Acquired Male Hypogonadism in the Post-Genomic Era—A Narrative Review
Abstract
:1. Introduction
2. Personalized -Omic Medicine in Post-Genomic Era
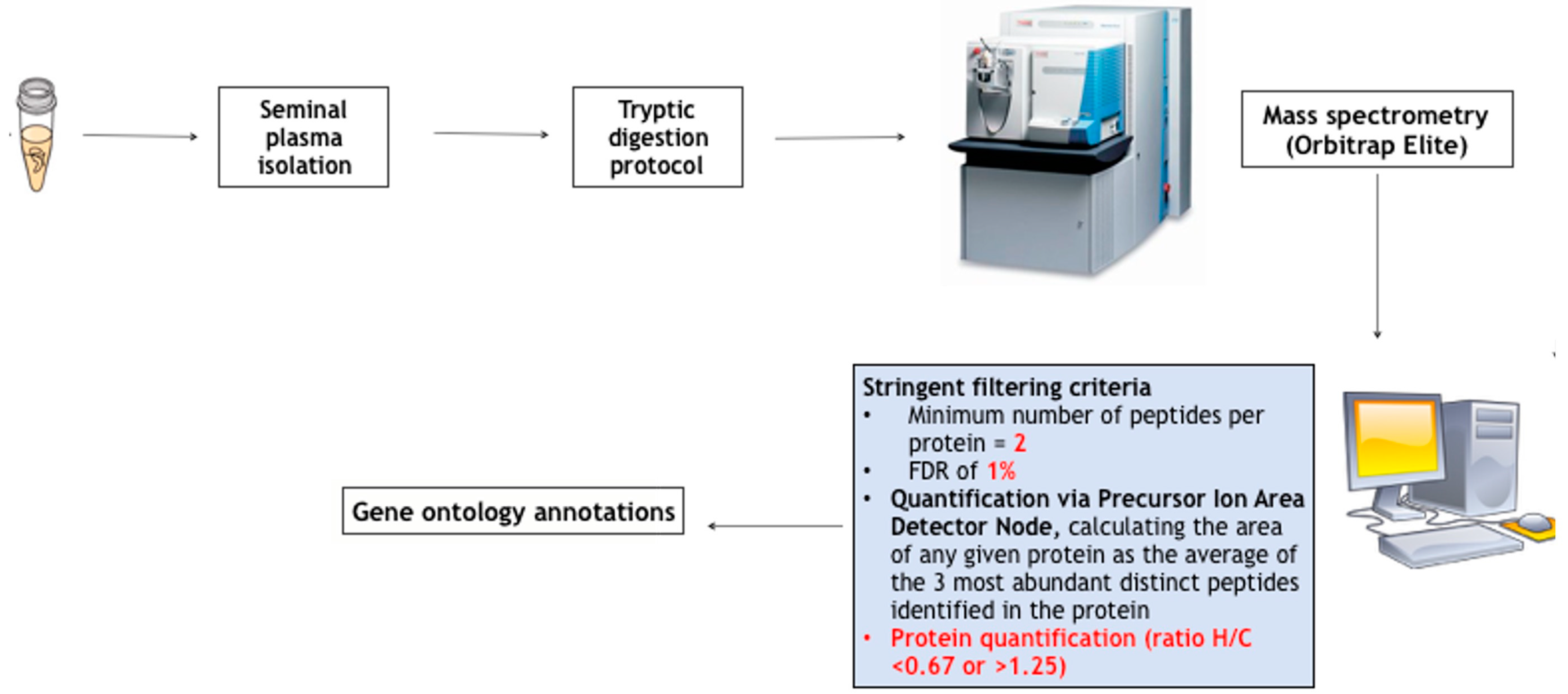
3. Seminal Proteomics in MH: Revealing Peripheral Markers of T Action
4. Sperm Proteomics in Hypogonadism: Unrevealing the Role of Testosterone in Spermatogenesis
5. Serum Metabolomics in Hypogonadism: Metabolic Differences between Functional and Primary Hypogonadism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men with Hypogonadism: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Aversa, A.; Calogero, A.; Ferlin, A.; Francavilla, S.; Lanfranco, F.; Pivonello, R.; Rochira, V.; Corona, G.; Maggi, M. Adult- and Late-Onset Male Hypogonadism: The Clinical Practice Guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2022, 45, 2385–2403. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Matsumoto, A.M. A Perspective on Middle-Aged and Older Men with Functional Hypogonadism: Focus on Holistic Management. J. Clin. Endocrinol. Metab. 2017, 102, 1067–1075. [Google Scholar] [CrossRef]
- Corona, G.; Goulis, D.G.; Huhtaniemi, I.; Zitzmann, M.; Toppari, J.; Forti, G.; Vanderschueren, D.; Wu, F.C.; Corona, G.; Goulis, D.G.; et al. European Academy of Andrology (EAA) Guidelines on Investigation, Treatment and Monitoring of Functional Hypogonadism in Males. Andrology 2020, 8, 970–987. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Balercia, G.; Bellastella, A.; Colao, A.; Fabbri, A.; Foresta, C.; Galdiero, M.; Gandini, L.; Krausz, C.; Lombardi, G.; et al. Epidemiology; Diagnosis, and Treatment of Male Hypogonadotropic Hypogonadism. J. Endocrinol. Investig. 2009, 32, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Milardi, D.; Baroni, S.; Urbani, A.; Pontecorvi, A. Why Do We Need New Markers for Male Hypogonadism and How Seminal Proteomics Might Solve the Problem? Protein Pept. Lett. 2020, 27, 1186–1191. [Google Scholar] [CrossRef]
- Arver, S.; Lehtihet, M. Current Guidelines for the Diagnosis of Testosterone Deficiency. In Advances in the Management of Testosterone Deficiency; KARGER: Basel, Switzerland, 2008; pp. 5–20. [Google Scholar]
- Facondo, P.; Di Lodovico, E.; Pezzaioli, L.C.; Cappelli, C.; Ferlin, A.; Delbarba, A. Usefulness of Routine Assessment of Free Testosterone for the Diagnosis of Functional Male Hypogonadism. Aging Male 2022, 25, 72–78. [Google Scholar] [CrossRef]
- Duarte, T.; Spencer, C. Personalized Proteomics: The Future of Precision Medicine. Proteomes 2016, 4, 29. [Google Scholar] [CrossRef]
- The International HapMap Consortium. A Haplotype Map of the Human Genome. Nature 2005, 437, 1299–1320. [Google Scholar] [CrossRef]
- The Cost of Sequencing a Human Genome. Available online: https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost (accessed on 1 July 2023).
