Understanding the Male Perspective: Evaluating Quality of Life and Psychological Distress in Serbian Men Undergoing Infertility Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethics Statement
2.3. Data Collection
2.3.1. General and Medical History Data
2.3.2. Anthropometric Assessment
2.3.3. Physical Activity Evaluation
2.3.4. Evaluation of Psycho-Emotional Disturbance
2.3.5. Health-Related Quality of Life Assessment
2.4. Semen Examination
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Infertility Prevalence Estimates: 1990–2021. 2023. Available online: https://apps.who.int/iris/bitstream/handle/10665/366700/9789240068315-eng.pdf (accessed on 1 May 2023).
- Mburu, G.; Kamuyango, A.; Kidula, N.; Kabra, R.; Thatte, N.; Kiarie, J.; Allotey, P. Fulfilment of fertility desires for the attainment of Global Sustainable Development Goals. BMJ Glob. Health 2023, 8, e012322. [Google Scholar] [CrossRef]
- Lotti, F.; Maggi, M. Sexual dysfunction and male infertility. Nat. Rev. Urol. 2018, 15, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Male infertility due to testicular disorders. J. Clin. Endocrinol. Metab. 2021, 106, e442. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Kothandaraman, N.; Agarwal, A.; Abu-Elmagd, M.; Al-Qahtani, M.H. Pathogenic landscape of idiopathic male infertility: New insight towards its regulatory networks. NPJ Genom. Med. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Khatun, A.; Rahman, M.S.; Pang, M.G. Clinical assessment of the male fertility. Obstet. Gynecol. Sci. 2018, 61, 179–191. [Google Scholar] [CrossRef]
- Patel, A.S.; Leong, J.Y.; Ramasamy, R. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: A systematic review. Arab J. Urol. 2018, 16, 96–102. [Google Scholar] [CrossRef]
- Choy, J.T.; Eisenberg, M.L. Male infertility as a window to health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef]
- Jensen, T.K.; Jacobsen, R.; Christensen, K.; Nielsen, N.C.; Bostofte, E. Good semen quality and life expectancy: A cohort study of 43,277 men. Am. J. Epidemiol. 2009, 170, 559–565. [Google Scholar] [CrossRef]
- Gannon, K.; Glover, L.; Abel, P. Masculinity, infertility, stigma and media reports. Soc. Sci. Med. 2004, 59, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Sylvest, R.; Fürbringer, J.K.; Pinborg, A.; Koert, E.; Bogstad, J.; Loessl, K.; Praetorius, L.; Schmidt, L. Low semen quality and experiences of masculinity and family building. Acta Obstet. Gynecol. Scand. 2018, 97, 727–733. [Google Scholar] [CrossRef]
