Optimization of High-Efficiency Tissue Culture Regeneration Systems in Gray Poplar
Abstract
:1. Introduction
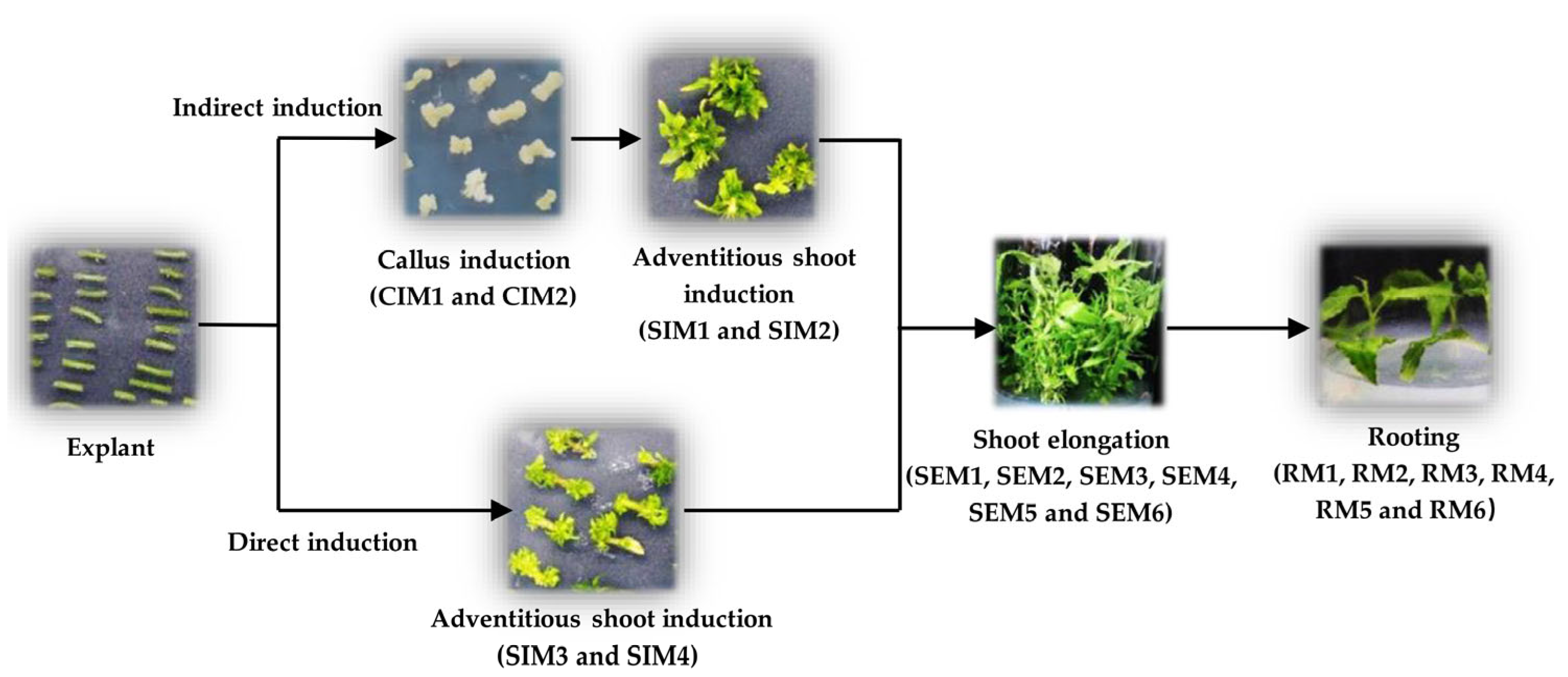
2. Materials and Methods
2.1. Plant Culture
2.2. Indirect Adventitious Shoot Induction
2.3. Direct Induction of Adventitious Shoots
2.4. Shoot Elongation Medium
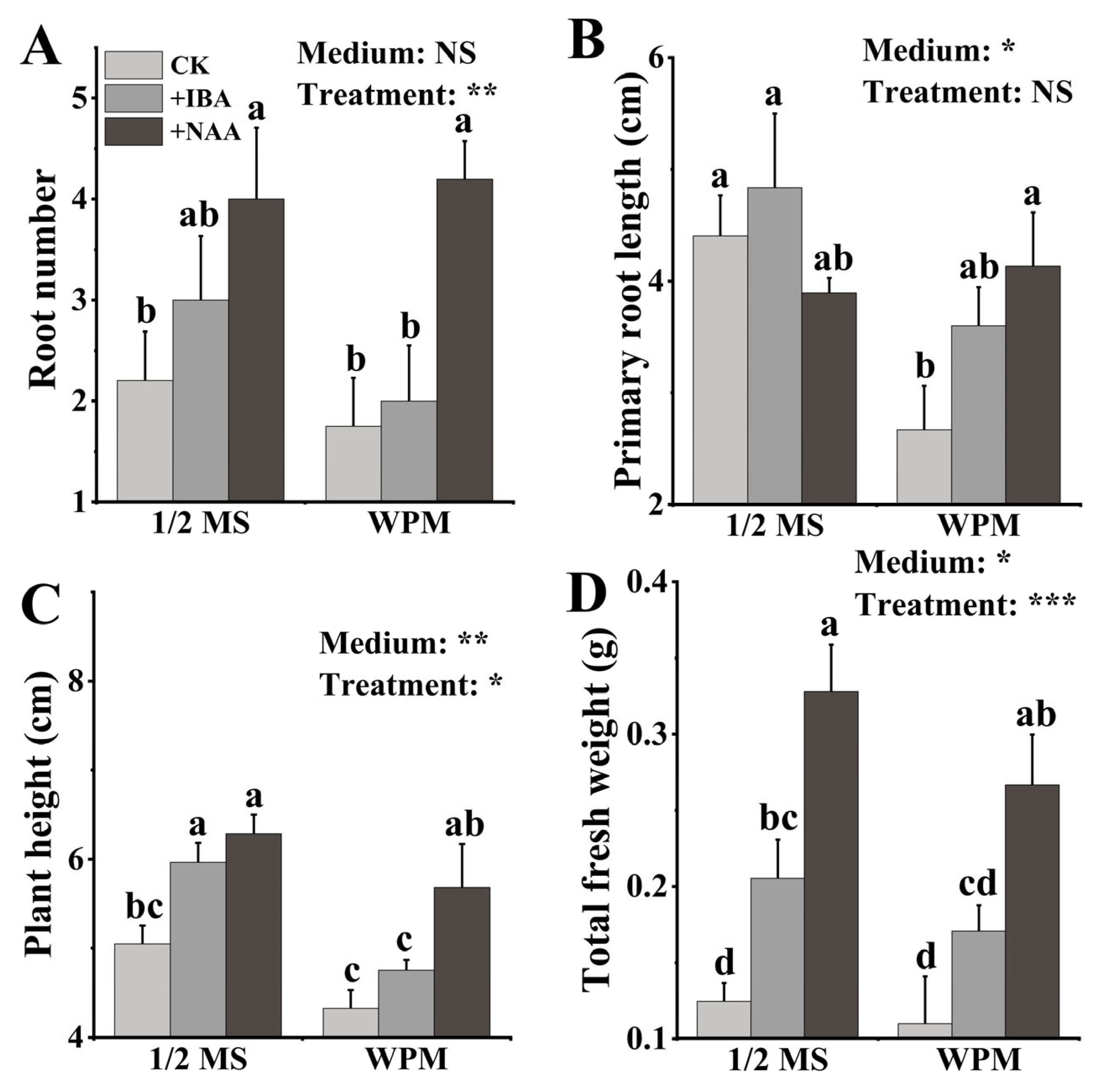
2.5. Rooting Medium
2.6. Data Statistics and Analysis
3. Results
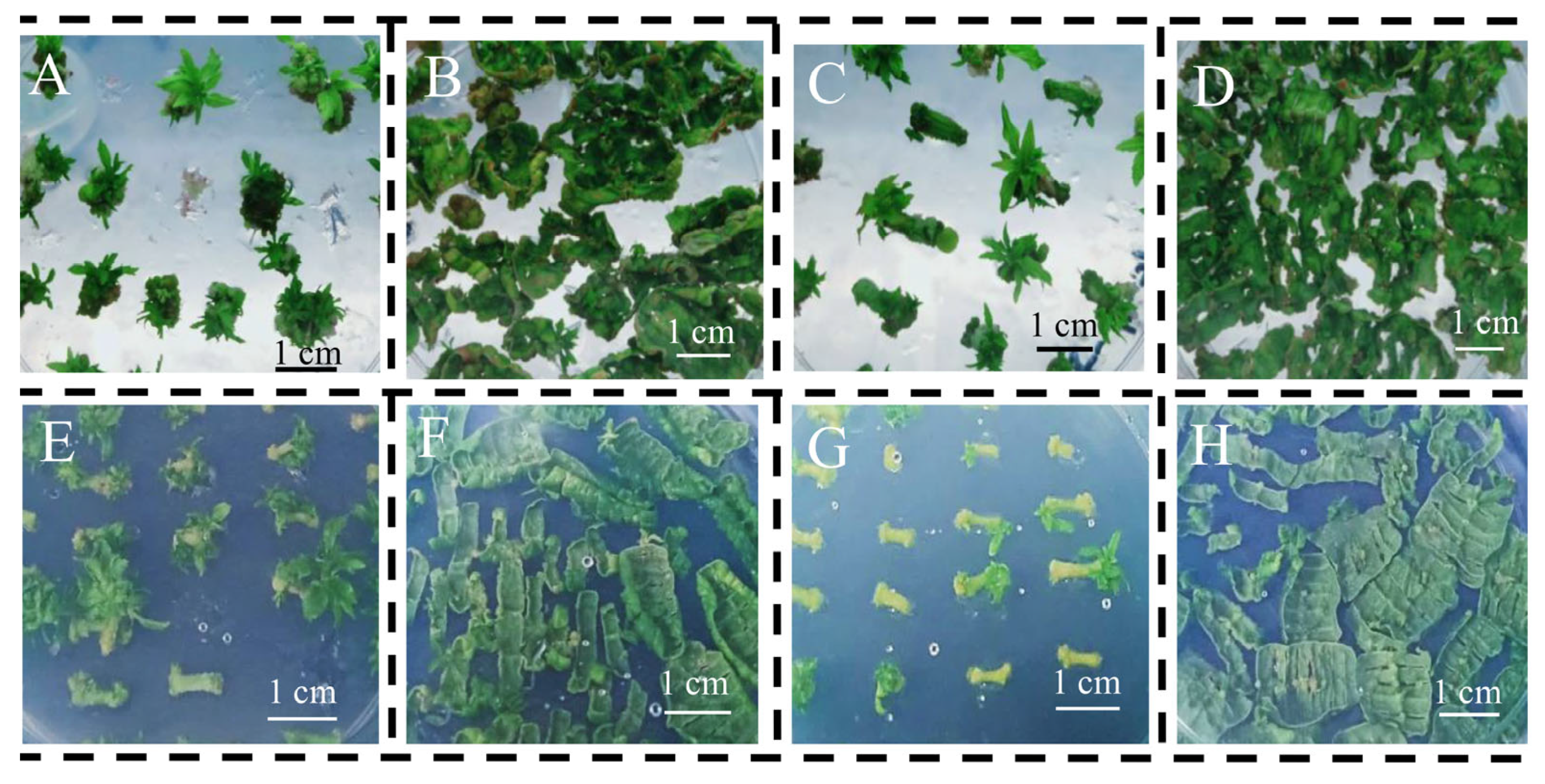
3.1. Indirect Induction of Adventitious Shoots
3.2. Direct Induction of Adventitious Shoots
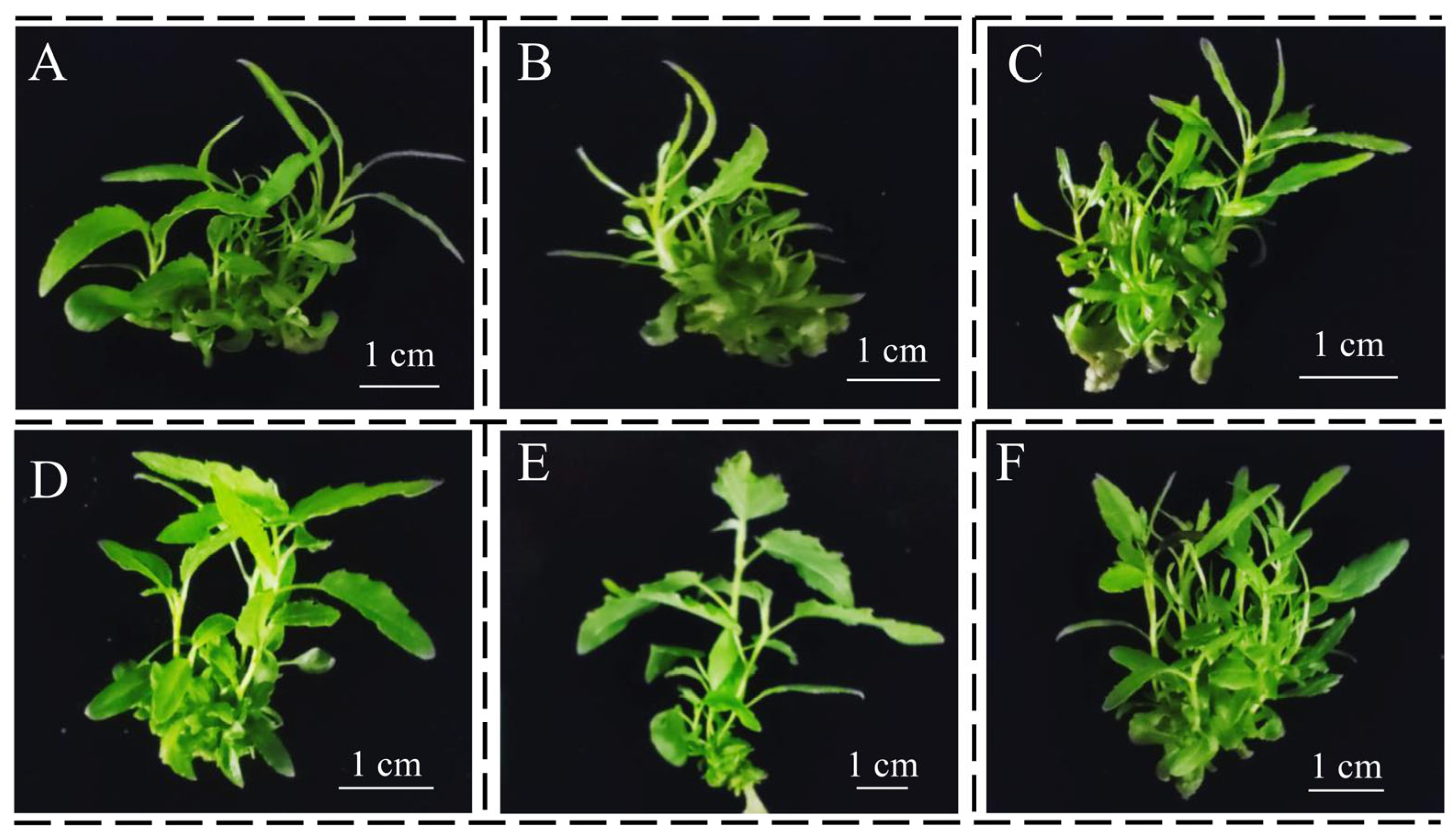
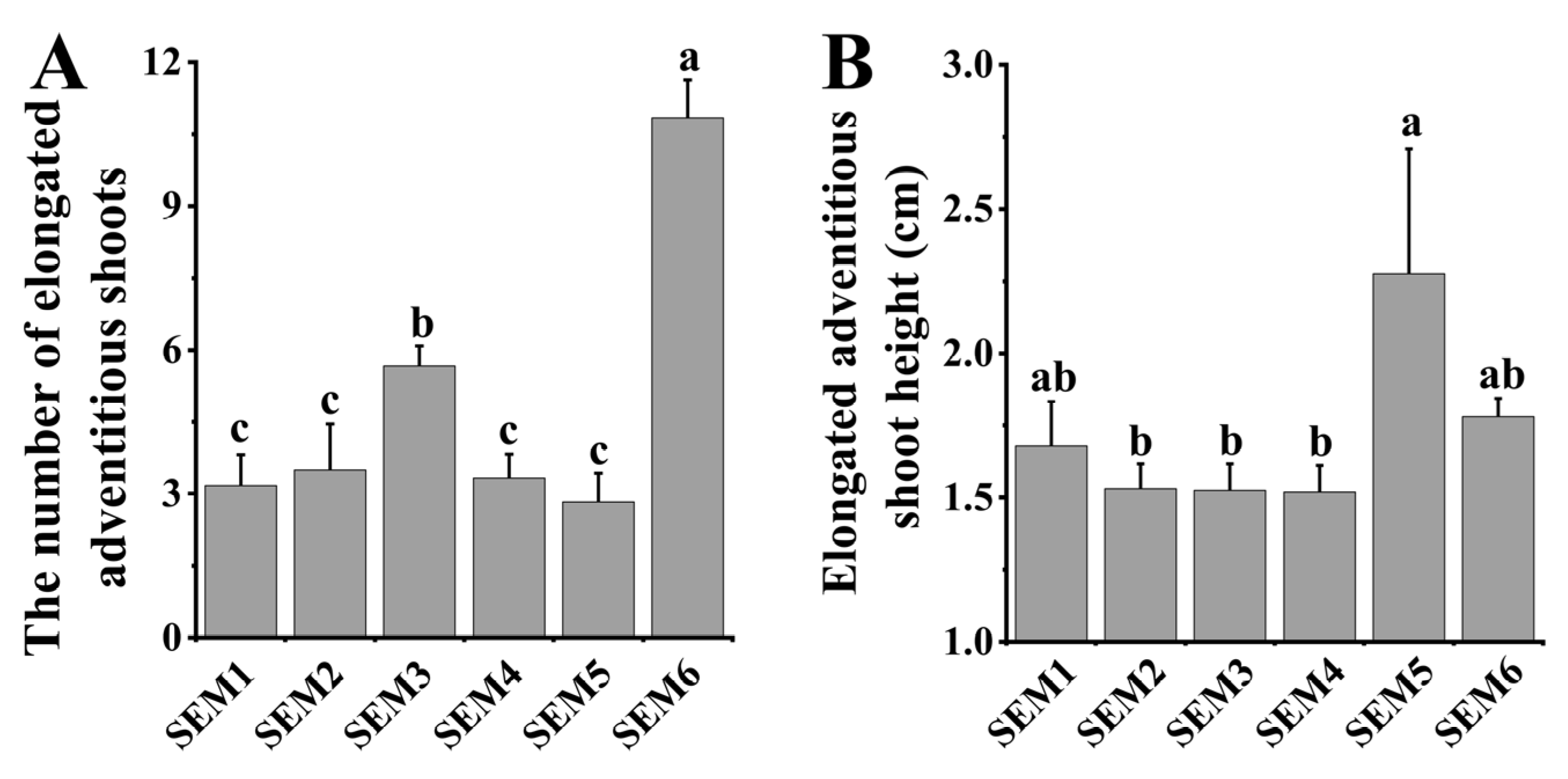
3.3. Shoot Elongation Medium
3.4. Rooting Medium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Zhou, J.-J.; Masclaux-Daubresse, C.; Wang, N.; Wang, H.; Zheng, B. Morphological and physiological responses to contrasting nitrogen regimes in Populus cathayana is linked to resources allocation and carbon/nitrogen partition. Environ. Exp. Bot. 2019, 162, 247–255. [Google Scholar] [CrossRef]
- Shi, W.; Zhou, J.; Li, J.; Ma, C.; Zhang, Y.; Deng, S.; Yu, W.; Luo, Z.-B. Lead exposure-induced defense responses result in low lead translocation from the roots to aerial tissues of two contrasting poplar species. Environ. Pollut. 2021, 271, 116346. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liang, Z.; Wu, M.; Mei, L. Genome-wide identification of BOR genes in poplar and their roles in response to various environmental stimuli. Environ. Exp. Bot. 2019, 164, 101–113. