Abstract
Gut dysbiosis has been associated with many chronic diseases, such as obesity, inflammatory bowel disease, and cancer. Gut dysbiosis triggers these diseases through the activation of the immune system by the endotoxins produced by gut microbiota, which leads to systemic inflammation. In addition to pre-/pro-/postbiotics, many natural products can restore healthy gut microbiota composition. Tocotrienol, which is a subfamily of vitamin E, has been demonstrated to have such effects. This scoping review presents an overview of the effects of tocotrienol on gut microbiota according to the existing scientific literature. A literature search to identify relevant studies was conducted using PubMed, Scopus, and Web of Science. Only original research articles which aligned with the review’s objective were examined. Six relevant studies investigating the effects of tocotrienol on gut microbiota were included. All of the studies used animal models to demonstrate that tocotrienol altered the gut microbiota composition, but none demonstrated the mechanism by which this occurred. The studies induced diseases known to be associated with gut dysbiosis in rats. Tocotrienol partially restored the gut microbiota compositions of the diseased rats so that they resembled those of the healthy rats. Tocotrienol also demonstrated strong anti-inflammatory effects in these animals. In conclusion, tocotrienol could exert anti-inflammatory effects by suppressing inflammation directly or partially by altering the gut microbiota composition, thus achieving its therapeutic effects.
1. Introduction
The human body contains many microorganisms, including bacteria, fungi, viruses, and protozoa, which are known collectively as microbiota [1]. The human gastrointestinal tract houses the largest number of these microorganisms, known as gut microbiota, which exert significant physiological and pathological influences on the host [2]. Bacteria, both commensal and pathogenic, represent the most significant type of gut microbiota [3]. The most dominant phyla of gut bacteria include Bacteroidetes, Firmicutes, Proteobacteria, Fusobacteria, Actinobacteria, and Verrucomicrobia. Notably, Bacteroidetes (gram-negative bacteria) and Firmicutes (gram-positive bacteria) represent 90% of the gut microbiota composition [4,5,6].
The gut microbiota influences the host metabolism through several mechanisms, such as enhancing gut integrity, modifying the intestinal epithelium, defending against pathogens, and modulating the host immune system [7,8]. Previous studies have shown that genetic background, lifestyle habits, health status (e.g., infection and inflammation), the use of xenobiotics (e.g., antibiotics, drugs, and food additives), hygiene, environmental factors, and diet (e.g., high sugar or low fiber) can cause gut dysbiosis [9].
Gut dysbiosis is broadly defined as an imbalance in the gut microbiota that results in decreased numbers and diversity of commensal bacteria and increased pathogenic bacteria [10]. Gut dysbiosis has been associated with many pathological conditions, such as inflammatory bowel disease (IBD), obesity, cardiovascular disease, metabolic syndrome, and cancer [11,12]. Since Firmicutes and Bacteroidetes represent the major phyla in the composition of gut microbiota, the Firmicutes-to-Bacteroidetes ratio (F/B ratio) can be used to determine whether there is gut dysbiosis [13]. An altered F/B ratio has been shown to have significant impacts on human health. For instance, compared with a healthy control group, the F/B ratio has been reported to decrease in patients with IBD, while it increases in patients with obesity [14]. It has been suggested that the F/B ratio influences metabolic potential, energy harvest, and weight regulation [15]. An increased F/B ratio has been associated with obesity [16]. Changes in F/B ratio have also been linked to the development of metabolic disorders, such as type 2 diabetes and non-alcoholic fatty liver disease [17]. Another study reported that the F/B ratio was negatively associated with osteoporosis [18].
Furthermore, gut microbiota generates short-chain fatty acids (SCFAs) such as butyrate by metabolizing starch and dietary fibers through fermentation [19]. SCFAs have important physiological roles because they enhance phagocytosis and chemotaxis, exert anti-inflammatory and anti-microbial effects, and alter gut integrity [20,21]. SCFAs such as butyrate prevent pathogens from entering the blood circulation by increasing the mucus production of the intestinal epithelial cells, the secretion of adenosine monophosphates, and oxygen attainability, and by reducing pro-inflammatory cytokines by inhibiting pro-inflammatory immune cells and activating anti-inflammatory immune cells [22]. SCFAs also enhance the functions of tight junction proteins, which is why tight junction dysfunction occurs during dysbiosis [23]. In gut dysbiosis, the synthesis of SCFAs by commensal bacteria is disrupted, and this loosens the tight junction of the intestinal epithelial barrier [24,25].
Dysbiosis can lead to chronic inflammation due to the increased amounts of intracellular pathogenic bacteria [26]. The pathogenic bacteria in the gut release endotoxins such as lipopolysaccharide (LPS), which activates the intestinal macrophages and causes them to produce pro-inflammatory cytokines (e.g., interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α)) [27]. The ensuing inflammation increases the permeability of the intestine. The increased intestinal permeability causes endotoxins such as bacterial LPS to enter the blood circulation, leading to the production of immunosuppressive proteins, and subsequently causing immune dysfunction and chronic diseases [28,29,30]. A previous study showed that there was an increase in mucus-degrading bacteria in IBD patients, and this might be responsible for the damage to the intestinal epithelial barrier [31].
