanding Indications of Liver Transplantation in Spain: Consensus Statement and Recommendations by the Spanish Society of Liver Transplantation. Transplantation 2020, 105, 602–607. [Google Scholar] [CrossRef]
- Tacke, F.; Kroy, D.C.; Barreiros, A.P.; Neumann, U.P. Liver transplantation in Germany. Liver Transplant. 2016, 22, 1136–1142. [Google Scholar] [CrossRef]
- Chandok, N.; Aljawad, M.; White, A.; Hernandez-Alejandro, R.; Marotta, P.; Yoshida, E.M. Liver transplantation for alcoholic liver disease among Canadian transplant centres: A national study. Can. J. Gastroenterol. 2013, 27, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Sandıkçı, B.; Paul, S.; Gampa, A.; Wang, J.; Te, H.; Pillai, A.; Reddy, K.G.; di Sabato, D.; Little, E.C.; et al. Liver transplantation for alcoholic hepatitis in the United States: Excellent outcomes with profound temporal and geographic variation in frequency. Am. J. Transplant. 2020, 21, 1039–1055. [Google Scholar] [CrossRef]
- Antonini, T.M.; Guillaud, O.; Dumortier, J.; Dharancy, S.; Saliba, F.; Mathurin, P.; Duclos-Vallée, J.-C.; Duvoux, C. Impact of a first study of early transplantation in acute alcoholic hepatitis: Results of a nationwide survey in french liver transplantation programs. Liver Transplant. 2018, 24, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, P.; Moreno, C.; Samuel, D.; Dumortier, J.; Salleron, J.; Durand, F.; Castel, H.; Duhamel, A.; Pageaux, G.-P.; Leroy, V.; et al. Early Liver Transplantation for Severe Alcoholic Hepatitis. N. Engl. J. Med. 2011, 365, 1790–1800. [Google Scholar] [CrossRef]
- Im, G.Y.; Kim-Schluger, L.; Shenoy, A.; Schubert, E.; Goel, A.; Friedman, S.L.; Florman, S.; Schiano, T.D. Early Liver Transplantation for Severe Alcoholic Hepatitis in the United States—A Single-Center Experience. Am. J. Transplant. 2016, 16, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Chen, P.-H.; Haugen, C.; Hernaez, R.; Gurakar, A.; Philosophe, B.; Dagher, N.; Moore, S.A.; Li, Z.; Cameron, A.M. Three-year Results of a Pilot Program in Early Liver Transplantation for Severe Alcoholic Hepatitis. Ann. Surg. 2017, 265, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Im, G.Y.; Rice, J.P.; Lazar, A.; Weinberg, E.; Han, H.; Maddur, H.; Ghobrial, R.M.; Therapondos, G.; Hsu, C.; et al. Faculty Opinions recommendation of Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology 2020, 155, 422–430. [Google Scholar] [CrossRef]
- Lee, B.P.; Im, G.Y.; Rice, J.P.; Lazar, A.; Weinberg, E.; Han, H.; Maddur, H.; Ghobrial, R.M.; Therapondos, G.; Hsu, C.; et al. Patterns of Alcohol Use After Early Liver Transplantation for Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol. 2022, 20, 409–418.e5. [Google Scholar] [CrossRef]
- Louvet, A.; Labreuche, J.; Moreno, C.; Vanlemmens, C.; Moirand, R.; Féray, C.; Dumortier, J.; Pageaux, G.-P.; Bureau, C.; Chermak, F.; et al. Early liver transplantation for severe alcohol-related hepatitis not responding to medical treatment: A prospective controlled study. Lancet Gastroenterol. Hepatol. 2022, 7, 416–425. [Google Scholar] [CrossRef]
- Germani, G.; Angrisani, D.; Addolorato, G.; Merli, M.; Mazzarelli, C.; Tarli, C.; Lattanzi, B.; Panariello, A.; Prandoni, P.; Craxì, L.; et al. Liver transplantation for severe alcoholic hepatitis: A multicenter Italian study. Am. J. Transplant. 2021, 22, 1191–1200. [Google Scholar] [CrossRef]
- Burra, P.; Samuel, D.; Sundaram, V.; Duvoux, C.; Petrowsky, H.; Terrault, N.; Jalan, R. Limitations of current liver donor allocation systems and the impact of newer indications for liver transplantation. J. Hepatol. 2021, 75, S178–S190. [Google Scholar] [CrossRef]
