Membrane Lipid Composition Influences the Hydration of Proton Half-Channels in FoF1-ATP Synthase
Abstract
1. Introduction
2. Materials and Methods
3. Results
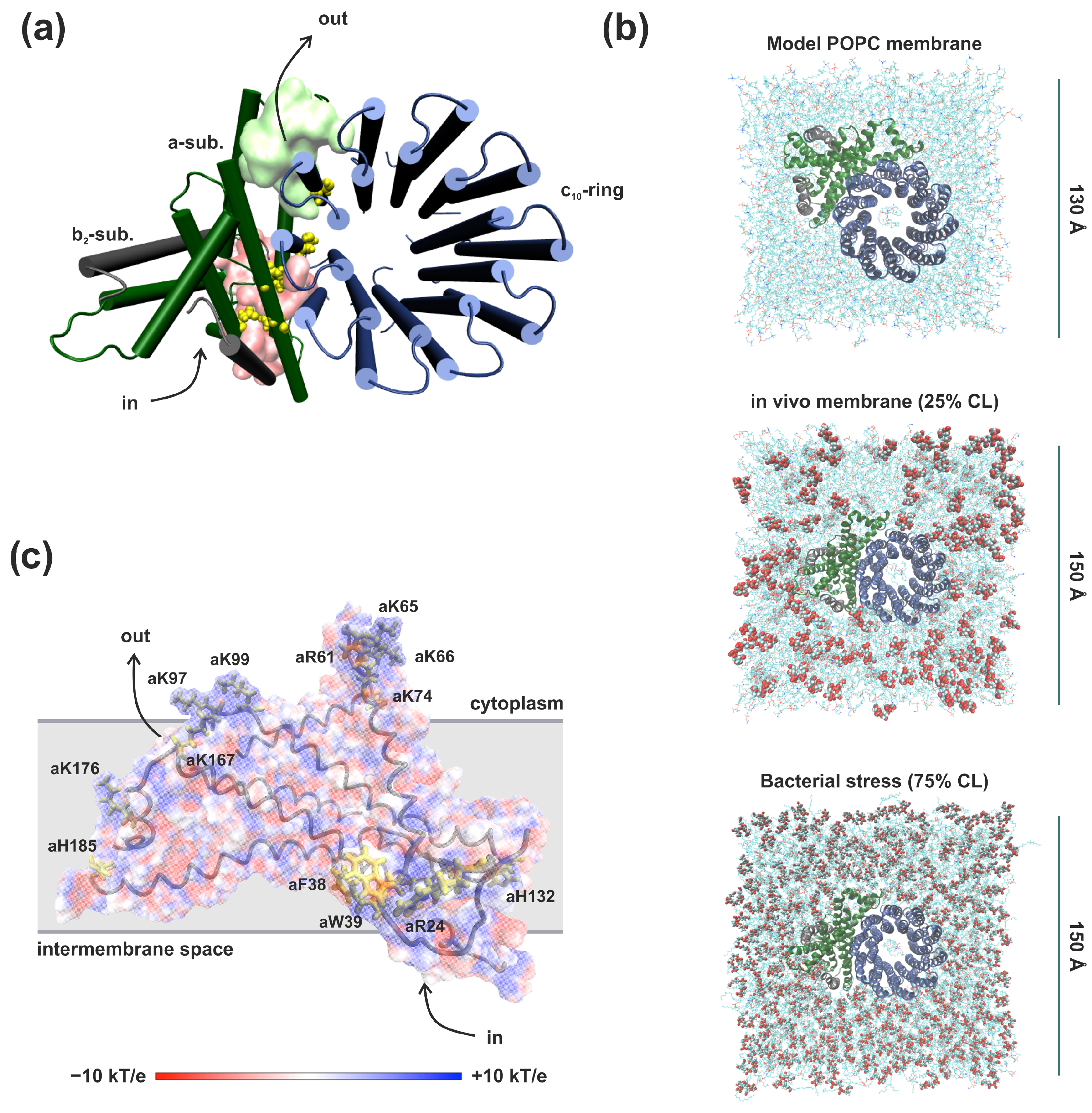
3.1. Proton Transport Pathway in the Fo Factor of E. coli ATP Synthase Embedded in Model POPC Bilayer
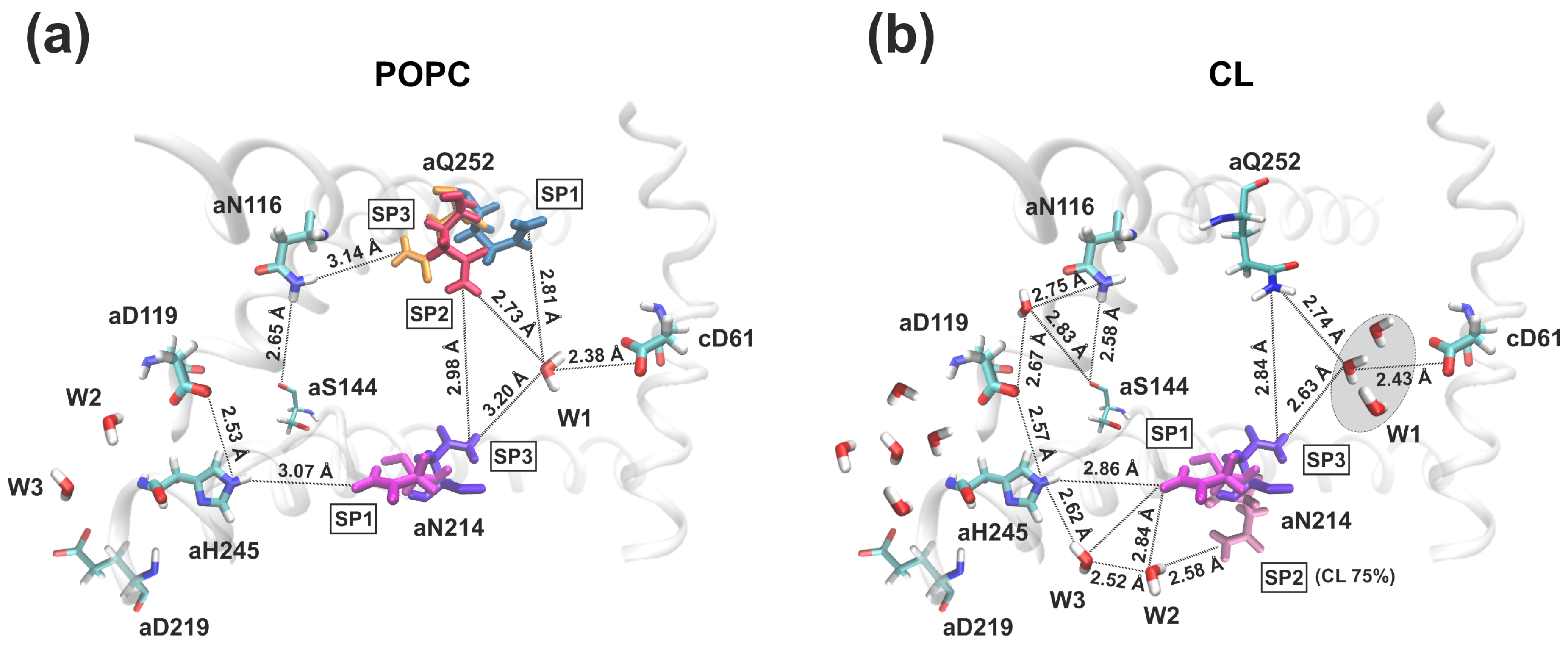
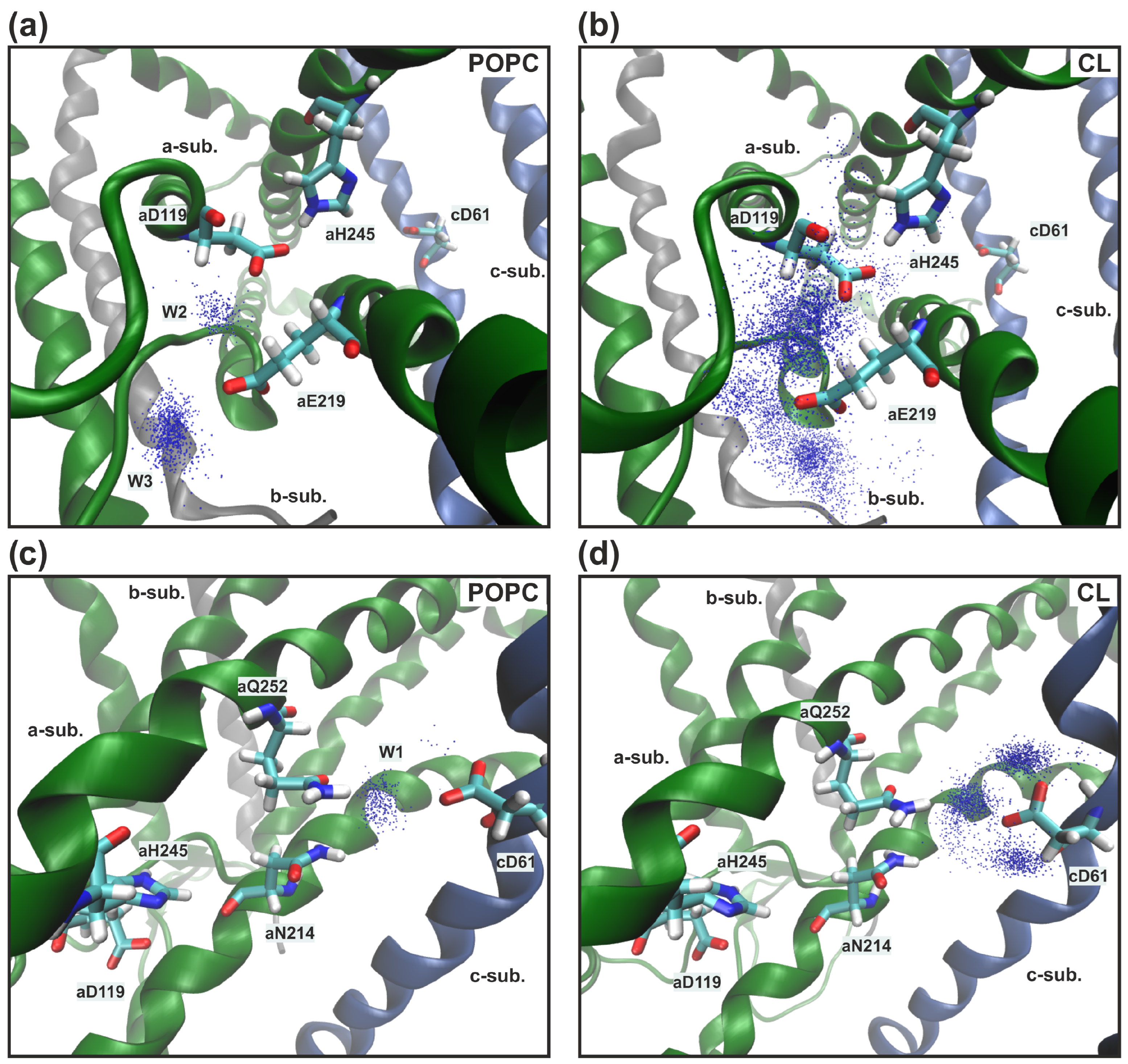
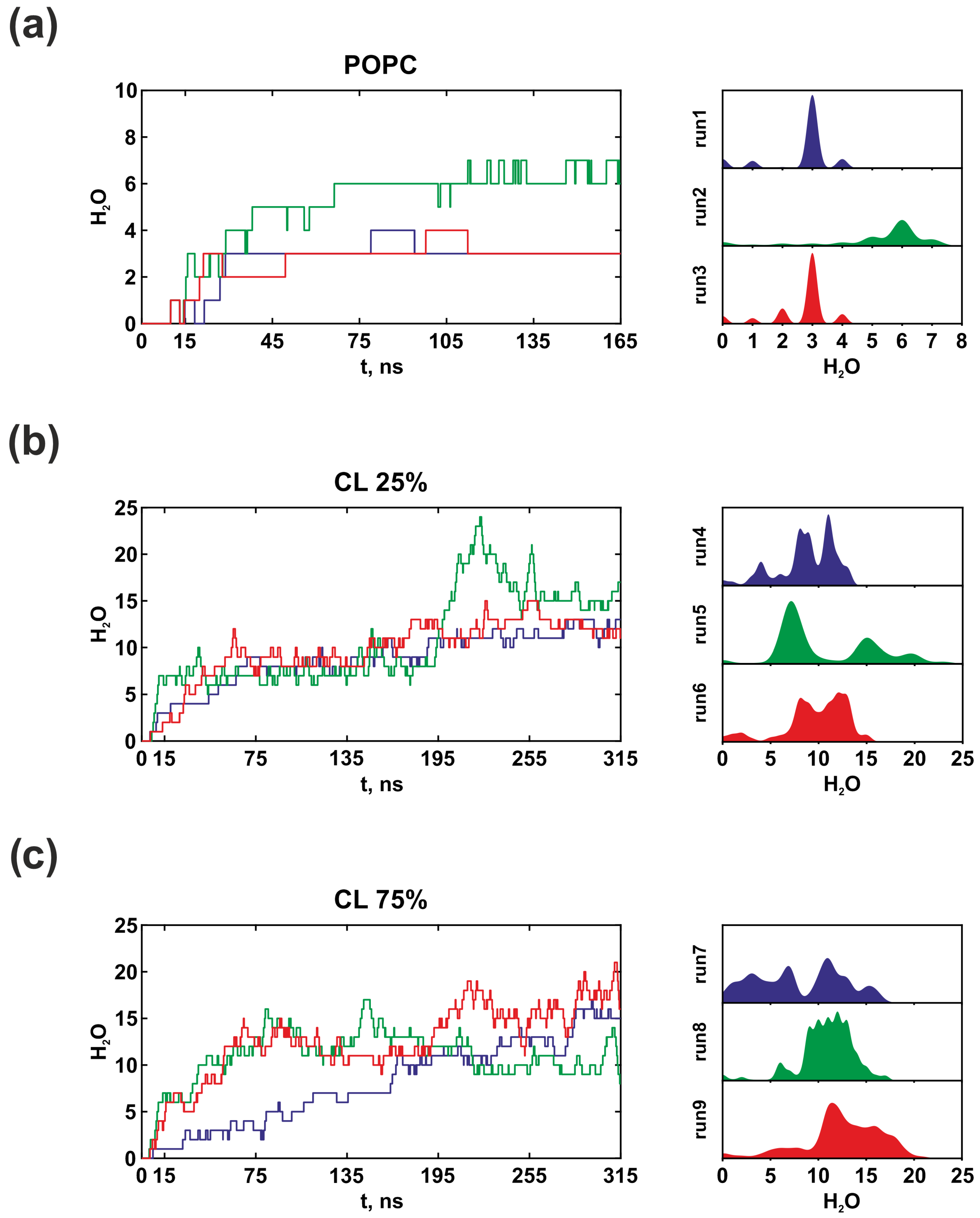
3.2. Membrane Lipid Composition Influence on the Structure and Hydration of the Proton Half-Channels
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Junge, W.; Lill, H.; Engelbrecht, S. ATP synthase: An electrochemical transducer with rotary mechanics. Trends Biochem. Sci. 1997, 11, 420–423. [Google Scholar] [CrossRef]
- Walker, J.E. The ATP synthase: The understood, the uncertain and the unknown. Biochem. Soc. Trans. 2013, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Moyle, J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature 1967, 213, 137–139. [Google Scholar] [CrossRef]
- Steed, P.R.; Fillingame, R.H. Subunit a Facilitates Aqueous Access to a Membrane-embedded Region of Subunit c in Escherichia coli F1F0 ATP Synthase. J. Biol. Chem. 2008, 283, 12365–12372. [Google Scholar] [CrossRef] [PubMed]
- Ivontsin, L.A.; Mashkovtseva, E.V.; Nartsissov, Y.R. Simulation of proton movement in FoF1-ATP synthase by quantum-mechanical approach. J. Phys. Conf. Ser. 2017, 784, 012021. [Google Scholar] [CrossRef]