- Fledderjohann, J.; Roberts, C. Missing men, missing infertility: The enactment of sex/gender in surveys in low-and middle-income countries. Popul. Horiz. 2018, 15, 66–87. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shi, L.; Wang, R.; Cui, L.; Wang, J.; Tang, M.; Qian, H.; Wei, M.; Wang, L.; Zhou, H.; et al. Global Research Trends on Infertility and Psychology From the Past Two Decades: A Bibliometric and Visualized Study. Front. Endocrinol. 2022, 13, 889845. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Guadagnoli, E.; Landrum, M.B.; Silliman, R.A.; Wolf, R.; Weeks, J.C. The treating physician as active gatekeeper in the recruitment of research subjects. Med. Care 2001, 39, 1339–1344. [Google Scholar] [CrossRef]
- Singh, S.; Wassenaar, D. Contextualising the role of the gatekeeper in social science research. S. Afr. J. Bioeth. Law 2016, 9, 42. [Google Scholar] [CrossRef]
- Jungwirth, A.; Diemer, T.; Kopa, Z.; Krausz, C.; Tournay, H. Eau Guidelines on Male Infertility; European Association of Urology, EAU Guidelines Office: Arnhem, The Netherlands, 2017. [Google Scholar]
- UNESCO Institute for Statistics. International Standard Classification of Education ISCED 2011; UNESCO Institute for Statistics: Montreal, QC, Canada, 2012; ISBN 978-92-9189-123-8. [Google Scholar]
- International Labour Office. International Standard Classification of Occupations 08 (ISCO-08); International Labour Office: Geneva, Switzerland, 2012. [Google Scholar]
- Abdoli, S.; Zahra Masoumi, S.; Kazemi, F. Environmental and occupational factors and higher risk of couple infertility: A systematic review study. East Fertil. Soc. J. 2022, 27, 33. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and Managing the Global Epidemic. In Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Healey, E.L.; Allen, K.D.; Bennell, K.; Bowden, J.L.; Quicke, J.G.; Smith, R. Self-Report Measures of Physical Activity. Arthritis Care Res. 2020, 72, 717–730. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Scholes, S.; Bridges, S.; Fat, L.N.; Mindell, J.S. Comparison of the Physical Activity and Sedentary Behaviour Assessment Questionnaire and the Short-Form International Physical Activity Questionnaire: An Analysis of Health Survey for England Data. PLoS ONE 2016, 11, e0151647. [Google Scholar] [CrossRef]
- Lovibond, P.; Lovibond, S. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.R.; Hope, D.A. A review of the tripartite model for understanding the link between anxiety and depression in youth. Clin. Psychol. Rev. 2008, 28, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.R.; Henry, J.D. The Depression Anxiety Stress Scales (DASS): Normative data and latent structure in a large non-clinical sample. Br. J. Clin. Psychol. 2003, 42, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.J.; Gandek, B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.W.; Lin, K.; Li, C.Y.; Yuan, C.W. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- JASP Team. JASP Version 0.17.2. 2023. Available online: https://jasp-stats.org/ (accessed on 1 May 2023).
- Kelter, R. Bayesian alternatives to null hypothesis significance testing in biomedical research: A non-technical introduction to Bayesian inference with JASP. BMC Med. Res. Methodol. 2020, 20, 142. [Google Scholar] [CrossRef]