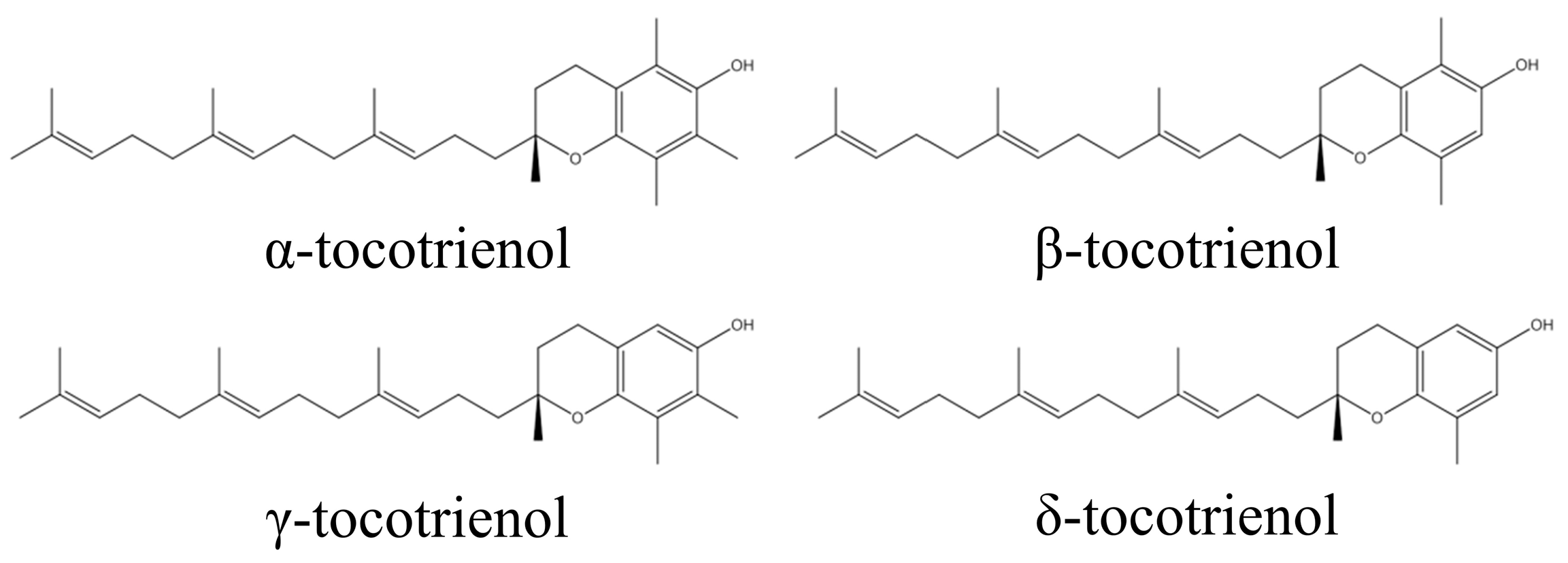
By recognizing that gut microbiota dysbiosis and the inflammation associated with it play a key role in causing pathological conditions such as obesity and IBD, it can be deduced that the use of natural compounds that can partially restore gut microbiota diversity to a balanced state, and which possess anti-inflammatory characteristics, could be useful in treating gut dysbiosis-associated diseases. Tocotrienol, a family of unsaturated forms of vitamin E, have exhibited potent anti-inflammatory activity [32]. Tocotrienol is divided into four specific isomers: α, β, γ, and δ (Figure 1) [33,34]. It can be found in many natural sources, such as palm oil, barley germ, coconut oil, rice bran oil, wheat germ, and annatto, in a variety of compositions [35]. The anti-inflammatory effects of tocotrienol have been well-recognized [36]. A previous study showed that δ-tocotrienol (δTE) supplementation reduced heart and liver inflammation in obese rats [37]. Our group has previously reported the anti-osteoporotic and anti-arthritic properties of tocotrienol [38,39,40,41]. Tocotrienol was also shown to enhance the efficacy of daptomycin against methicillin-resistant Staphylococcus aureus in an infected wound model [42].
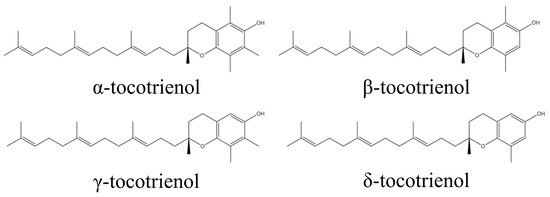
Figure 1.
Molecular structure of tocotrienol.
The anti-inflammatory effects of tocotrienol have been well studied. Tocotrienol suppresses the nuclear factor κB (NF-κB) activation pathway, which is closely associated with inflammation [43]. This leads to the inhibition of the downstream activator protein-1 and the suppression of pro-inflammatory cytokines, such as IL-2a, IL-12, IL-18, IL-6, and TNF-α [44,45]. The expression of cyclo-oxygenase 2 (COX-2), one of the main mediators of inflammation, is also suppressed by tocotrienol [46]. As mentioned above, LPS from gram-negative bacteria stimulates COX-2 expression [47] and activates the NF-κB pathway [48]. Thus, tocotrienol can suppress LPS-induced inflammation. Tocotrienol also exerts anti-inflammatory activity by suppressing the release of nitric oxide (NO) by blocking LPS-stimulated inducible nitric oxide synthase (iNOS) and COX-2 expression without affecting COX-1 [49]. Overall, the anti-inflammatory effects of tocotrienol can be exerted through the inhibition of iNOS, COX-2, and NF-ĸB expression.
Given how gut microbiota dysbiosis plays a vital role in the pathogenesis of various diseases, the influence of tocotrienol on the composition of gut microbiota has gained attention because it may explain the many health beneficial effects of this compound. Tocotrienol is reported to improve gut dysbiosis and the F/B ratio in mice with type 2 diabetes [50]. Therefore, tocotrienol could be a potential treatment for gut dysbiosis. This review aims to summarize the effects of tocotrienol on gut microbiota as reported in the recent literature. We hope that this review will advocate the practical application of tocotrienol in correcting gut dysbiosis and improving health.