- Allegretti, M.; Klusch, N.; Mills, D.J.; Vonck, J.; Kühlbrandt, W.; Davies, K.M. Horizontal membrane-intrinsic a-helices in the stator a-subunit of an F-type ATP synthase. Nature 2015, 521, 237–240. [Google Scholar] [CrossRef]
- Hahn, A.; Vonck, J.; Mills, D.J.; Meier, T.; Kühlbrandt, W. Structure, mechanism, and regulation of the chloroplast ATP synthase. Science 2018, 360, eaat4318. [Google Scholar] [CrossRef] [PubMed]
- Sobti, M.; Walshe, J.L.; Wu, D.; Ishmukhametov, R.; Zeng, Y.C.; Robinson, C.V.; Berry, R.M.; Stewart, A.G. Cryo-EM structures provide insight into how E. coli F1Fo ATP synthase accommodates symmetry mismatch. Nat. Commun. 2020, 11, 2615. [Google Scholar] [CrossRef]
- Kühlbrandt, W. Structure and mechanisms of F-type ATP synthases. Annu. Rev. Biochem. 2019, 88, 515–549. [Google Scholar] [CrossRef] [PubMed]
- Mashkovtseva, E.; Boronovsky, S.; Nartsissov, Y. Combined mathematical methods in the description of the FoF1-ATP synthase catalytic cycle. Math. Biosci. 2013, 243, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.X.; Ernst, A.M.; Wieland, F.; Brügger, B. Specificity of intramembrane protein-lipid interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004705. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Benedictis, V.; Ruggiero, F.M.; Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 2014, 1837, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Glukhov, E.; Stark, M.; Burrows, L.L.; Deber, C.M. Basis for Selectivity of Cationic Antimicrobial Peptides for Bacterial versus Mammalian Membranes. J. Biol. Chem. 2005, 280, 33960–33967. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, M.S.; Shannon, R.J.; Draghi, F.; Hirst, J. Interactions between phospholipids and NADH: Ubiquinone oxidoreductase (complex I) from bovine mitochondria. Biochemistry 2006, 45, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Arnarez, C.; Mazat, J.P.; Elezgaray, J.; Marrink, S.J.; Periole, X. Evidence for cardiolipin binding sites on the membrane-exposed surface of the cytochrome bc1. J. Am. Chem. Soc. 2013, 135, 3112–3120. [Google Scholar] [CrossRef]
- Arnarez, C.; Marrink, S.J.; Periole, X. Identification of cardiolipin binding sites on cytochrome c oxidase at the entrance of proton channels. Sci. Rep. 2013, 3, 1263. [Google Scholar] [CrossRef]
- Van den Brink-van der Laan, E.; Killian, J.; de Kruijff, B. Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim. Biophys. Acta 2004, 1666, 275–288. [Google Scholar] [CrossRef]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 2005, 280, 29403–29408. [Google Scholar] [CrossRef]
- Jussupow, A.; Luca, A.D.; Kaila, V.R.I. How cardiolipin modulates the dynamics of respiratory complex I. Sci. Adv. 2019, 5, eaav1850. [Google Scholar] [CrossRef]
- Yi, Q.; Yao, S.; Ma, B.; Cang, X. The effects of cardiolipin on the structural dynamics of the mitochondrial ADP/ATP carrier in its cytosol-open state. J. Lipid Res. 2022, 63, 100227. [Google Scholar] [CrossRef]