- van Doorn, J.; van den Bergh, D.; Böhm, U.; Dablander, F.; Derks, K.; Draws, T.; Etz, A.; Evans, N.J.; Gronau, Q.F.; Haaf, J.M.; et al. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychon. Bull. Rev. 2021, 28, 813–826. [Google Scholar] [CrossRef]
- Warchol-Biedermann, K. The Etiology of Infertility Affects Fertility Quality of Life of Males Undergoing Fertility Workup and Treatment. Am. J. Mens. Health 2021, 15, 1557988320982167. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Su, P.; Liu, J.; Shi, K.; Hao, Z.; Zhou, J.; Liang, C. Relationship between sexual dysfunction and psychological burden in men with infertility: A large observational study in China. J. Sex. Med. 2013, 10, 1935–1942. [Google Scholar] [CrossRef]
- Bold, J.; Swinburne, D. Pre-Conceptual Guidelines for Men: A Review of Male Infertility Experience, including Nutrition and Lifestyle Factors. Dietetics 2022, 1, 164–181. [Google Scholar] [CrossRef]
- Arya, S.T.; Dibb, B. The experience of infertility treatment: The male perspective. Hum. Fertil. 2016, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C. Novel concepts in male factor infertility: Clinical and laboratory perspectives. J. Assist. Reprod. Genet. 2016, 33, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, R.; Vaiarelli, A.; Dovere, L.; Cimadomo, D.; Ubaldi, N.; Ferrero, S.; Rienzi, L.; Lombardo, F.; Lenzi, A.; Tournaye, H.; et al. Severe male factor in in vitro fertilization: Definition, prevalence, and treatment. An update. Asian J. Androl. 2022, 24, 125. [Google Scholar]
- Punjani, N.; Kang, C.; Lamb, D.J.; Schlegel, P.N. Current updates and future perspectives in the evaluation of azoospermia: A systematic review. Arab J. Urol. 2021, 19, 206. [Google Scholar] [CrossRef]
- Jarow, J.P.; Espeland, M.A.; Lipshultz, L.I. Evaluation of the azoospermic patient. J. Urol. 1989, 142, 62–65. [Google Scholar] [CrossRef]
- Cocuzza, M.; Alvarenga, C.; Pagani, R. The epidemiology and etiology of azoospermia. Clinics 2013, 68, 15–26. [Google Scholar] [CrossRef]
- Topuz, B.; Ebiloğlu, T.; Sarıkaya, S.; Coğuplugil, A.E.; Bedir, S.; Karataş, Ö.F. Evaluation of depression, anxiety and quality of life in patients with non-obstructive azoospermia. Rev. Int. Androl. 2021, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Age, the environment and our reproductive future: Bonking baby boomers and the future of sex. Reproduction 2013, 147, S1–S11. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Curi, S.M.; Ariagno, J.I.; Chenlo, P.H.; Mendeluk, G.R.; Pugliese, M.N.; Sardi Segovia, L.M.; Repetto, H.E.H.; Blanco, A.M. Asthenozoospermia: Analysis of a large population. Arch. Androl. 2003, 49, 343–349. [Google Scholar] [CrossRef]