2. Methodology
This scoping review was developed using the Arksey and O’Malley (2005) framework [51], and it was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews [52] (Supplementary Table S1). The following steps were implemented: (1) defining the research question; (2) gathering the relevant studies; (3) selecting the studies; (4) extracting the data; (5) reporting the results.
2.1. Defining the Research Question
The research question was as follows: what are the effects of tocotrienol on gut microbiota? Since vitamin E isomers are usually present together as a mixture in natural sources, natural tocotrienol mixtures containing tocopherols were considered in this review. Gut microbiota is a broad term which includes various commensal and pathogenic bacteria. Their diversity was emphasized in this review.
2.2. Identifying Relevant Studies
A literature search was performed via three electronic databases (PubMed, Scopus, and Web of Science) in April 2023 using the following search string: tocotrienol AND (gut OR intestinal OR gastrointestinal) AND (microbiome OR microbiota). All primary studies examining the impact of tocotrienol-rich fraction (TRF) on the gut microbiota were taken into consideration. Articles without relevant results or primary data (e.g., reviews, letters to the editor, perspectives, books, and book chapters) were not considered. Additionally, due to the preliminary nature of the data they present and their potential to duplicate data from full articles, conference abstracts and proceedings were excluded. Articles not written in English were excluded.
2.3. Study Selection
Endnote X9 (Clarivate, London, UK) was used to compile the literature. The search results from the three electronic databases were downloaded. Endnote X9 was used to identify and eliminate duplicate entries, and the list was then reviewed manually. A.K. and S.O.E. reviewed the titles and abstracts of the articles to find pertinent studies. The whole texts of the chosen publications were then acquired and reviewed using the inclusion and exclusion criteria. To identify studies that were overlooked during the search, the reference lists of the included publications were checked. To resolve any disagreements, the opinions of other authors were solicited.
2.4. Extracting the Data
A.K. and S.O.E. extracted the pertinent data, such as the names of the researchers, publication years, study designs (disease models utilized, type of tocotrienol, dosages, treatment period), and key findings from the chosen studies using a standardized table.
2.5. Collating, Summarising, and Reporting the Results
Instead of synthesizing any specific factors, the scoping review method was used to describe the search results due to the heterogeneity of the studies included and the variables of interest that were revealed. The fundamental goal of a scoping review is to give an overview of a field’s advancements. The study designs, amount of tocotrienol (dose and treatment time) used in each study, disease models, and key findings were therefore summarized and are presented below. Research gaps and the role of tocotrienol in managing chronic diseases by preventing gut dysbiosis were explored.
3. Results
3.1. Study Selection
The literature search was carried out using three electronic databases and found 44 items (PubMed = 13; Scopus = 19; and Web of Science = 12). After removing 17 duplicates, 27 items were subjected to title and abstract screening. Twenty-one items were excluded for various reasons (not within the scope = 10; not an original research article = 11). Six items were subjected to full-text screening, and all were included in this review. The article selection process is illustrated in Figure 2.
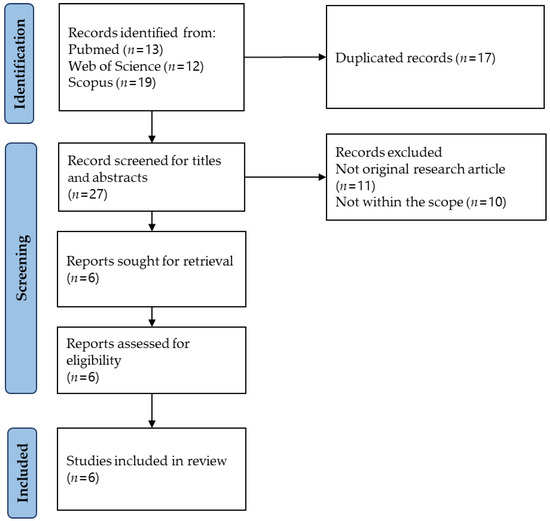
Figure 2.
Article selection process.
3.2. Study Characteristics
In a study using mice with azoxymethane (AOM)/dextran sodium sulphate (DSS)-induced colitis-associated colon cancer, an oral δ/γ-tocotrienol (8/1 ratio) 0.035% (~2.2 μmoles daily) and δ-tocotrienol-13-COOH 0.04% (~2.3 μmoles daily) diet was given for 2 months [53]. Two studies supplemented mice with high-fat diet-induced obesity using 400 or 800 mg/kg annatto tocotrienol for 14 weeks [54,55]. One study induced non-alcoholic fatty liver disease in the mice and supplemented them with a mixture of berberine, tocotrienols, and chlorogenic acid (140 mg/kg diet for 24 weeks) [56]. Another study altered the gut microbiota composition of the male mice using antibiotics and supplemented them with α-, γ-, and δ-tocopherol, as well as γ- and δ-tocotrienol, each at a dose of 75 mg/kg for 17 days [57]. Another study fed severe combined immunodeficient (SCID) mice, which had received human colon cancer cell grafts (HT-29 and HCT-116), with essential turmeric oil–curcumin (ETO–Cur) and tocotrienol-rich fraction (TRF) for 34 days [58].
The diversity of the gut microbiota was analyzed in each study. Firstly, operational taxonomic units (OTUs) were used as a measure of community richness via Quantitative Insights Into Microbial Ecology (QIIME) [56,58] and Quantitative Insights Into Microbial Ecology 2 (QIIME 2) [53,54,55]. Next, a redundancy analysis (RDA) was performed to test whether there were alterations in the gut microbiota compositions of the study groups [54,55]. For further analysis, α-diversity and β-diversity were evaluated. Alpha-diversity assesses the within-group diversity using different metrics, such as Shannon indices [56,58], Faith’s phylogenetic diversity (PD), and Pielou evenness [53,54,55]. Beta-diversity involves the comparison of diversity between groups where a principal coordinate analysis (PCoA) has been performed using unweighted UniFrac, weighted UniFrac, Bray Curtis distance [56,58], and Jaccard matrices [53]. Only one study performed a canonical correspondence analysis (CCA) using PAST3 (PAleontological STatistics) to determine the correlation between the relative abundances (at the species level) of gut microbial communities and the environmental variables, including the treatment given [53].