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J. Growth performance, photosynthesis, and root characteristics are associated with nitrogen use efficiency in six poplar species. Environ. Exp. Bot. 2019, 164, 40–51. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Luo, J.; Cao, X.; Qu, L.; Gai, Y.; Jiang, X.; Liu, T.; Bai, H.; Janz, D. N-fertilization has different effects on the growth, carbon and nitrogen physiology, and wood properties of slow-and fast-growing Populus species. J. Exp. Bot. 2012, 63, 6173–6185. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Aldén, T.; Sitbon, F.; Anthony Little, C.; Chalupa, V.; Sandberg, G.; Olsson, O. Spatial pattern of cauliflower mosaic virus 35S promoter-luciferase expression in transgenic hybrid aspen trees monitored by enzymatic assay and non-destructive imaging. Transgenic Res. 1992, 1, 209–220. [Google Scholar] [CrossRef]
- Wei, F.; Zhao, F.-f.; Tian, B.-m. In vitro regeneration of Populus tomentosa from petioles. J. For. Res. 2017, 28, 465–471. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Liu, H.; Tang, R.; Zhang, H. An efficient Agrobacterium-mediated transformation and regeneration system for leaf explants of two elite aspen hybrid clones Populus alba × P. berolinensis and Populus davidiana × P. bolleana. Plant Cell Rep. 2011, 30, 2037–2044. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar]
- Jansson, S.; Douglas, C.J. Populus: A model system for plant biology. Annu. Rev. Plant Biol. 2007, 58, 435–458. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Zhao, K.; Yao, W.; Li, R.; Zhou, B.; Jiang, T. Over-expression of ERF38 gene enhances salt and osmotic tolerance in transgenic poplar. Front. Plant Sci. 2019, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Q.; Wang, W.; Wang, X.; Lu, M. Salt tolerance conferred by over-expression of OsNHX1 gene in Poplar 84K. Chin. Sci. Bull. 2005, 50, 225–229. [Google Scholar] [CrossRef]