- Shahrokhi, S.Z.; Salehi, P.; Alyasin, A.; Taghiyar, S.; Deemeh, M.R. Asthenozoospermia: Cellular and molecular contributing factors and treatment strategies. Andrologia 2020, 52, e13463. [Google Scholar] [CrossRef]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef]
- Chaudhuri, G.R.; Das, A.; Kesh, S.B.; Bhattacharya, K.; Dutta, S.; Sengupta, P.; Syamal, A.K. Obesity and male infertility: Multifaceted reproductive disruption. Middle East Fertil. Soc. J. 2022, 27, 8. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Cabler, S.; McAlister, D.A.; Sabanegh, E.; Agarwal, A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 2010, 7, 153–161. [Google Scholar] [CrossRef]
- Keszthelyi, M.; Gyarmathy, V.A.; Kaposi, A.; Kopa, Z. The potential role of central obesity in male infertility: Body mass index versus waist to hip ratio as they relate to selected semen parameters. BMC Public Health 2020, 20, 307. [Google Scholar] [CrossRef]
- Mykhailivna Kozopas, N.; Ihorivna Chornenka, O.; Zinoviyovych Vorobets, M.; Yevhenivna Lapovets, L.; Vasylivna Maksymyuk, H. Body Mass Index and Sperm Quality: Is there a Relationship? J. Hum. Reprod. Sci. 2020, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Sun, J.; Wang, J.Y.; Ping, Z.G.; Liu, L. Does obesity based on body mass index affect semen quality?—A meta-analysis and systematic review from the general population rather than the infertile population. Andrologia 2021, 53, e14099. [Google Scholar] [CrossRef]
- Szatmári, A.; Helembai, K.; Zádori, J.; Kovács, I. Paramedical counselling in infertility treatment: Its effects on anxio-depressive symptom severity, perceived stress and self-esteem. Heliyon 2022, 8, e09827. [Google Scholar] [CrossRef] [PubMed]
- Zorn, B.; Auger, J.; Velikonja, V.; Kolbezen, M.; Meden-Vrtovec, H. Psychological factors in male partners of infertile couples: Relationship with semen quality and early miscarriage. Int. J. Androl. 2008, 31, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, J.; Qi, Y.; Wang, P.; Jiang, R.; Li, H. Assessment on Occurrences of Depression and Anxiety and Associated Risk Factors in the Infertile Chinese Men. Am. J. Mens. Health 2017, 11, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Volgsten, H.; Skoog Svanberg, A.; Ekselius, L.; Lundkvist, Ö.; Sundström Poromaa, I. Prevalence of psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Hum. Reprod. 2008, 23, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Baldini, M.P.; Scarduelli, C.; Bommarito, F.; Ambrosio, S.; D’Orsi, C.; Torretta, R.; Bonizzoni, M.; Ragni, G. Prevalence and incidence of depressive and anxious symptoms in couples undergoing assisted reproductive treatment in an Italian infertility department. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 235–241. [Google Scholar] [CrossRef]
- Sherrard, W. The fertility problem inventory: Measuring perceived infertility-related stress. Fertil. Steril. 1999, 72, 54–62. [Google Scholar]