- Pennisi, E. ENCODE Project Writes Eulogy for Junk DNA. Science 2012, 337, 1159–1161. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Sanchez, J.-C.; Gooley, A.A.; Appel, R.D.; Humphery-Smith, I.; Hochstrasser, D.F.; Williams, K.L. Progress with Proteome Projects: Why All Proteins Expressed by a Genome Should Be Identified and How to Do It. Biotechnol. Genet. Eng. Rev. 1996, 13, 19–50. [Google Scholar] [CrossRef] [PubMed]
- Boja, E.S.; Rodriguez, H. The Path to Clinical Proteomics Research: Integration of Proteomics, Genomics, Clinical Laboratory and Regulatory Science. Ann. Lab. Med. 2011, 31, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Pierconti, F.; Pontecorvi, A. Proteomics for the Identification of Biomarkers in Testicular Cancer–Review. Front. Endocrinol. 2019, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Castagnola, M.; Marana, R. Proteomics of Human Seminal Plasma: Identification of Biomarker Candidates for Fertility and Infertility and the Evolution of Technology. Mol. Reprod. Dev. 2013, 80, 350–357. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Piras, C.; Atzori, L.; Fanos, V. Slotting Metabolomics into Routine Precision Medicine. Expert Rev. Precis. Med. Drug Dev. 2021, 6, 173–187. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond Biomarkers and towards Mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Brooks, D.E. Influence of androgens on the weights of the male accessory reproductive organs and on the activities of mitochondrial enzymes in the epididymis of the rat. J. Endocrinol. 1979, 82, 293–303. [Google Scholar] [CrossRef]
- Green, S.M.; Mostaghel, E.A.; Nelson, P.S. Androgen Action and Metabolism in Prostate Cancer. Mol. Cell. Endocrinol. 2012, 360, 3–13. [Google Scholar] [CrossRef]
- Ma, C.; Yoshioka, M.; Boivin, A.; Gan, L.; Takase, Y.; Labrie, F.; St-Amand, J. Atlas of Dihydrotestosterone Actions on the Transcriptome of Prostate in Vivo. Prostate 2009, 69, 293–316. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Giampietro, A.; Messana, I.; Castagnola, M.; Marana, R.; De Marinis, L.; Pontecorvi, A. Novel Biomarkers of Androgen Deficiency From Seminal Plasma Profiling Using High-Resolution Mass Spectrometry. J. Clin. Endocrinol. Metab. 2014, 99, 2813–2820. [Google Scholar] [CrossRef]
- Grande, G.; Vincenzoni, F.; Mancini, F.; Barrachina, F.; Giampietro, A.; Castagnola, M.; Urbani, A.; Oliva, R.; Milardi, D.; Pontecorvi, A. Quantitative Analysis of the Seminal Plasma Proteome in Secondary Hypogonadism. J. Clin. Med. 2019, 8, 2128. [Google Scholar] [CrossRef] [PubMed]
- Behre, H.M.; Kliesch, S.; Schädel, F.; Nieschlag, E. Clinical Relevance of Scrotal and Transrectal Ultrasonography in Andrological Patients. Int. J. Androl. 1995, 18 (Suppl. S2), 27–31. [Google Scholar] [PubMed]