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the Osmotic Stress Responses of Bacteria. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, R.; Munari, F.; Becker, S.; Zweckstetter, M. Interaction of the intermembrane space domain of Tim23 protein with mitochondrial membranes. J. Biol. Chem. 2014, 289, 34620–34626. [Google Scholar] [CrossRef]
- Beyer, K.; Nuscher, B. Specific cardiolipin binding interferes with labeling of sulfhydryl residues in the adenosine diphosphate/adenosine triphosphate carrier protein from beef heart mitochondria. Biochemistry 1996, 35, 15784–15790. [Google Scholar] [CrossRef]
- Zardeneta, G.; Horowitz, P.M. Physical characterization of a reactivatable liposome-bound rhodanese folding intermediate. Biochemistry 1993, 32, 13941–13948. [Google Scholar] [CrossRef]
- Acehan, D.; Malhotra, A.; Xu, Y.; Ren, M.; Stokes, D.L.; Schlame, M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 2011, 100, 2184–2192. [Google Scholar] [CrossRef]
- Eble, K.S.; Coleman, W.B.; Hantgan, R.R.; Cunningham, C.C. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J. Biol. Chem. 1990, 265, 19434–19440. [Google Scholar] [CrossRef]
- Mehdipour, A.R.; Hummer, G. Cardiolipin puts the seal on ATP synthase. Proc. Natl. Acad. Sci. USA 2016, 113, 8568–8570. [Google Scholar] [CrossRef]
- Duncan, A.L.; Robinson, A.J.; Walker, J.E. Cardiolipin binds selectively but transiently to conserved lysine residues in the rotor of metazoan ATP synthases. Proc. Natl. Acad. Sci. USA 2016, 113, 8687–8692. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Nett, J.H.; Trumpower, B.L.; Hunte, C. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 2001, 20, 6591–6600. [Google Scholar] [CrossRef]