- Chi, H.J.; Park, I.H.; Sun, H.G.; Kim, J.W.; Lee, K.H. Psychological distress and fertility quality of life (FertiQoL) in infertile Korean women: The first validation study of Korean FertiQoL. Clin. Exp. Reprod. Med. 2016, 43, 174–180. [Google Scholar] [CrossRef]
- Nachtigall, R.D.; Becker, G.; Wozny, M. The effects of gender-specific diagnosis on men’s and women’s response to infertility. Fertil. Steril. 1992, 57, 113–121. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Programme on Mental Health: WHOQOL User Manual; 2012 revision; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Theofilou, P. Quality of life: Definition and measurement. Eur. J. Psychol. 2013, 9, 150–162. [Google Scholar] [CrossRef]
- Belladelli, F.; Muncey, W.; Seranio, N.; Eisenberg, M.L. Counseling for the man with severe male infertility. Curr. Opin. Urol. 2023, 33, 5–9. [Google Scholar] [CrossRef]
- Ragni, G.; Mosconi, P.; Baldini, M.P.; Somigliana, E.; Vegetti, W.; Caliari, I.; Nicolosi, A.E. Health-related quality of life and need for IVF in 1000 Italian infertile couples. Hum. Reprod. 2005, 20, 1286–1291. [Google Scholar] [CrossRef]
- Rashidi, B.; Montazeri, A.; Ramezanzadeh, F.; Shariat, M.; Abedinia, N.; Ashrafi, M. Health-related quality of life in infertile couples receiving IVF or ICSI treatment. BMC Health Serv. Res. 2008, 8, 186. [Google Scholar] [CrossRef]
- Shindel, A.W.; Nelson, C.J.; Naughton, C.K.; Ohebshalom, M.; Mulhall, J.P. Sexual Function and Quality of Life in the Male Partner of Infertile Couples: Prevalence and Correlates of Dysfunction. J. Urol. 2008, 179, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Masoumi, S.Z.; Keramat, A.; Pooralajal, J.; Shobeiri, F. Assessment of questionnaires measuring quality of life in infertile couples: A systematic review. J. Reprod. Infertil. 2013, 14, 110–119. [Google Scholar] [PubMed]
- Bernd, M.; Schick, M.; Rösner, S.; Germeyer, A.; Strowitzki, T.; Moessner, M.; Bauer, S.; Ditzen, B.; Wischmann, T. Predictors for the Early Termination of a Psychological Intervention During Treatment with Assisted Reproductive Technologies. Geburtshilfe Frauenheilkd. 2020, 80, 190. [Google Scholar] [CrossRef] [PubMed]
- Salazar Mederos, A.M.; Gutiérrez Hernández, P.R.; Ortega González, Y.; Hess Medler, S. Depressive ranges in infertile couples with male factor. Rev. Int. Androl. 2023, 21, 100324. [Google Scholar] [CrossRef]
- Yamanaka-Altenstein, M. Demand-oriented Cognitive-behavioural Intervention for Couples with Infertility (FERTIFIT): A Pilot Study about Development, Feasibility and Acceptance. Psychother. Psychosom. Med. Psychol. 2023, 73, 197–205. [Google Scholar]
- Li, Y.; Zhang, X.; Shi, M.; Guo, S.; Wang, L. Resilience acts as a moderator in the relationship between infertility-related stress and fertility quality of life among women with infertility: A cross-sectional study. Health Qual. Life Outcomes 2019, 17, 38. [Google Scholar] [CrossRef]