- Vickram, S.; Rohini, K.; Anbarasu, K.; Dey, N.; Jeyanthi, P.; Thanigaivel, S.; Issac, P.K.; Arockiaraj, J. Semenogelin, a Coagulum Macromolecule Monitoring Factor Involved in the First Step of Fertilization: A Prospective Review. Int. J. Biol. Macromol. 2022, 209, 951–962. [Google Scholar] [CrossRef]
- Tomar, A.K.; Sooch, B.S.; Raj, I.; Singh, S.; Yadav, S. Interaction Analysis Identifies Semenogelin I Fragments as New Binding Partners of PIP in Human Seminal Plasma. Int. J. Biol. Macromol. 2013, 52, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.d.I.; Waheed, A.; Yadav, S.; Singh, T.P.; Ahmad, F. Prolactin Inducible Protein in Cancer, Fertility and Immunoregulation: Structure, Function and Its Clinical Implications. Cell. Mol. Life Sci. 2009, 66, 447–459. [Google Scholar] [CrossRef]
- Martinez-Heredia, J.; de Mateo, S.; Vidal-Taboada, J.M.; Ballesca, J.L.; Oliva, R. Identification of Proteomic Differences in Asthenozoospermic Sperm Samples. Hum. Reprod. 2008, 23, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Anklesaria, J.H.; Jagtap, D.D.; Pathak, B.R.; Kadam, K.M.; Joseph, S.; Mahale, S.D. Prostate Secretory Protein of 94 Amino Acids (PSP94) Binds to Prostatic Acid Phosphatase (PAP) in Human Seminal Plasma. PLoS ONE 2013, 8, e58631. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Messana, I.; Pontecorvi, A.; De Marinis, L.; Castagnola, M.; Marana, R. Proteomic Approach in the Identification of Fertility Pattern in Seminal Plasma of Fertile Men. Fertil. Steril. 2012, 97, 67–73. [Google Scholar] [CrossRef]
- Piomboni, P.; Gambera, L.; Serafini, F.; Campanella, G.; Morgante, G.; De Leo, V. Sperm Quality Improvement after Natural Anti-Oxidant Treatment of Asthenoteratospermic Men with Leukocytospermia. Asian J. Androl. 2008, 10, 201–206. [Google Scholar] [CrossRef]
- Grande, G.; Barrachina, F.; Soler-Ventura, A.; Jodar, M.; Mancini, F.; Marana, R.; Chiloiro, S.; Pontecorvi, A.; Oliva, R.; Milardi, D. The Role of Testosterone in Spermatogenesis: Lessons from Proteome Profiling of Human Spermatozoa in Testosterone Deficiency. Front. Endocrinol. 2022, 13, 852661. [Google Scholar] [CrossRef]
- Stanton, P.G.; Sluka, P.; Foo, C.F.H.; Stephens, A.N.; Smith, A.I.; McLachlan, R.I.; O’Donnell, L. Proteomic Changes in Rat Spermatogenesis in Response to In Vivo Androgen Manipulation; Impact on Meiotic Cells. PLoS ONE 2012, 7, e41718. [Google Scholar] [CrossRef] [PubMed]
- Vague, J.; Sardo, J. Hypogonadism with Spermatogenesis (Fertile Eunuch Syndrome) (Author’s Transl). Sem. Hop. 1982, 58, 767–774. [Google Scholar] [PubMed]
- Kishimoto, Y.; Hiraiwa, M.; O’Brien, J.S. Saposins: Structure, Function, Distribution, and Molecular Genetics. J. Lipid Res. 1992, 33, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Collard, M.W.; Sylvester, S.R.; Tsuruta, J.K.; Griswold, M.D. Biosynthesis and Molecular Cloning of Sulfated Glycoprotein 1 Secreted by Rat Sertoli Cells: Sequence Similarity with the 70-Kilodalton Precursor to Sulfatide/GM1 Activator. Biochemistry 1988, 27, 4557–4564. [Google Scholar] [CrossRef]