3.3. Study Outcomes
The essential turmeric oil–curcumin–tocotrienol-rich fraction (ETO–Cur–TRF) increased the OTUs, the β-diversity, and the species number diversity in the SCID mice engrafted with human colon cancer cells. This increased microbial diversity indicated the successful prevention of gut dysbiosis. At the phylum level, both the control and ETO–Cur–TRF groups showed increased Bacteroides and Firmicutes, while an increase in Proteobacteria and Actinobacteria could only be observed in the ETO–Cur–TRF group. The gut microbial composition showed that the Porphymonadaceae, Rickenellaceae, Lactobacillaeceae, Desulphovibrionaceae, Enterobacteriaceae, and Bifidobacteriaceae families had increased in number, but that the Bacteroidaceae family had decreased in number in the ETO–Cur–TRF group compared with the negative control group. Clostridium XIVa, Lactobacillus, and Aliistipes also increased in number in the ETO–Cur–TRF group. To determine whether the gut microbial changes were reflected in the tumors, DNA was extracted from the tumors of the SCID mice, and quantitative polymerase chain reaction (qPCR) was performed. The results showed increased amounts of Bifidobacteria, Lactobacillus, and Clostridium IV in the ETO–Cur–TRF group. Tumor growth inhibition was more effective in the ETO–Cur–TRF group than in other groups, which received individual supplements [58].
The mice with colitis-associated colon cancer treated with a δ/γ-tocotrienol (8/1 ratio) 0.035% (~2.2 μM) or δ-tocotrienol-COOH 0.04% (~2.3 μM) diet showed decreases in their F/B ratios [53]. This change was associated with decreased tumor size (for both the δ-tocotrienol-COOH- and the δ/γ-tocotrienol-supplemented groups) and tumor formation rate (for the tocotrienol-COOH-supplemented group) [53]. The 800 mg/kg annatto tocotrienol diet significantly increased the F/B ratios of the high-fat diet-fed mice [55]. Concurrently, the mice demonstrated a decrease in fat pad weight, circulating glucose, and adipokine and IL-6 levels [55]. Another study, in which obese mice were treated with annatto tocotrienol (400 mg/kg diet) and green tea polyphenols (GTP, 0.5% w/v in drinking water), only found a decrease in the Firmicutes phyla in the mice treated with green tea polyphenols and those which received the combined treatment [54]. The same study found an improvement in skeletal parameters and a reduction in white adipose tissue in the groups which received annatto tocotrienol and GTP, both individually and in combination. However, the skeletal effects were mostly a result of the GTP [54]. The F/B ratio was partially restored in a non-alcoholic fatty liver disease mouse model which was given a plant extract consisting of 5.27 mg of tocotrienols along with 87.84 mg of berberine and 5.28 mg of chlorogenic acid as part of a 140 mg/kg diet [56]. The plant extract reduced their fasting blood glucose and prevented hyperinsulinemia, though it did not prevent liver steatosis [56]. However, since a mixture of compounds was used in this study, the effects of tocotrienol alone could not be determined.
Microbiota richness, represented by α-diversity, was not significantly altered in any of the studies after supplementation with tocotrienol alone or mixtures containing tocotrienol [53,54,55,56,58]. The studies that used tocotrienol alone were scrutinized to determine the specific bacterial population modified by tocotrienol. In one study, annatto tocotrienol reduced the amounts of Firmicutes as well as the amounts of bacteria in the Ruminococcus lactaris, Dorea longicatena, and Lachnospiraceae families in mice fed with a high-fat diet [55]. Delta-tocotrienol has been shown to increase the amounts of the Streptococccaceae, Bacteroides, and Lactococcus bacteria, as well as the amount of Parabacteroides goldsteinii CL02T12C30 in the mice with colon cancer who received the supplement compared with those that did not. A significant increase in Eubacterium coprostanoligenes and a decrease in the Clostridiales vadinBB60 group was observed in the mice who received the δ-tocotrienol supplement, but not in the mice who received the δ-T3-13-COOH supplement (a metabolite of δ-tocotrienol). It is still unclear how δ-T3-13-COOH and δ-tocotrienol interact with gut microbiota, so there is no valid explanation for this discrepancy. Furthermore, additional studies are required to determine whether these compounds can modulate gut microbiota in a non-disease model [53].
On the other hand, another study reported that the administration of antibiotics depleted the gut commensal bacteria, but increased the bioavailability of δ- and γ-tocotrienol by 150 and 157%, respectively. This was further evidenced by the lack of tocotrienol or tocopherol in the fecal and urine samples [57]. These observations show that the presence of tocotrienol-metabolizing gut microbiota reduces the bioavailability of tocotrienol.
A summary of the effects of tocotrienol on gut microbiota is presented in Table 1.

Table 1.
Effects of tocotrienol on gut microbiota.