- Yu, L.; Xiong, D.; Han, Z.; Liang, Y.; Tian, C. The mitogen-activated protein kinase gene CcPmk1 is required for fungal growth, cell wall integrity and pathogenicity in Cytospora chrysosperma. Fungal Genet. Biol. 2019, 128, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shang, X.; Zhang, X.; Shao, W.; Ren, L.; Li, G.; Zhu, M.; Wang, R. In vitro regeneration and Agrobacterium-mediated genetic transformation of Caragana korshinskii. For. Res. 2023, 3, 14. [Google Scholar]
- Zhou, T.; Lin, Y.; Lin, Y.; Luo, J.; Ding, J. Regeneration and Agrobacterium-mediated genetic transformation of twelve Eucalyptus species. For. Res. 2022, 2, 15. [Google Scholar] [CrossRef]
- Gözükirmizi, N.; Bajroviç, K.; İpekçi, Z.; Boydak, M.; Akalp, T.; Tunçtaner, K.; Balkan, H.; Tanrıyar, H.; Çalıkoėlu, M.; Oėraş, T. Genotype differencies in direct plant regeneration from stem explants of Populus tremula in Turkey. J. For. Res. 1998, 3, 123–126. [Google Scholar] [CrossRef]
- Ferreira, S.; Batista, D.; Serrazina, S.; Pais, M.S. Morphogenesis induction and organogenic nodule differentiation in Populus euphratica Oliv. leaf explants. Plant Cell Tissue Organ Cult. 2009, 96, 35–43. [Google Scholar] [CrossRef]
- Aggarwal, G.; Gaur, A.; Srivastava, D.K. Establishment of high frequency shoot regeneration system in Himalayan poplar (Populus ciliata Wall. ex Royle) from petiole explants using Thidiazuron cytokinin as plant growth regulator. J. For. Res. 2015, 26, 651–656. [Google Scholar] [CrossRef]