- Lucey, M.R.; Brown, K.A.; Everson, G.T.; Fung, J.J.; Gish, R.; Keeffe, E.B.; Kneteman, N.M.; Lake, J.R.; Martin, P.; McDiarmid, S.V.; et al. Minimal criteria for placement of adults on the liver transplant waiting list: A report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transplant. Surg. 1997, 3, 628–637. [Google Scholar] [CrossRef]
- Testino, G.; Burra, P.; Bonino, F.; Piani, F.; Sumberaz, A.; Peressutti, R.; Castiglione, A.G.; Patussi, V.; Fanucchi, T.; Ancarani, O.; et al. Acute alcoholic hepatitis, end stage alcoholic liver disease and liver transplantation: An Italian position statement. World J. Gastroenterol. 2014, 20, 14642–14651. [Google Scholar] [CrossRef]
- Burra, P.; Belli, L.S.; Corradini, S.G.; Volpes, R.; Marzioni, M.; Giannini, E.; Toniutto, P. Common issues in the management of patients in the waiting list and after liver transplantation. Dig. Liver Dis. 2017, 49, 241–253. [Google Scholar] [CrossRef]
- DiMartini, A.; Day, N.; Dew, M.A.; Javed, L.; Fitzgerald, M.G.; Jain, A.; Fung, J.J.; Fontes, P. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transplant. 2006, 12, 813–820. [Google Scholar] [CrossRef]
- Lee, B.P.; Vittinghoff, E.; Hsu, C.; Han, H.; Therapondos, G.; Fix, O.K.; Victor, D.W.; Dronamraju, D.; Im, G.Y.; Voigt, M.D.; et al. Predicting Low Risk for Sustained Alcohol Use After Early Liver Transplant for Acute Alcoholic Hepatitis: The Sustained Alcohol Use Post–Liver Transplant Score. Hepatology 2018, 69, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.M.; Dukewich, M.; Jakhete, N.; Stonesifer, E.; Im, G.Y.; Lucey, M.R.; Shetty, K.; Rice, J.P.; Victor, D.W.; Ghobrial, M.R.; et al. Early Liver Transplantation for Severe Alcohol-Associated Hepatitis and a History of Prior Liver Decompensation. Am. J. Gastroenterol. 2022, 117, 1990–1998. [Google Scholar] [CrossRef]
- Lee, B.P.; Roth, N.; Rao, P.; Im, G.Y.; Vogel, A.S.; Hasbun, J.; Roth, Y.; Shenoy, A.; Arvelakis, A.; Ford, L.; et al. Artificial intelligence to identify harmful alcohol use after early liver transplant for alcohol-associated hepatitis. Am. J. Transplant. 2022, 22, 1834–1841. [Google Scholar] [CrossRef]
- Bittermann, T.M.; Mahmud, N.; Weinberg, E.M.; Reddy, K.R. Rising Trend in Waitlisting for Alcoholic Hepatitis with More Favorable Outcomes Than Other High Model for End-stage Liver Disease in the Current Era. Transplantation 2022, 106, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Im, G.Y.; Vogel, A.S.; Florman, S.; Nahas, J.; Friedman, S.L.; Aqui, S.; Ford, L.; Mirza, O.; Kim-Schluger, L.; Schiano, T.D. Extensive Health Care Utilization and Costs of an Early Liver Transplantation Program for Alcoholic Hepatitis. Liver Transplant. 2021, 28, 27–38. [Google Scholar] [CrossRef]
- Lee, B.P.; Im, G.Y.; Rice, J.P.; Weinberg, E.; Hsu, C.; Fix, O.K.; Therapondos, G.; Han, H.; Victor, D.W.; Eswaran, S.; et al. Underestimation of Liver Transplantation for Alcoholic Hepatitis in the National Transplant Database. Liver Transplant. 2019, 25, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Bittermann, T.; Mahmud, N.; Abt, P. Trends in Liver Transplantation for Acute Alcohol-Associated Hepatitis During the COVID-19 Pandemic in the US. JAMA Netw. Open 2021, 4, e2118713. [Google Scholar] [CrossRef]
- Patel, P.; Wang, J.; Pillai, A. CON: Liver Transplant Should Not Be Performed in Patients with Acute Alcoholic Hepatitis. Clin. Liver Dis. 2020, 16, 182–185. [Google Scholar] [CrossRef]
- Stroh, G.; Rosell, T.; Dong, F.; Forster, J. Early Liver Transplantation for Patients with Acute Alcoholic Hepatitis: Public Views and the Effects on Organ Donation. Am. J. Transplant. 2015, 15, 1598–1604. [Google Scholar] [CrossRef]