4. Discussion
Both commensal and pathogenic bacteria reside in our gut. The commensal bacteria regulate the mucosal immune system while the pathogenic bacteria cause immune dysfunction [59]. The disturbance of the composition of the gut microbiota can lead to disease. For instance, apart from the well-recognized F/B ratio, an increase in the abundance of Proteobacteria and facultative bacteria of the Enterobacteriaceae family is associated with dysbiosis as their numbers are minimal in healthy humans [60]. Commensal bacteria, which come, for example, from the Firmicutes phylum, maintain the intestinal epithelial barrier by producing SCFAs, such as butyrate [61]. In gut dysbiosis, SCFA-producing bacteria are depleted [62] and mucus-degrading bacteria increase [31], thus increasing intestinal permeability due to intestinal epithelial barrier dysfunction [63]. The dysfunction provides an opportunity for LPS from pathogenic bacteria to enter the blood circulation [64]. The LPS then triggers the immune response by interacting with CD4 membrane receptors to generate proinflammatory cytokines such as IL-6, IL-1, and TNF-α [65]. According to a previous study, LPS-induced inflammation is closely related to type 2 diabetes, cardiovascular diseases, and non-alcoholic steatohepatitis [66]. Tocotrienol could counteract dysbiosis and its associated immune dysregulation. Tocotrienol can decrease the F/B ratio in high-fat diet mice [55] and in colitis-associated colon cancer mice [53]. In another study, tocotrienol with essential turmeric oil increased the beta diversity and the species number diversity in SCID mice. This increase in microbiota diversity is further supported by a significant increase in the phyla of Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria [58]. Additionally, tocotrienol increases the abundance of Firmicutes and Bacteroidetes phyla in mice with type 2 diabetes [50].
The alteration of gut microbiota has been associated with many chronic diseases. Recent studies have shown that the F/B ratio increases in overweight or obese patients [67] and decreases in IBD patients [68]. In the current review, gut dysbiosis has been established in several mouse disease models, such as colitis-associated colon cancer [53], obesity [54,55], and non-alcoholic fatty liver disease [56]. Tocotrienol has been shown to prevent or reverse these diseases by correcting the associated gut dysbiosis. The included studies show that parts of tocotrienol’s mechanisms of action can be attributed to its effects on gut microbiota. In mice with AOM/DSS-induced colon cancer, oral supplementation with a δ/γ-tocotrienol 0.035% (~2.2 μmoles daily) and δ-tocotrienol-13-COOH 0.04% (~2.3 μmoles daily) diet led to a decrease in pro-inflammatory cytokines, such as GM-CSF and MCP-1, in the δ-tocotrienol-13-COOH-treated group, and a decrease in IL-1β in the δ-T3-treated group compared with the negative control group [53]. It should be noted that tocotrienol is an anti-inflammatory agent by itself [32]. Unless the gut microbiota is suppressed, it is difficult to determine whether the suppression of inflammation is due to the direct action of tocotrienol or whether it occurs indirectly through the gut microbiota.
The relationship between gut microbiota and tocotrienol is two-way. Although tocotrienol can alter the composition of gut microbiota, gut microbiota also metabolizes tocotrienol. A study has demonstrated that the presence of gut microbiota decreases the bioavailability of tocotrienol because tocotrienol is partially metabolized by the gut microbiota [57]. Since gut microbiota also degrade the side-chain of vitamin E, which subsequently decreases its bioavailability, it would be useful to discover ways of enhancing the bioavailability of tocotrienol without affecting the balance of the gut microbiota. Tocotrienol suffers from low bioavailability because the presence of α-tocopherol transfer protein in the liver facilitates the transport of α-tocopherol (rather than tocotrienol) into the circulatory system [69,70]. Currently, emulsification is a popular method of increasing the bioavailability of tocotrienol, although some components, such as synthetic surfactants, may cause gut irritation [71]. Further studies on the bioavailability of vitamin E in the intestines will be needed to study the effects of intestinal microbiota on vitamin E [57].
The anti-inflammatory actions of tocotrienol have been well-recognized [36]. Our previous study showed that annatto tocotrienol (60 and 100 mg/kg/day for 12 weeks) reduced circulating IL-1α and IL-6 levels in male rats fed with a high-fat, high-carbohydrate diet [72]. It has also been shown to reduce liver inflammation by suppressing toll-like receptor activation and increasing the expression of IL-10 (an anti-inflammatory cytokine) in the same groups of rats [73]. However, it is difficult to determine whether the anti-inflammatory effects of tocotrienol observed in these studies are mediated by alterations in the composition of gut microbiota. This is because tocotrienol has direct immuno-modulatory effects. Recent studies have shown that α-tocotrienol is involved in Th17 differentiation in vitro and in vivo through the IL-6/Janus kinase/STAT3 pathway [74]. On the other hand, γ-tocotrienol has been shown to raise circulating CD4+/CD8+ T-cells and natural killer cells, but to suppress regulatory T-cells in mice with mammary cancer. CD4+ T-cells were found to have infiltrated the tumors of γ-tocotrienol-fed mice [75]. These studies have shown that the immuno-modulatory effects of γ-tocotrienol could be dependent on disease status.
The mechanisms by which tocotrienol acts on microorganisms are not clearly understood. The mevalonate pathway, which is governed by the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), is critical in synthesizing isopentenyl diphosphate for peptidoglycan cell walls, as well as ubiquinones and menaquinones for the electron transport chain, especially in G+C gram-positive bacteria [76]. This pathway may potentially be affected by tocotrienol since studies have shown that tocotrienol suppresses mammalian HMGR expression at the post-translational level [77]. However, its efficacy in suppressing bacterial HMGR is yet to be validated. Efflux pumps form an important bacterial multidrug resistance mechanism [78]. Alpha-tocopherol, the more common form of vitamin E, has been shown to inhibit the efflux pumps of methicillin-resistant Staphylococcus aureus [79]. Currently, there is no direct evidence showing that tocotrienol can inhibit bacterial efflux pumps, but it has been shown to suppress the P-gp protein and mdr1 mRNA in breast cancer cells [80]. Overall, the microbial modulating mechanism of tocotrienol remains speculative at the moment and warrants further investigation.