- Maheshwari, P.; Kovalchuk, I. Efficient shoot regeneration from internodal explants of Populus angustifolia, Populus balsamifera and Populus deltoids. New Biotechnol. 2011, 28, 778–787. [Google Scholar] [CrossRef]
- Hai, G.; Jia, Z.; Xu, W.; Wang, C.; Cao, S.; Liu, J.; Cheng, Y. Characterization of the Populus PtrCesA4 promoter in transgenic Populus alba × P. glandulosa. Plant Cell Tissue Organ Cult. 2016, 124, 495–505. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, J.; Hu, J.; Ling, J. Mini review: Application of the somatic embryogenesis technique in conifer species. For. Res. 2022, 2, 18. [Google Scholar] [CrossRef]
- Yan, X.; Wang, K.; Zheng, K.; Zhang, L.; Ye, Y.; Qi, L.; Zhu, M. Efficient organogenesis and taxifolin production system from mature zygotic embryos and needles in larch. For. Res. 2023, 3, 4. [Google Scholar] [CrossRef]
- Guo, M.; Yu, Q.; Li, D.; Xu, K.; Di, Z.; Zhang, Y.; Yu, Y.; Zheng, J.; Zhang, Y. In vitro propagation, shoot regeneration, callus induction, and suspension from lamina explants of Sorbus caloneura. For. Res. 2023, 3, 7. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, J.; Chen, Y.; Ding, L.; Wei, J.; Wang, H. An improved and efficient method of Agrobacterium syringe infiltration for transient transformation and its application in the elucidation of gene function in poplar. BMC Plant Biol. 2021, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Sun, Y.; Yuan, L.; Tian, Q.; Luo, K. The chitinase gene (Bbchit1) from Beauveria