- Sylvester, S.R.; Morales, C.; Oko, R.; Griswold, M.D. Sulfated Glycoprotein-1 (Saposin Precursor) in the Reproductive Tract of the Male Rat. Biol. Reprod. 1989, 41, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.R.; Clermont, Y. Phagocytosis and Endocytosis in Sertoli Cells of the Rat. Bull Assoc. Anat. 1991, 75, 157–162. [Google Scholar]
- Morales, C.; Clermont, Y.; Hermo, L. Nature and Function of Endocytosis in Sertoli Cells of the Rat. Am. J. Anat. 1985, 173, 203–217. [Google Scholar] [CrossRef]
- Morales, C.; Clermont, Y.; Nadler, N.J. Cyclic Endocytic Activity and Kinetics of Lysosomes in Sertoli Cells of the Rat: A Morphometric Analysis. Biol. Reprod. 1986, 34, 207–218. [Google Scholar] [CrossRef]
- Igdoura, S.A.; Morales, C.R.; Hermo, L. Differential Expression of Cathepsins B and D in Testis and Epididymis of Adult Rats. J. Histochem. Cytochem. 1995, 43, 545–557. [Google Scholar] [CrossRef]
- Igdoura, S.A.; Morales, C.R. Role of Sulfated Glycoprotein-1 (SGP-1) in the Disposal of Residual Bodies by Sertoli Cells of the Rat. Mol. Reprod. Dev. 1995, 40, 91–102. [Google Scholar] [CrossRef]
- La Sala, G.; Marazziti, D.; Di Pietro, C.; Golini, E.; Matteoni, R.; Tocchini-Valentini, G.P. Modulation of Dhh Signaling and Altered Sertoli Cell Function in Mice Lacking the GPR37-Prosaposin Receptor. FASEB J. 2015, 29, 2059–2069. [Google Scholar] [CrossRef]
- Rosenthal, A.L.; Igdoura, S.A.; Morales, C.R.; Hermo, L. Hormonal Regulation of Sulfated Glycoprotein-1 Synthesis by Nonciliated Cells of the Efferent Ducts of Adult Rats. Mol. Reprod. Dev. 1995, 40, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Hammerstedt, R.H.; Cramer, P.G.; Barbato, G.F.; Amann, R.P.; O’Brien, J.S.; Griswold, M.D. A Fragment of Prosaposin (SGP-1) from Rooster Sperm Promotes Sperm-Egg Binding and Improves Fertility in Chickens. J. Androl. 2001, 22, 361–375. [Google Scholar]
- Fujita, A.; Nakamura, K.; Kato, T.; Watanabe, N.; Ishizaki, T.; Kimura, K.; Mizoguchi, A.; Narumiya, S. Ropporin, a Sperm-Specific Binding Protein of Rhophilin, That Is Localized in the Fibrous Sheath of Sperm Flagella. J. Cell Sci. 2000, 113 Pt 1, 103–112. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Wei, B.; Lai, Y.; Yan, Q.; Gui, Y.; Cai, Z. Functional Expression of Ropporin in Human Testis and Ejaculated Spermatozoa. J. Androl. 2011, 32, 26–32. [Google Scholar] [CrossRef]
- Amer, M.K.; Mostafa, R.M.; Fathy, A.; Saad, H.M.; Mostafa, T. Ropporin Gene Expression in Infertile Asthenozoospermic Men with Varicocele Before and After Repair. Urology 2015, 85, 805–808. [Google Scholar] [CrossRef]