Furthermore, the disease models that have been used to study the effect of tocotrienol on gut microbiota are very limited. Similar studies should be undertaken, but with other disease models. Gut microbiota plays a significant role in diseases such as type 2 diabetes, osteoporosis, and cardiovascular diseases [81,82,83], wherein tocotrienol has demonstrated protective effects. It will be interesting to see whether the protective effects of tocotrienol against these diseases are modulated by gut microbiota. Although rodent models are commonly used in gut microbiota research because they share some physiological and anatomical characteristics with humans (particularly the gastrointestinal system), some variations between the two organisms have been observed. For example, humans have a small caecum in which no fermentation occurs, while mice have a large caecum in which prominent fermentation activities occur [84]. These differences should be considered during experimental design and interpretation. The phylogenetic makeup of the bacteria in both humans and rodents is similar: the two main phyla found in the intestinal tract are Firmicutes and Bacteroidetes [85]. Previous studies have also found that rodents and humans share around 80 microbial genera. However, there are variations in genera; some, for instance, have been found in humans, but not in rodents [86]. Therefore, a clinical trial would be necessary to ensure the translatability of rodent data to humans. A search was performed in the ClinicalTrials.gov registry using the search string specified in Section 2.2 in May 2023. Two clinical trials investigating the effects of annatto tocotrienol on gut microbiota in postmenopausal women with obesity (identifier: NCT03705845; status: recruiting) and sarcopenia (identifier: NCT03708354; status: withdrawn due to difficulties in recruiting) were found. Two additional studies investigating the effects of tocotrienol and carotene-rich red palm oil on inflammation and gut health in adults (identifier: NCT05791370; status: completed, but report not found) and the effects of red palm oil-containing biscuits on gut microbiota in children with vitamin A deficiency (identifier: NCT03256123; status: unknown, and report not found) were also found. The publication of the findings from these studies will help us to understand the effects of tocotrienol on human gut microbiota in the future.
In this review, the literature search was performed using three electronic databases. Since unpublished materials and grey literature were not searched, potential studies and, in particular, studies which produced negative results, could have been missed. A limited number of studies was retrieved from the search despite the use of a broad search string, indicating that more intensive research on this topic is necessary.
5. Conclusions
In conclusion, preclinical studies have shown that tocotrienol can restore gut microbiota diversity in various disease models. Gut dysbiosis plays a vital role in the pathogenesis of many diseases, and tocotrienol has been shown to alter the abundance of certain bacterial species depending on the disease model. However, the mechanism by which tocotrienol modulates gut microbiota remains elusive. The improvements in health status observed in the various disease models summarized in this review could be due to the restoration of gut microbiota composition, and the suppression of inflammation could have resulted from the correction of gut dysbiosis or the direct action of tocotrienol. Due to the limited evidence from the clinical trials, it cannot be confirmed whether tocotrienol-induced gut microbial diversity changes can be achieved in patients with disease associated with gut dysbiosis. More research should be carried out to validate whether tocotrienol can be used to manage gut dysbiosis, which is responsible for the pathogenesis of many diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13091882/s1, Table S1: PRISMA checklist for scoping reviews.
Author Contributions
Conceptualization, K.-Y.C. and A.K.; methodology, K.-Y.C., A.K. and S.O.E.; validation, N.M. (Norazlina Mohamed), N.M. (Norliza Muhammad), S.K.W., A.H., K.-L.P. and D.C.H.C.; formal analysis, K.-Y.C., A.K. and S.O.E.; investigation, K.-Y.C., A.K. and S.O.E.; resources, K.-Y.C.; data curation, A.K.; writing—original draft preparation, A.K. and K.-Y.C.; writing—review and editing, N.M. (Norazlina Mohamed), N.M. (Norliza Muhammad), S.K.W., A.H., K.-L.P. and D.C.H.C.; visualization, A.K.; supervision, K.-Y.C.; project administration, K.-Y.C.; funding acquisition, K.-Y.C., N.M. (Norazlina Mohamed), N.M. (Norliza Muhammad), S.K.W., A.H., K.-L.P. and D.C.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Higher Education, Malaysia through the Fundamental Research Grant Scheme (FRGS/1/2022/SKK10/UKM/02/3).
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
No applicable.