- Wenger, L.M. Beyond ballistics: Expanding our conceptualization of men’s health-related help seeking. Am. J. Mens. Health 2011, 5, 488–499. [Google Scholar] [CrossRef]
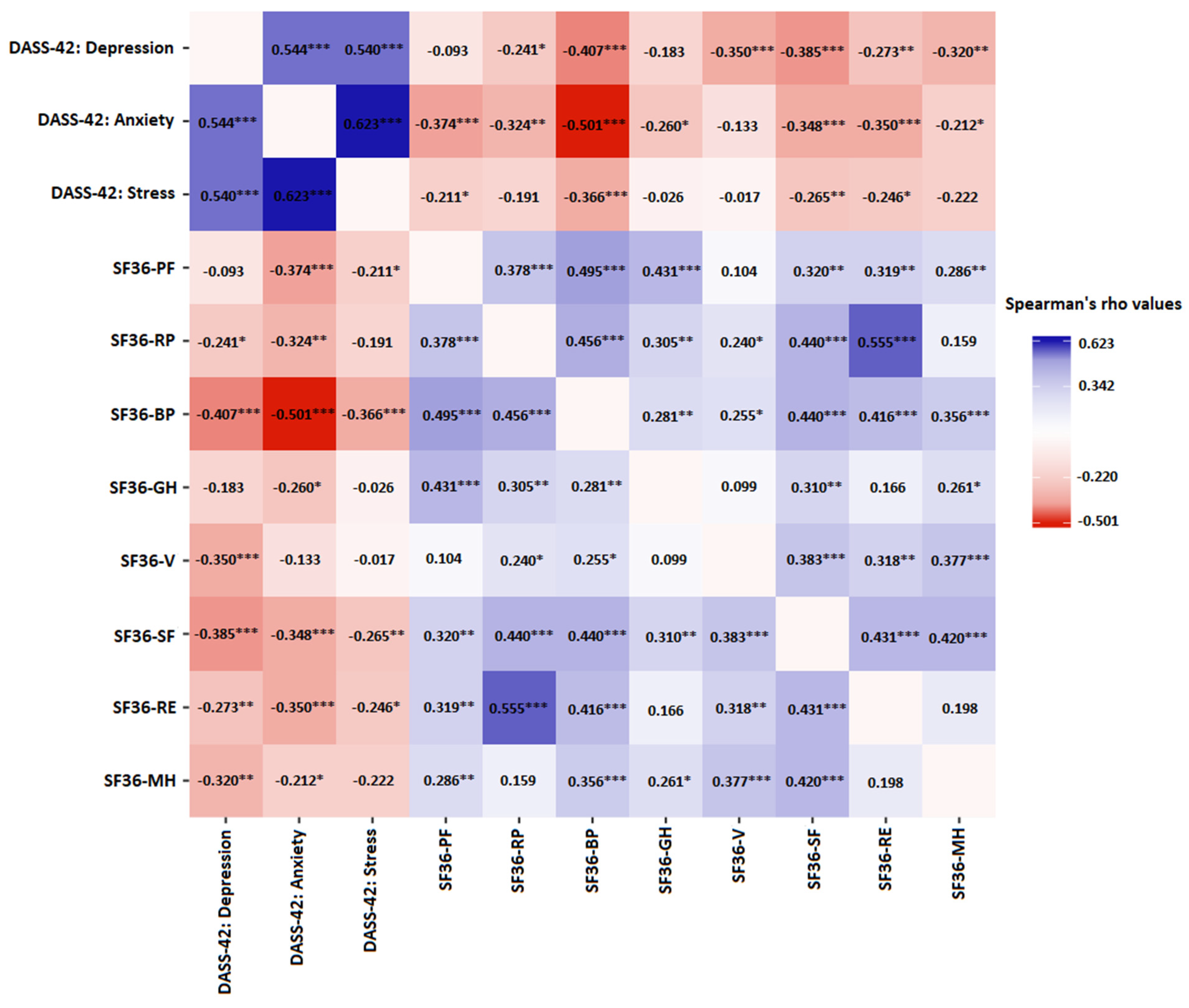

| Characteristics | Patients Treated for Infertility < 2 Years; n = 50 | Patients Treated for Infertility ≥ 2 Years; n = 46 | p 1 | Total Sample n = 96 |
|---|---|---|---|---|
| Age, years, ± SD | 36.74 ± 5.56 | 38.72 ± 5.78 | 0.193 | 37.69 ± 5.72 |
| Body Mass Index (BMI), kg/m2, ± SD | 27.60 ± 4.50 | 27.85 ± 3.45 | 0.331 | 27.72 ± 4.01 |
| Highest level of formal education *, n (%) | ||||
| ISCED 0/1: Less than primary/primary education | 4 (8.0) | 1 (2.17) | 0.279 | 5 (5.2) |
| ISCED 2/3: Lower/upper secondary education | 22 (44.0) | 26 (56.52) | 48 (50.0) | |
| ISCED 4–8: Post-secondary non-tertiary/tertiary education ** | 24 (48.0) | 19 (41.30) | 43 (44.8) | |
| Occupational profile ***, n (%) | ||||
| Unemployed | 1 (2.0) | 1 (2.2) | 0.915 | 2 (2.1) |
| Skilled agricultural, forestry, and fishery workers | 1 (2.0) | 0 (0.0) | 1 (1.0) | |
| Elementary occupations | 5 (10.0) | 3 (6.5) | 8 (8.3) | |
| Plant and machine operators, assemblers/Craft workers | 8 (16.0) | 7 (15.2) | 15 (15.6) | |
| Technicians/Clerical support/Service and sales workers | 19 (38.0) | 21 (45.6) | 40 (41.7) | |
| Professionals/Managers | 10 (20.0) | 9 (19.6) | 19 (19.8) | |
| Other | 6 (12.0) | 5 (10.9) | 11 (11.5) | |
| Residential region, n (%) | ||||
| Belgrade (capital city) region | 40 (80.0) | 39 (84.8) | 0.539 | 79 (82.3) |
| Other geographical regions | 10 (20.0) | 7 (15.1) | 17 (17.7) | |
| Self-reported socio-economic status, n (%) | ||||
| High | 7 (14.0) | 7 (15.2) | 0.412 | 14 (14.6) |
| Middle | 41 (82.0) | 34 (73.9) | 75 (78.1) | |
| Low | 2 (4.0) | 5 (10.9) | 7 (7.3) | |
| Currently smoking, n (%) | ||||
| Yes | 18 (36.0) | 10 (21.7) | 0.125 | 28 (29.2) |
| No | 32 (64.0) | 36 (78.3) | 68 (70.8) | |
| Alcohol consumption, n (%) | ||||
| Yes | 28 (56.0) | 26 (56.5) | 0.959 | 54 (56.3) |
| No | 22 (44.0) | 20 (43.5) | 42 (43.8) | |
| History of sexually transmitted diseases, n (%) | ||||
| Yes | 9 (18.0) | 4 (8.7) | 0.183 | 13 (15.5) |
| No | 41 (82.0) | 42 (91.3) | 83 (86.5) | |
| History of urological surgery, n (%) | ||||
| Yes | 8 (16.0) | 7 (15.2) | 0.916 | 15 (15.6) |
| No | 42 (84.0) | 39 (84.8) | 81 (84.4) | |
| Having at least one child, n (%) | ||||
| Yes | 10 (20.0) | 4 (8.7) | 0.117 | 14 (14.6) |
| No | 40 (80.0) | 42 (91.3) | 82 (85.4) | |
| Duration of infertility treatment, months, ± SD | 13.90 ± 2.86 | 57.52 ± 36.91 | NA | 34.80 ± 33.61 |
| Semen Analysis Category | Criteria | Patients Treated for Infertility < 2 Years; N = 50 n (%) | Patients Treated for Infertility ≥ 2 Years; N = 46 n (%) | p * | Total Sample; N = 96 n (%) |