- Schomerus, G.; Leonhard, A.; Manthey, J.; Morris, J.; Neufeld, M.; Kilian, C.; Speerforck, S.; Winkler, P.; Corrigan, P.W. The stigma of alcohol-related liver disease and its impact on healthcare. J. Hepatol. 2022, 77, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Kilian, C.; Manthey, J.; Carr, S.; Hanschmidt, F.; Rehm, J.; Speerforck, S.; Schomerus, G. Stigmatization of people with alcohol use disorders: An updated systematic review of population studies. Alcohol. Clin. Exp. Res. 2021, 45, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, S.; Andersson, J. Liver health literacy and social stigma of liver disease: A general population e-survey. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101750. [Google Scholar] [CrossRef]
- Speerforck, S.; Schomerus, G. Reducing Substance Use Stigma in Health Care. In The Stigma of Substance Use Disorders; Schomerus, G., Corrigan, P.W., Eds.; Cambridge University Press: Cambridge, UK, 2022; pp. 232–251. [Google Scholar] [CrossRef]


| Therapy | Outcome | Reference/Trial |
|---|---|---|
| Anti-TNFα | Higher mortality rates due to increased risk of infection. | [52,53] |
| Pentoxifylline | No benefit on mortality over placebo, even when administered with steroid therapy. | [44] |
| IL-1 inhibitor (Anakinra) | No survival advantages were reported compared with steroid therapy. | [54] |
| N-acetylcysteine | In combination with a steroid, it improves survival. | [55,56] |
| Methadoxin | Improved survival at 3 and 6 months in combination with steroid. | [57] |
| Fecal microbiota transplantation (FMT) | Effective and safe in patients with severe AH, resulting in improved 1-year survival. | [58] |
| Authors (Year) | Number of Patients | Study Design | Location | Patients’ Selection Criteria | Selection Rate | Survival Rate in eLT |
|---|---|---|---|---|---|---|
| Mathurin et al., 2011 [65] | 28 | Prospective Case–Control Study | France and Belgium. Multicentric |
| <2% | 77 ± 8% at 6 months and 719% at 24 months |
| Im et al., 2016 [66] | 9 (2/9 underwent liver-kidney LT for hemodialysis > 12 w) | Prospective Case–Control Study | United States. Monocentric |
| 21% | 89% at 6 months |
| Lee, B.P. 2017 [67] | 17 | Retrospective pilot study | United States. Monocentric |
| 6.3% | 100% at 6 months |
| Lee, B.P. 2018 [68] | 147 | Retrospective | United States. Multicentric (12 centers) |
| 35.9% | 94% at 1 year and 84% at 3 years. |
| Lee B.P. 2022 [69] | 153 | Retrospective | United States. Multicentric (11 centers) |
| Not reported | 95%, 88% and 82%. At 1, 3 and 5 years |
| Louvet 2022 [70] | 68 | Prospective, non-randomized non-inferiority controlled trial | France and Belgium. Multicentric (2 centers) |
| 68% | 89% at 2 years |
| Germani 2022 [71] | 16 | Prospective | Italy-multicentric (4 centers) |
| 38.5% | 100% at 2 years |
| Salt Score | |
|---|---|
| >10 drinks per day at diagnosis | +4 points |
| >2 failed rehabilitation attempts | +4 points |
| Any episodes of alcohol-related legal issue | +2 points |
| History of illicit substance abuse (except for THC) | +1 Point |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germani, G.; D’Arcangelo, F.; Grasso, M.; Burra, P. Advances and Controversies in Acute Alcohol-Related Hepatitis: From Medical Therapy to Liver Transplantation. Life 2023, 13, 1802. https://doi.org/10.3390/life13091802
Germani G, D’Arcangelo F, Grasso M, Burra P. Advances and Controversies in Acute Alcohol-Related Hepatitis: From Medical Therapy to Liver Transplantation. Life. 2023; 13(9):1802. https://doi.org/10.3390/life13091802
Chicago/Turabian StyleGermani, Giacomo, Francesca D’Arcangelo, Marco Grasso, and Patrizia Burra. 2023. "Advances and Controversies in Acute Alcohol-Related Hepatitis: From Medical Therapy to Liver Transplantation" Life 13, no. 9: 1802. https://doi.org/10.3390/life13091802
APA StyleGermani, G., D’Arcangelo, F., Grasso, M., & Burra, P. (2023). Advances and Controversies in Acute Alcohol-Related Hepatitis: From Medical Therapy to Liver Transplantation. Life, 13(9), 1802. https://doi.org/10.3390/life13091802