- Rowlett, V.W.; Mallampalli, V.W.K.P.S.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of membrane phospholipid alterations in Escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017, 199, e00849-16. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.R.; Sutcliffe, I.C.; Bendell, D.; Cummings, S.P. The modification of the membrane of Oceanomonas baumannii(T) when subjected to both osmotic and organic solvent stress. Fems. Microbiol. Lett. 2000, 189, 149–154. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Fukamachi, T.; Saito, H.; Kobayashi, H. Respiration and the F1Fo-ATPase Enhance Survival under Acidic Conditions in Escherichia coli. PLoS ONE 2012, 7, e52577. [Google Scholar] [CrossRef]
- Ivontsin, L.A.; Mashkovtseva, E.V.; Nartsissov, Y.R. Insights on the proton translocation pathways in FoF1-ATP synthase using molecular dynamics simulations. Arch. Biochem. Biophys. 2022, 717, 109135. [Google Scholar] [CrossRef]
- Anandakrishnan, R.; Aguilar, B.; Onufriev, A. H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012, 40, W537–W541. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Davila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward Realistic Biological Membrane Simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, E.M.; Mittal, J.; Feig, M.; MacKerell, A.D. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theor. Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Ivontsin, L.A.; Mashkovtseva, E.V.; Nartsissov, Y.R. Quantum-mechanical analysis of amino acid residues function in the proton transport during FoF1-ATP synthase catalytic cycle. J. Phys. Conf. Ser. 2017, 917, 042004. [Google Scholar] [CrossRef]