bassiana enhances resistance to Cytospora chrysosperma in Populus tomentosa Carr. Biotechnol. Lett. 2010, 32, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.S.; Ge, X.L.; Wang, R.; Yang, H.F.; Bai, Y.E.; Guo, Y.H.; Zhang, J.; Lu, M.Z.; Zhao, S.T.; Wang, L.Q. An efficient Agrobacterium-mediated transformation method for hybrid poplar 84K (Populus alba × P. glandulosa) using calli as explants. Int. J. Mol. Sci. 2022, 23, 2216. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, A.; Zhang, J.; Amirian, R.; Zhuge, Q. An efficient Agrobacterium-mediated transformation system for poplar. Int. J. Mol. Sci. 2014, 15, 10780–10793. [Google Scholar] [CrossRef]
- Muhr, M.; Prüfer, N.; Paulat, M.; Teichmann, T. Knockdown of strigolactone biosynthesis genes in Populus affects BRANCHED 1 expression and shoot architecture. New Phytol. 2016, 212, 613–626. [Google Scholar] [CrossRef]
- Zhou, H.; Song, X.; Wei, K.; Zhao, Y.; Jiang, C.; Wang, J.; Tang, F.; Lu, M. Growth-regulating factor 15 is required for leaf size control in Populus. Tree Physiol. 2019, 39, 381–390. [Google Scholar] [CrossRef]
- Li, S.; Zhen, C.; Xu, W.; Wang, C.; Cheng, Y. Simple, rapid and efficient transformation of genotype Nisqually-1: A basic tool for the first sequenced model tree. Sci. Rep. 2017, 7, 2638. [Google Scholar] [CrossRef]
- Luo, J.; Nvsvrot, T.; Wang, N. Comparative transcriptomic analysis uncovers conserved pathways involved in adventitious root formation in poplar. Physiol. Mol. Biol. Plants 2021, 27, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Barkla, B.J.; Vera-Estrella, R.; Pantoja, O. Growing Arabidopsis in vitro: Cell suspensions, in vitro culture, and regeneration. In Arabidopsis Protocols; Springer: Berlin/Heidelberg, Germany, 2014; pp. 53–62. [Google Scholar]