Acknowledgments
We thank Universiti Kebangsaan Malaysia and the Ministry of Higher Education, Malaysia for supporting the researchers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yao, Y.; Cai, X.; Fei, W.; Ren, F.; Wang, F.; Luan, X.; Chen, F.; Zheng, C. Regulating Gut Microbiome: Therapeutic Strategy for Rheumatoid Arthritis During Pregnancy and Lactation. Front. Pharmacol. 2020, 11, 594042. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.M.; Lampe, J.W.; Gibson, G. Targeted Approaches for In Situ Gut Microbiome Manipulation. J. Parenter. Enter. Nutr. 2020, 44, 581–588. [Google Scholar] [CrossRef]
- Seong, C.N.; Kang, J.W.; Lee, J.H.; Seo, S.Y.; Woo, J.J.; Park, C.; Bae, K.S.; Kim, M.S. Taxonomic hierarchy of the phylum Firmicutes and novel Firmicutes species originated from various environments in Korea. J. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Baumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Siranosian, B.A.; Brooks, E.F.; Andermann, T.; Rezvani, A.R.; Banaei, N.; Tang, H.; Bhatt, A.S. Rare transmission of commensal and pathogenic bacteria in the gut microbiome of hospitalized adults. Nat. Commun. 2022, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Strukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Montenegro, J.; Armet, A.M.; Willing, B.P.; Deehan, E.C.; Fassini, P.G.; Mota, J.F.; Walter, J.; Prado, C.M. Exploring the Influence of Gut Microbiome on Energy Metabolism in Humans. Adv. Nutr. 2023, 14, 840–857. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Caudet, J.; Trelis, M.; Cifre, S.; Soriano, J.M.; Rico, H.; Merino-Torres, J.F. Interplay between Intestinal Bacterial Communities and Unicellular Parasites in a Morbidly Obese Population: A Neglected Trinomial. Nutrients 2022, 14, 3211. [Google Scholar] [CrossRef]
- Huang, R.; Liu, P.; Bai, Y.; Huang, J.; Pan, R.; Li, H.; Su, Y.; Zhou, Q.; Ma, R.; Zong, S.; et al. Changes in the gut microbiota of osteoporosis patients based on 16S rRNA gene sequencing: A systematic review and meta-analysis. J. Zhejiang Univ. Sci. B 2022, 23, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef] [PubMed]
- Saleri, R.; Borghetti, P.; Ravanetti, F.; Cavalli, V.; Ferrari, L.; De Angelis, E.; Andrani, M.; Martelli, P. Effects of different short-chain fatty acids (SCFA) on gene expression of proteins involved in barrier function in IPEC-J2. Porc. Health Manag. 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Perez-Reytor, D.; Puebla, C.; Karahanian, E.; Garcia, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.D.; Wang, Y.D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Belancic, A. Gut microbiome dysbiosis and endotoxemia—Additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes. Med. 2020, 20, 100302. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef]
- Malavolta, M.; Pierpaoli, E.; Giacconi, R.; Basso, A.; Cardelli, M.; Piacenza, F.; Provinciali, M. Anti-inflammatory Activity of Tocotrienols in Age-related Pathologies: A SASPected Involvement of Cellular Senescence. Biol. Proced. Online 2018, 20, 22. [Google Scholar] [CrossRef]
- Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Fahami, N.A.M.; Mohamed, I.N.; Shuid, A.N.; Saad, Q.M.; Abdullah, A.; et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients 2020, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L.; Soelaiman, I.N. Tocotrienol and Its Role in Chronic Diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Springer: Berlin/Heidelberg, Germany, 2016; Volume 928, pp. 97–130. [Google Scholar]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef]
- Khor, B.-H.; Tiong, H.-C.; Tan, S.C.; Wong, S.K.; Chin, K.-Y.; Karupaiah, T.; Ima-Nirwana, S.; Abdul Gafor, A.H. Effects of tocotrienols supplementation on markers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2021, 16, e0255205. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Ward, L.C.; Fong, C.W.; Yap, W.N.; Brown, L. Anti-inflammatory gamma- and delta-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur. J. Nutr. 2017, 56, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Self-emulsified annatto tocotrienol improves bone histomorphometric parameters in a rat model of oestrogen deficiency through suppression of skeletal sclerostin level and RANKL/OPG ratio. Int. J. Med. Sci. 2021, 18, 3665–3673. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharmacother. 2021, 137, 111368. [Google Scholar] [CrossRef]
- Shen, C.L.; Klein, A.; Chin, K.Y.; Mo, H.; Tsai, P.; Yang, R.S.; Chyu, M.C.; Ima-Nirwana, S. Tocotrienols for bone health: A translational approach. Ann. N. Y. Acad. Sci. 2017, 1401, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Tejpal Singh, H.S.; Aminuddin, A.A.; Pang, K.-L.; Ekeuku, S.O.; Chin, K.-Y. The Role of Tocotrienol in Arthritis Management—A Scoping Review of Literature. Pharmaceuticals 2023, 16, 385. [Google Scholar]
- Pierpaoli, E.; Orlando, F.; Cirioni, O.; Simonetti, O.; Giacometti, A.; Provinciali, M. Supplementation with tocotrienols from Bixa orellana improves the in vivo efficacy of daptomycin against methicillin-resistant Staphylococcus aureus in a mouse model of infected wound. Phytomedicine 2017, 36, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. γ-Tocotrienol Inhibits Nuclear Factor-κB Signaling Pathway through Inhibition of Receptor-interacting Protein and TAK1 Leading to Suppression of Antiapoptotic Gene Products and Potentiation of Apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, T.; Xu, Y.; Luo, Y.; Zhong, X.; Shi, L.; Hu, T.; Guo, T.; Nie, Y.; Luo, F.; et al. δ-Tocotrienol, Isolated from Rice Bran, Exerts an Anti-Inflammatory Effect via MAPKs and PPARs Signaling Pathways in Lipopolysaccharide-Stimulated Macrophages. Int. J. Mol. Sci. 2018, 19, 3022. [Google Scholar] [CrossRef]
- Qureshi, A.A. Tocotrienols: Exciting Biological and Pharmacological Properties of Tocotrienols and other Naturally Occurring Compounds, Part I. Ann. Clin. Case Rep. 2022, 7, 2194. [Google Scholar] [PubMed]