- Uhrin, P.; Schöfer, C.; Zaujec, J.; Ryban, L.; Hilpert, M.; Weipoltshammer, K.; Jerabek, I.; Pirtzkall, I.; Furtmüller, M.; Dewerchin, M.; et al. Male Fertility and Protein C Inhibitor/Plasminogen Activator Inhibitor-3 (PCI): Localization of PCI in Mouse Testis and Failure of Single Plasminogen Activator Knockout to Restore Spermatogenesis in PCI-Deficient Mice. Fertil. Steril. 2007, 88, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Marlar, R.A.; Griffin, J.H. Deficiency of Protein C Inhibitor in Combined Factor V/VIII Deficiency Disease. J. Clin. Investig. 1980, 66, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nishioka, J.; Hashimoto, S. Protein C Inhibitor. Purification from Human Plasma and Characterization. J. Biol. Chem. 1983, 258, 163–168. [Google Scholar] [CrossRef] [PubMed]
- España, F.; Berrettini, M.; Griffin, J.H. Purification and Characterization of Plasma Protein C Inhibitor. Thromb. Res. 1989, 55, 369–384. [Google Scholar] [CrossRef]
- Meijers, J.C.M.; Kanters, D.H.A.J.; Vlooswijk, R.A.A.; Van Erp, H.E.; Hessing, M.; Bouma, B.N. Inactivation of Human Plasma Kallikrein and Factor XIa by Protein C Inhibitor. Biochemistry 1988, 27, 4231–4237. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.R.; Cooper, S.T.; Church, F.C.; Esmon, C.T. Protein C Inhibitor Is a Potent Inhibitor of the Thrombin-Thrombomodulin Complex. J. Biol. Chem. 1995, 270, 25336–25339. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Huber, K.; Wojta, J.; Stingl, L.; Espana, F.; Griffin, J.H.; Binder, B.R. Complex Formation between Urokinase and Plasma Protein C Inhibitor in Vitro and in Vivo. Blood 1989, 74, 722–728. [Google Scholar] [CrossRef] [PubMed]
- España, F.; Estellés, A.; Fernández, P.J.; Gilabert, J.; Sánchez-Cuenca, J.; Griffin, J.H. Evidence for the Regulation of Urokinase and Tissue Type Plasminogen Activators by the Serpin, Protein C Inhibitor, in Semen and Blood Plasma. Thromb. Haemost. 1993, 70, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Hermans, J.M.; Jones, R.; Stone, S.R. Rapid Inhibition of the Sperm Protease Acrosin by Protein C Inhibitor. Biochemistry 1994, 33, 5440–5444. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Geiger, M.; Ecke, S.; Bielek, E.; Donner, P.; Eberspacher, U.; Schleuning, W.D.; Binder, B.R. Inhibition of Acrosin by Protein C Inhibitor and Localization of Protein C Inhibitor to Spermatozoa. Am. J. Physiol.-Cell Physiol. 1994, 267, C466–C472. [Google Scholar] [CrossRef]
- Ecke, S.; Geiger, M.; Resch, I.; Jerabek, I.; Sting, L.; Maier, M.; Binder, B.R. Inhibition of Tissue Kallikrein by Protein C Inhibitor. Evidence for Identity of Protein C Inhibitor with the Kallikrein Binding Protein. J. Biol. Chem. 1992, 267, 7048–7052. [Google Scholar] [CrossRef]
- España, F.; Gilabert, J.; Estellés, A.; Romeu, A.; Aznar, J.; Cabo, A. Functionally Active Protein C Inhibitor/Plasminogen Activator Inhibitor-3 (PCI/PAI-3) Is Secreted in Seminal Vesicles, Occurs at High Concentrations in Human Seminal Plasma and Complexes with Prostate-Specific Antigen. Thromb. Res. 1991, 64, 309–320. [Google Scholar] [CrossRef]