- Maheshwari, P.; Kovalchuk, I. Agrobacterium-mediated stable genetic transformation of Populus angustifolia and Populus balsamifera. Front. Plant Sci. 2016, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-J.; Podila, G.K.; Chiang, V.L. Agrobacterium-mediated transformation of quaking aspen (Populus tremuloides) and regeneration of transgenic plants. Plant Cell Rep. 1994, 14, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ma, S.; Kong, X.; Takano, T.; Liu, S. Efficient Agrobacterium-mediated transformation of hybrid poplar Populus davidiana Dode × Populus bollena Lauche. Int. J. Mol. Sci. 2013, 14, 2515–2528. [Google Scholar] [CrossRef] [PubMed]
- Coleman, G.D.; Ernst, S.G. In vitro shoot regeneration of Populus deltoides: Effect of cytokinin and genotype. Plant Cell Rep. 1989, 8, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.; Tomaz, M.; Arruda, S.; Demétrio, C.G.B.; Venables, W.; Martinelli, A.P. Callus sieving is effective in improving synchronization and frequency of somatic embryogenesis in Citrus sinensis. Biol. Plant. 2011, 55, 703–707. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, C.; Li, Y.; Wang, P.; Li, Y.; Kang, X. Induction of somatic embryogenesis by anther-derived callus culture and plantlet ploidy determination in poplar (Populus × beijingensis). Plant Cell Tissue Organ Cult. 2015, 120, 949–959. [Google Scholar] [CrossRef]