- Kuhad, A.; Chopra, K. Attenuation of diabetic nephropathy by tocotrienol: Involvement of NFkB signaling pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef]
- Feng, L.; Xia, Y.; Garcia, G.E.; Hwang, D.; Wilson, C.B. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J. Clin. Investig. 1995, 95, 1669–1675. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Liu, P.L.; Ng, L.T. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol. Nutr. Food Res. 2008, 52, 921–929. [Google Scholar] [CrossRef]
- Elmassry, M.; Chung, E.; Hamood, A.; Shen, C.L. Supplementation of Geranylgeraniol and Tocotrienols to High-Fat Diet Shifts the Gut Microbiome Composition and Function in Type 2 Diabetic Mice. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), 393. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Y.; Im, S.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Vitamin E delta-tocotrienol and metabolite 13′-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J. Nutr. Biochem. 2021, 89, 108567. [Google Scholar] [CrossRef] [PubMed]
- Elmassry, M.M.; Chung, E.; Cao, J.J.; Hamood, A.N.; Shen, C.L. Osteoprotective effect of green tea polyphenols and annatto-extracted tocotrienol in obese mice is associated with enhanced microbiome vitamin K(2) biosynthetic pathways. J. Nutr. Biochem. 2020, 86, 108492. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Elmassry, M.M.; Kottapalli, P.; Kottapalli, K.R.; Kaur, G.; Dufour, J.M.; Wright, K.; Ramalingam, L.; Moustaid-Moussa, N.; Wang, R.; et al. Metabolic benefits of annatto-extracted tocotrienol on glucose homeostasis, inflammation, and gut microbiome. Nutr. Res. 2020, 77, 97–107. [Google Scholar] [CrossRef]
- Cossiga, V.; Lembo, V.; Nigro, C.; Mirra, P.; Miele, C.; D’Argenio, V.; Leone, A.; Mazzone, G.; Veneruso, I.; Guido, M.; et al. The Combination of Berberine, Tocotrienols and Coffee Extracts Improves Metabolic Profile and Liver Steatosis by the Modulation of Gut Microbiota and Hepatic miR-122 and miR-34a Expression in Mice. Nutrients 2021, 13, 1281. [Google Scholar] [CrossRef]
- Ran, L.; Liu, A.B.; Lee, M.J.; Xie, P.; Lin, Y.; Yang, C.S. Effects of antibiotics on degradation and bioavailability of different vitamin E forms in mice. Biofactors 2019, 45, 450–462. [Google Scholar] [CrossRef]
- Farhana, L.; Sarkar, S.; Nangia-Makker, P.; Yu, Y.; Khosla, P.; Levi, E.; Azmi, A.; Majumdar, A.P.N. Natural agents inhibit colon cancer cell proliferation and alter microbial diversity in mice. PLoS ONE 2020, 15, e0229823. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef]
- Tan, C.; Wu, Q.; Wang, H.; Gao, X.; Xu, R.; Cui, Z.; Zhu, J.; Zeng, X.; Zhou, H.; He, Y.; et al. Dysbiosis of Gut Microbiota and Short-Chain Fatty Acids in Acute Ischemic Stroke and the Subsequent Risk for Poor Functional Outcomes. J. Parenter. Enter. Nutr. 2021, 45, 518–529. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Tan, Y.; Yu, D.; Qiu, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity. Front. Nutr. 2022, 9, 886902. [Google Scholar] [CrossRef]
- Ikeda, T.; Kamohara, H.; Suda, S.; Nagura, T.; Tomino, M.; Sugi, M.; Wajima, Z. Comparative Evaluation of Endotoxin Activity Level and Various Biomarkers for Infection and Outcome of ICU-Admitted Patients. Biomedicines 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997, 185, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Serban, D.E. Microbiota in Inflammatory Bowel Disease Pathogenesis and Therapy: Is It All About Diet? Nutr. Clin. Pract. 2015, 30, 760–779. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Gornicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Y.; Tey, B.T.; Chan, E.S.; Lai, O.M.; Chang, H.W.; Tan, T.B.; Liu, Y.; Wang, Y.; Tan, C.P. Stabilization and Release of Palm Tocotrienol Emulsion Fabricated Using pH-Sensitive Calcium Carbonate. Foods 2021, 10, 358. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ahmad, F.; Ima-Nirwana, S. Regulation of inflammatory response and oxidative stress by tocotrienol in a rat model of non-alcoholic fatty liver disease. J. Funct. Foods 2020, 74, 104209. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, X.; Lei, Y.; Xia, W.; Cai, F.; Zhu, D.; An, Y.; Xi, Y.; Niu, X.; Wang, Z.; et al. gamma-Tocotrienol inhibits T helper 17 cell differentiation via the IL-6/JAK/STAT3 signaling pathway. Mol. Immunol. 2022, 151, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Anandha Rao, J.S.; Ramdas, P.; Ng, M.H.; Kannan Kutty, M.; Selvaduray, K.R.; Radhakrishnan, A.K. Reduced infiltration of regulatory T cells in tumours from mice fed daily with gamma-tocotrienol supplementation. Clin. Exp. Immunol. 2021, 206, 161–172. [Google Scholar] [CrossRef]
- Bose, S.; Steussy, C.N.; López-Pérez, D.; Schmidt, T.; Kulathunga, S.C.; Seleem, M.N.; Lipton, M.; Mesecar, A.D.; Rodwell, V.W.; Stauffacher, C.V. Targeting Enterococcus faecalis HMG-CoA reductase with a non-statin inhibitor. Commun. Biol. 2023, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Pearce, B.C.; Clark, R.W.; Gordon, D.A.; Wright, J.J. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 1993, 268, 11230–11238. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Pereira, R.L.; Costa Mdo, S.; Braga, M.F.; Limaverde, P.W.; Andrade, J.C.; Siqueira-Junior, J.P.; Coutinho, H.D.; et al. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J. 2016, 15, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fan, J.; Fan, Z.; Zhang, K. γ-Tocotrienol reverses multidrug resistance of breast cancer cells through the regulation of the γ-Tocotrienol-NF-κB-P-gp axis. J. Steroid Biochem. Mol. Biol. 2021, 209, 105835. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Hua, F.; Ding, W. Gut Microbiome and Osteoporosis. Aging Dis. 2020, 11, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Models Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).