- Christensson, A.; Lilja, H. Complex Formation between Protein C Inhibitor and Prostate-Specific Antigen in Vitro and in Human Semen. Eur. J. Biochem. 1994, 220, 45–53. [Google Scholar] [CrossRef]
- Uhrin, P.; Dewerchin, M.; Hilpert, M.; Chrenek, P.; Schöfer, C.; Zechmeister-Machhart, M.; Krönke, G.; Vales, A.; Carmeliet, P.; Binder, B.R.; et al. Disruption of the Protein C Inhibitor Gene Results in Impaired Spermatogenesis and Male Infertility. J. Clin. Investig. 2000, 106, 1531–1539. [Google Scholar] [CrossRef]
- Bornstein, P.; Sage, E.H. Matricellular Proteins: Extracellular Modulators of Cell Function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Raines, E.W.; Lane, T.F.; Iruela-Arispe, M.L.; Ross, R.; Sage, E.H. The Extracellular Glycoprotein SPARC Interacts with Platelet-Derived Growth Factor (PDGF)-AB and -BB and Inhibits the Binding of PDGF to Its Receptors. Proc. Natl. Acad. Sci. USA 1992, 89, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Kupprion, C.; Motamed, K.; Sage, E.H. SPARC (BM-40, Osteonectin) Inhibits the Mitogenic Effect of Vascular Endothelial Growth Factor on Microvascular Endothelial Cells. J. Biol. Chem. 1998, 273, 29635–29640. [Google Scholar] [CrossRef] [PubMed]
- Motamed, K.; Blake, D.J.; Angello, J.C.; Allen, B.L.; Rapraeger, A.C.; Hauschka, S.D.; Sage, E.H. Fibroblast Growth Factor Receptor-1 Mediates the Inhibition of Endothelial Cell Proliferation and the Promotion of Skeletal Myoblast Differentiation by SPARC: A Role for Protein Kinase A. J. Cell. Biochem. 2003, 90, 408–423. [Google Scholar] [CrossRef]
- Francki, A.; Motamed, K.; McClure, T.D.; Kaya, M.; Murri, C.; Blake, D.J.; Carbon, J.G.; Sage, E.H. SPARC Regulates Cell Cycle Progression in Mesangial Cells via Its Inhibition of IGF-Dependent Signaling. J. Cell. Biochem. 2003, 88, 802–811. [Google Scholar] [CrossRef]
- Pazin, D.E.; Albrecht, K.H. Developmental Expression of Smoc1 and Smoc2 Suggests Potential Roles in Fetal Gonad and Reproductive Tract Differentiation. Dev. Dyn. 2009, 238, 2877–2890. [Google Scholar] [CrossRef]
- Westbrook, V.A.; Diekman, A.B.; Klotz, K.L.; Khole, V.V.; von Kap-Herr, C.; Golden, W.L.; Eddy, R.L.; Shows, T.B.; Stoler, M.H.; Lee, C.-Y.G.; et al. Spermatid-Specific Expression of the Novel X-Linked Gene Product SPAN-X Localized to the Nucleus of Human Spermatozoa. Biol. Reprod. 2000, 63, 469–481. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Liang, J.; He, D. Comparative Proteomic Study between Human Normal Motility Sperm and Idiopathic Asthenozoospermia. World J. Urol. 2013, 31, 1395–1401. [Google Scholar] [CrossRef]
- Malcher, A.; Rozwadowska, N.; Stokowy, T.; Kolanowski, T.; Jedrzejczak, P.; Zietkowiak, W.; Kurpisz, M. Potential Biomarkers of Nonobstructive Azoospermia Identified in Microarray Gene Expression Analysis. Fertil. Steril. 2013, 100, 1686–1694. [Google Scholar] [CrossRef]