- Balestrazzi, A.; Carbonera, D.; Confalonieri, M. Agrobacterium tumefaciens-mediated transformation of elite white poplar (Populus alba L.) and regeneration of transgenic plants. J. Genet. Breed. 2000, 54, 263–270. [Google Scholar]
- Luo, J.; Shi, W.; Li, H.; Janz, D.; Luo, Z.-B. The conserved salt-responsive genes in the roots of Populus × canescens and Arabidopsis thaliana. Environ. Exp. Bot. 2016, 129, 48–56. [Google Scholar] [CrossRef]
- Li, Z.; Deng, S.; Zhu, D.; Wu, J.; Zhou, J.; Shi, W.; Fayyaz, P.; Luo, Z.-B.; Luo, J. Proteomic reconfigurations underlying physiological alterations in poplar roots in acclimation to changing nitrogen availability. Environ. Exp. Bot. 2023, 211, 105367. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Luo, J.; Ma, C.; Li, S.; Qu, L.; Gai, Y.; Jiang, X.; Janz, D.; Polle, A. A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus × canescens. Plant Physiol. 2013, 162, 424–439. [Google Scholar] [CrossRef]
- Filichkin, S.; Meilan, R.; Busov, V.; Ma, C.; Brunner, A.; Strauss, S. Alcohol-inducible gene expression in transgenic Populus. Plant Cell Rep. 2006, 25, 660–667. [Google Scholar] [CrossRef]
- Song, J.; Lu, S.; Chen, Z.-Z.; Lourenco, R.; Chiang, V.L. Genetic transformation of Populus trichocarpa genotype Nisqually-1: A functional genomic tool for woody plants. Plant Cell Physiol. 2006, 47, 1582–1589. [Google Scholar] [CrossRef]


| Types of Medium | Explant | Callus Induction Rate/% | Adventitious Shoot Induction Ratio/% | ||
|---|---|---|---|---|---|
| CIM1 and SIM1 | Old leaves | 15/15 | 100.0 | 1/14 | 7.1 |
| Old stems | 13/14 | 92.9 | 4/30 | 13.3 | |
| Young leaves | 11/11 | 100.0 | 8/36 | 22.2 | |