|---|---|---|---|---|---|
| Normozoospermia | Sperm concentration exceeding the lower reference limit of 15 × 106 spermatozoa/mL of ejaculate | 23 (46.0) | 18 (39.1) | 0.497 | 41 (42.7) |
| Azoospermia | Absence of spermatozoa in the ejaculate | 7 (14.0) | 3 (6.5) | 0.230 | 10 (10.4) |
| Oligozoospermia | Sperm concentration below the lower reference limit of 15 × 106 spermatozoa/mL of ejaculate | 20 (40.0) | 25 (54.4) | 0.159 | 45 (46.9) |
| Mild | 10–15 × 106 spermatozoa/mL of ejaculate | 3 (6.0) | 2 (4.4) | 0.171 | 5 (5.2) |
| Moderate | 5–10 × 106 spermatozoa/mL of ejaculate | 9 (18.0) | 6 (13.0) | 15 (15.6) | |
| Severe | <5 × 106 spermatozoa/mL of ejaculate | 8 (16.0) | 17 (36.9) | 25 (26.0) | |
| Asthenospermia | <40% total sperm motility or <32% rapid progressive motility | 8 (16.0) | 23 (50.0) | <0.001 | 31 (32.3) |
| Teratozoospermia | <4% of morphologically normal spermatozoa | 13 (26.0) | 20 (43.5) | 0.072 | 33 (34.4) |
| Pyospermia | >1.0 × 106 leucocytes per mL of ejaculate | 4 (8.0) | 5 (10.9) | 0.630 | 9 (9.4) |
| Semen volume | |||||
| Hypospermia | <1.5 mL | 5 (10.0) | 6 (13.0) | 0.703 | 11 (11.5) |
| Normospermia | 1.5–6.0 mL | 41 (82.0) | 38 (82.6) | 79 (82.3) | |
| Hyperspermia | >6.0 mL | 4 (8.0) | 2 (4.4) | 6 (6.3) |
| Psychosocial Distress Domain Based on DASS-42* | Scale Score | p 1 | Bayes Factor (BF10) | Conventional Severity Categories, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ± SD | Median | IQR | Normal | Mild | Moderate | Severe | Extremely Severe | |||
| Depression | 0.116 | 0.582 | ||||||||
| Patients treated for infertility < 2 years; n = 50 | 3.76 ± 4.38 | 2.50 | 1.00–5.00 | 45 (46.9) | 3 (3.1) | 2 (2.1) | - | - | ||
| Patients treated for infertility ≥ 2 years; n = 46 | 5.34 ± 5.48 | 4.00 | 1.25–7.00 | 38 (39.5) | 4 (4.2) | 2 (2.1) | 2 (2.1) | - | ||
| Total sample, n = 96 | 4.52 ± 4.98 | 3.00 | 1.00–6.00 | 83 (86.5) | 7 (7.3) | 4 (4.2) | 2 (2.1) | - | ||
| Anxiety | 0.605 | 0.244 | ||||||||
| Patients treated for infertility < 2 years; n = 50 | 4.12 ± 4.94 | 3.00 | 1.00–5.00 | 42 (43.8) | 3 (3.1) | 3 (3.1) | 1 (1.0) | 1 (1.0) | ||
| Patients treated for infertility ≥ 2 years; n = 46 | 3.63 ± 3.09 | 2.50 | 2.00–5.00 | 43 (44.8) | 1 (1.0) | 1 (1.0) | 1 (1.0) | - | ||
| Total sample, n = 96 | 3.89 ± 4.15 | 3.00 | 1.00–5.00 | 85 (88.5) | 4 (4.2) | 4 (4.2) | 2 (2.1) | 1 (1.0) | ||
| Stress | 0.364 | 0.261 | ||||||||
| Patients treated for infertility < 2 years; n = 50 | 10.36 ± 8.11 | 9.00 | 4.25–13.75 | 39 (40.6) | 3 (3.1) | 4 (4.2) | 3 (3.1) | 1 (1.0) | ||
| Patients treated for infertility ≥ 2 years; n = 46 | 11.24 ± 7.59 | 11.00 | 6.00–14.00 | 35 (36.5) | 4 (4.2) | 5 (5.2) | 2 (2.1) | - | ||
| Total sample, n = 96 | 10.78 ± 7.83 | 10.00 | 5.00–14.00 | 74 (77.1) | 7 (7.3) | 9 (9.4) | 5 (5.2) | 1 (1.0) | ||