- D’Amours, O.; Frenette, G.; Bordeleau, L.-J.; Allard, N.; Leclerc, P.; Blondin, P.; Sullivan, R. Epididymosomes Transfer Epididymal Sperm Binding Protein 1 (ELSPBP1) to Dead Spermatozoa During Epididymal Transit in Bovine1. Biol. Reprod. 2012, 87, 94. [Google Scholar] [CrossRef]
- Pinke, L.A.; Swanlund, D.J.; Hensleigh, H.C.; McCarthy, J.B.; Roberts, K.P.; Pryor, J.L. Analysis of Fibronectin on Human Sperm. J. Urol. 1997, 158, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Leone, E.; Festa, R.; Grande, G.; Silvestrini, A.; de Marinis, L.; Pontecorvi, A.; Maira, G.; Littarru, G.P.; Meucci, E. Effects of Testosterone on Antioxidant Systems in Male Secondary Hypogonadism. J. Androl. 2008, 29, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Vena, W.; Pizzocaro, A.; Giagulli, V.A.; Francomano, D.; Rastrelli, G.; Mazziotti, G.; Aversa, A.; Isidori, A.M.; Pivonello, R.; et al. Testosterone Supplementation and Bone Parameters: A Systematic Review and Meta-Analysis Study. J. Endocrinol. Investig. 2022, 45, 911–926. [Google Scholar] [CrossRef]
- Foresta, C.; Ferlin, A.; Lenzi, A.; Montorsi, P. The Great Opportunity of the Andrological Patient: Cardiovascular and Metabolic Risk Assessment and Prevention. Andrology 2017, 5, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Hackett, G.; Cole, N.; Bhartia, M.; Kennedy, D.; Raju, J.; Wilkinson, P.; The BLAST Study Group. Testosterone Replacement Therapy Improves Metabolic Parameters in Hypogonadal Men with Type 2 Diabetes but Not in Men with Coexisting Depression: The BLAST Study. J. Sex. Med. 2014, 11, 840–856. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.H.; Arver, S.; Behre, H.M.; Buvat, J.; Meuleman, E.; Moncada, I.; Morales, A.M.; Volterrani, M.; Yellowlees, A.; Howell, J.D.; et al. Testosterone Replacement in Hypogonadal Men with Type 2 Diabetes and/or Metabolic Syndrome (the TIMES2 Study). Diabetes Care 2011, 34, 828–837. [Google Scholar] [CrossRef]
- Hamilton, E.J.; Gianatti, E.; Strauss, B.J.; Wentworth, J.; Lim-Joon, D.; Bolton, D.; Zajac, J.D.; Grossmann, M. Increase in Visceral and Subcutaneous Abdominal Fat in Men with Prostate Cancer Treated with Androgen Deprivation Therapy. Clin. Endocrinol. 2011, 74, 377–383. [Google Scholar] [CrossRef]
- Rastrelli, G.; Carter, E.L.; Ahern, T.; Finn, J.D.; Antonio, L.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; Keevil, B.; et al. Development of and Recovery from Secondary Hypogonadism in Aging Men: Prospective Results from the EMAS. J. Clin. Endocrinol. Metab. 2015, 100, 3172–3182. [Google Scholar] [CrossRef]
- Camacho, E.M.; Huhtaniemi, I.T.; O’Neill, T.W.; Finn, J.D.; Pye, S.R.; Lee, D.M.; Tajar, A.; Bartfai, G.; Boonen, S.; Casanueva, F.F.; et al. Age-Associated Changes in Hypothalamic–Pituitary–Testicular Function in Middle-Aged and Older Men Are Modified by Weight Change and Lifestyle Factors: Longitudinal Results from the European Male Ageing Study. Eur. J. Endocrinol. 2013, 168, 445–455. [Google Scholar] [CrossRef]