| Young stems | 16/20 | 80.0 | 13/31 | 41.9 | |
| CIM2 and SIM2 | Old leaves | 33/33 | 100.0 | 15/72 | 20.8 |
| Old stems | 12/14 | 85.7 | 0 | 0.0 | |
| Young leaves | 49/49 | 100.0 | 29/68 | 42.7 | |
| Young stems | 16/16 | 100.0 | 0 | 0.0 | |
| Types of Medium | Explant | Number of Days to Induce Adventitious Shoots/d | Adventitious Shoot Differentiation Rate/% | |
|---|---|---|---|---|
| SIM3 | Old leaves | 45 | 20/20 | 100.0 |
| Old stems | 45 | 19/19 | 100.0 | |
| Young leaves | 45 | 19/19 | 100.0 | |
| Young stems | 45 | 18/19 | 94.7 | |
| SIM4 | Old leaves | 52 | 56/56 | 100.0 |
| Old stems | 52 | 31/31 | 100.0 | |
| Young leaves | 52 | 50/50 | 100.0 | |
| Young stems | 52 | 24/25 | 96.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, H.; Guan, L.; Li, Z.; Wang, H.; Luo, J. Optimization of High-Efficiency Tissue Culture Regeneration Systems in Gray Poplar. Life 2023, 13, 1896. https://doi.org/10.3390/life13091896
Li H, Wang H, Guan L, Li Z, Wang H, Luo J. Optimization of High-Efficiency Tissue Culture Regeneration Systems in Gray Poplar. Life. 2023; 13(9):1896. https://doi.org/10.3390/life13091896
Chicago/Turabian StyleLi, Huanhuan, Hang Wang, Lianke Guan, Zihui Li, Hua Wang, and Jie Luo. 2023. "Optimization of High-Efficiency Tissue Culture Regeneration Systems in Gray Poplar" Life 13, no. 9: 1896. https://doi.org/10.3390/life13091896
APA StyleLi, H., Wang, H., Guan, L., Li, Z., Wang, H., & Luo, J. (2023). Optimization of High-Efficiency Tissue Culture Regeneration Systems in Gray Poplar. Life, 13(9), 1896. https://doi.org/10.3390/life13091896