| Health-Related Quality of Life Domain Based on SF-36 | Total Sample, n = 96 | Patients Treated for Infertility < 2 Years; n = 50 | Patients Treated for Infertility ≥ 2 Years; n = 46 | p 1 | Bayes Factor (BF10) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ± SD | Median | IQR | ± SD | Median | IQR | ± SD | Median | IQR | |||
| Physical functioning | 90.16 ± 17.75 | 95.00 | 90.00–100.00 | 90.30 ± 17.24 | 95.00 | 86.20–100.00 | 90.00 ± 18.47 | 95.00 | 90.00–100.00 | 0.676 | 0.245 |
| Role physical | 87.50 ± 26.66 | 90.00 | 85.00–100.00 | 89.50 ± 22.07 | 90.00 | 85.00–100.00 | 85.33 ± 30.99 | 90.00 | 85.00–100.00 | 0.750 | 0.285 |
| Bodily pain | 75.34 ± 21.68 | 87.00 | 62.00–90.00 | 77.34 ± 21.61 | 90.00 | 74.00–90.00 | 73.17 ± 21.78 | 84.00 | 62.00–90.00 | 0.146 | 0.375 |
| General health | 74.67 ± 17.24 | 77.00 | 64.25–87.00 | 72.76 ± 17.85 | 77.00 | 62.75–84.25 | 76.74 ± 16.49 | 80.00 | 65.50–89.25 | 0.317 | 0.408 |
| Vitality | 65.42 ± 11.14 | 65.00 | 60.00–75.00 | 66.80 ± 10.14 | 65.00 | 60.00–75.00 | 63.91 ± 12.06 | 62.50 | 55.00–75.00 | 0.230 | 0.424 |
| Social functioning | 83.59 ± 17.07 | 87.50 | 75.00–100.00 | 83.25 ± 18.49 | 87.50 | 75.00–100.00 | 83.96 ± 17.81 | 87.50 | 75.00–100.00 | 0.893 | 0.227 |
| Role emotional | 84.03 ± 30.58 | 87.50 | 66.67–100.00 | 80.00 ± 24.51 | 87.50 | 75.00–100.00 | 77.54 ± 35.18 | 87.50 | 66.67–100.00 | 0.050 | 0.569 |
| Mental health | 49.00 ± 6.25 | 48.00 | 44.00–52.00 | 49.36 ± 5.58 | 50.00 | 45.00–52.00 | 48.61 ± 6.96 | 48.00 | 44.00–55.00 | 0.352 | 0.262 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čegar, B.; Šipetić Grujičić, S.; Bjekić, J.; Vuksanović, A.; Bojanić, N.; Bartolović, D.; Jovanović, D.; Zeković, M. Understanding the Male Perspective: Evaluating Quality of Life and Psychological Distress in Serbian Men Undergoing Infertility Treatment. Life 2023, 13, 1894. https://doi.org/10.3390/life13091894
Čegar B, Šipetić Grujičić S, Bjekić J, Vuksanović A, Bojanić N, Bartolović D, Jovanović D, Zeković M. Understanding the Male Perspective: Evaluating Quality of Life and Psychological Distress in Serbian Men Undergoing Infertility Treatment. Life. 2023; 13(9):1894. https://doi.org/10.3390/life13091894
Chicago/Turabian StyleČegar, Bojan, Sandra Šipetić Grujičić, Jovana Bjekić, Aleksandar Vuksanović, Nebojša Bojanić, Daniela Bartolović, Darko Jovanović, and Milica Zeković. 2023. "Understanding the Male Perspective: Evaluating Quality of Life and Psychological Distress in Serbian Men Undergoing Infertility Treatment" Life 13, no. 9: 1894. https://doi.org/10.3390/life13091894
APA StyleČegar, B., Šipetić Grujičić, S., Bjekić, J., Vuksanović, A., Bojanić, N., Bartolović, D., Jovanović, D., & Zeković, M. (2023). Understanding the Male Perspective: Evaluating Quality of Life and Psychological Distress in Serbian Men Undergoing Infertility Treatment. Life, 13(9), 1894. https://doi.org/10.3390/life13091894