- Grossmann, M.; Fui, M.N.T.; Cheung, A.S. Late-onset Hypogonadism: Metabolic Impact. Andrology 2020, 8, 1519–1529. [Google Scholar] [CrossRef]
- Grossmann, M. Hypogonadism and Male Obesity: Focus on Unresolved Questions. Clin. Endocrinol. 2018, 89, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.; Gevi, F.; Belardo, A.; Zolla, L. Metabolic Patterns in Insulin-Sensitive Male Hypogonadism. Cell Death Dis. 2018, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.; Belardo, A.; Savino, R.; Rinalducci, S.; Zolla, L. Testosterone Replacement Therapy in Insulin-sensitive Hypogonadal Men Restores Phosphatidylcholine Levels by Regulation of Arachidonic Acid Metabolism. J. Cell Mol. Med. 2020, 24, 8266–8269. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Fanelli, G.; Zolla, L. Metabolic Patterns in Insulin-Resistant Male Hypogonadism. Cell Death Dis. 2018, 9, 671. [Google Scholar] [CrossRef]
- Zolla, L.; Grande, G.; Milardi, D. Plasma Metabonomics in Insulin-Resistant Hypogonadic Patients Induced by Testosterone Treatment. Int. J. Mol. Sci. 2022, 23, 7754. [Google Scholar] [CrossRef]
- Grande, G.; De Toni, L.; Garolla, A.; Milardi, D.; Ferlin, A. Plasma metabolomics in male primary and functional hypogonadism. Front. Endocrinol. 2023, 14, 1165741. [Google Scholar] [CrossRef]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-Omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights 2020, 14, 117793221989905. [Google Scholar] [CrossRef]

| Protein | Gene |
|---|---|
| Semenogelin-1 | SEMG1 |
| Semenogelin-2 | SEMG2 |
| Prolactine-inducible protein | PIP |
| Prostatic acid phosphatase | PPAP |
| Lactotransferrin | TRFL |
| Primary Hypogonadism | Functional Hypogonadism | |||
|---|---|---|---|---|
| Metabolic Pathway | Before TRT | After TRT | Before TRT | After TRT |
| Glycolysis | decreased | increased | highly decreased | increased |
| Gluconeogenesis | inactive | inactive | increased | decreased |
| Ketone body formation | inactive | increased | highly increased | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, G.; Graziani, A.; De Toni, L.; Garolla, A.; Milardi, D.; Ferlin, A. Acquired Male Hypogonadism in the Post-Genomic Era—A Narrative Review. Life 2023, 13, 1854. https://doi.org/10.3390/life13091854
Grande G, Graziani A, De Toni L, Garolla A, Milardi D, Ferlin A. Acquired Male Hypogonadism in the Post-Genomic Era—A Narrative Review. Life. 2023; 13(9):1854. https://doi.org/10.3390/life13091854
Chicago/Turabian StyleGrande, Giuseppe, Andrea Graziani, Luca De Toni, Andrea Garolla, Domenico Milardi, and Alberto Ferlin. 2023. "Acquired Male Hypogonadism in the Post-Genomic Era—A Narrative Review" Life 13, no. 9: 1854. https://doi.org/10.3390/life13091854