- Sugo, Y.; Ishikita, H. Mechanism of asparagine-mediated proton transfer in photosynthetic reaction centers. Biochemistry 2023, 62, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Nartsissov, Y.R.; Mashkovtseva, E.V. Application of rigid body mechanics to theoretical description of rotation within F0F1-ATP synthase. J. Theor. Biol. 2006, 242, 300–308. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivontsin, L.A.; Mashkovtseva, E.V.; Nartsissov, Y.R. Membrane Lipid Composition Influences the Hydration of Proton Half-Channels in FoF1-ATP Synthase. Life 2023, 13, 1816. https://doi.org/10.3390/life13091816
Ivontsin LA, Mashkovtseva EV, Nartsissov YR. Membrane Lipid Composition Influences the Hydration of Proton Half-Channels in FoF1-ATP Synthase. Life. 2023; 13(9):1816. https://doi.org/10.3390/life13091816
Chicago/Turabian StyleIvontsin, Leonid A., Elena V. Mashkovtseva, and Yaroslav R. Nartsissov. 2023. "Membrane Lipid Composition Influences the Hydration of Proton Half-Channels in FoF1-ATP Synthase" Life 13, no. 9: 1816. https://doi.org/10.3390/life13091816
APA StyleIvontsin, L. A., Mashkovtseva, E. V., & Nartsissov, Y. R. (2023). Membrane Lipid Composition Influences the Hydration of Proton Half-Channels in FoF1-ATP Synthase. Life, 13(9), 1816. https://doi.org/10.3390/life13091816





