Operative Findings of over 5000 Microvascular Decompression Surgeries for Hemifacial Spasm: Our Perspective and Current Updates
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Overview of HFS
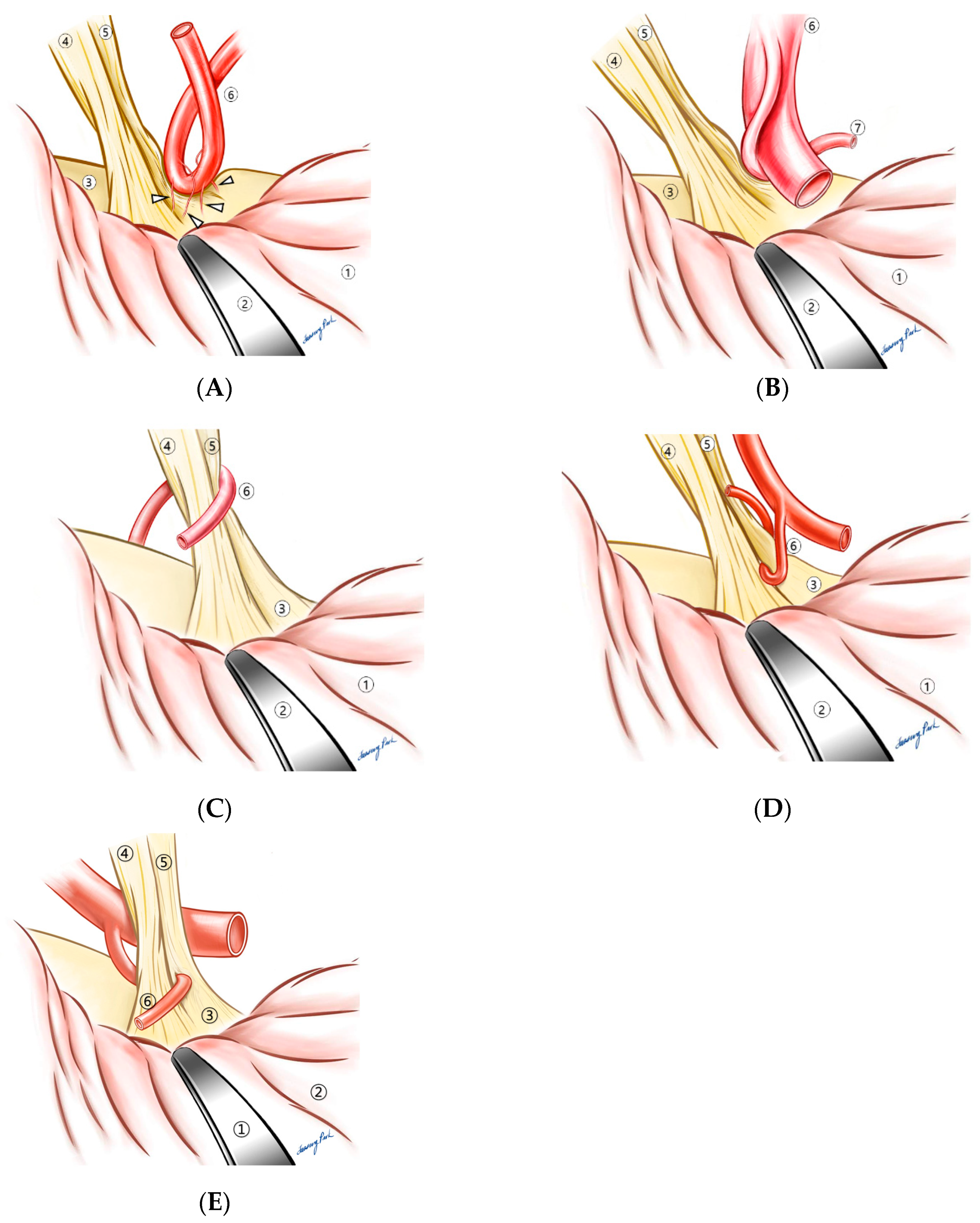
4.2. Compressive Patterns
4.3. Brief Summary of Current Updates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Møller, A.R. The cranial nerve vascular compression syndrome: II. A review of pathophysiology. Acta Neurochir. 1991, 113, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R.; Jannetta, P.J. On the origin of synkinesis in hemifacial spasm: Results of intracranial recordings. J. Neurosurg. 1984, 61, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.G.; Jannetta, P.J.; Bissonette, D.J.; Shields, P.T.; Larkins, M.V.; Jho, H.D. Microvascular decompression for hemifacial spasm. J. Neurosurg. 1995, 82, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R.; Jannetta, P.J. Microvascular decompression in hemifacial spasm: Intraoperative electrophysiological observations. Neurosurgery 1985, 16, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.W.; Kong, D.-S.; Park, K. Microvascular decompression for hemifacial spasm: Long-term outcome and prognostic factors, with emphasis on delayed cure. Neurosurg. Rev. 2013, 36, 297–302. [Google Scholar] [CrossRef]
- Park, J.S.; Kong, D.-S.; Lee, J.-A.; Park, K. Hemifacial spasm: Neurovascular compressive patterns and surgical significance. Acta Neurochir. 2008, 150, 235–241. [Google Scholar] [CrossRef]
- Lee, S.; Joo, K.M.; Park, K. Challenging microvascular decompression surgery for hemifacial spasm. World Neurosurg. 2021, 151, e94–e99. [Google Scholar] [CrossRef]
- Fang, L.-J.; Wang, C.-Y. Bibliometric analysis of studies on the treatment of hemifacial spasm. Front. Neurol. 2022, 13, 931551. [Google Scholar] [CrossRef]
- Park, K.; Park, J.S. Hemifacial Spasm: A Comprehensive Guide; Springer Nature: London, UK, 2020. [Google Scholar]
- Pawlowski, M.; Gess, B.; Evers, S. The Babinski-2 sign in hemifacial spasm. Mov. Disord. 2013, 28, 1298–1300. [Google Scholar] [CrossRef]
- Chan, L.-L.; Ng, K.-M.; Fook-Chong, S.; Lo, Y.-L.; Tan, E.-K.J.N. Three-dimensional MR volumetric analysis of the posterior fossa CSF space in hemifacial spasm. Neurology 2009, 73, 1054–1057. [Google Scholar] [CrossRef]
- Nilsen, B.; Le, K.-D.; Dietrichs, E. Prevalence of hemifacial spasm in Oslo, Norway. Neurology 2004, 63, 1532–1533. [Google Scholar] [CrossRef] [PubMed]
- Auger, R.G.; Whisnant, J.P. Hemifacial spasm in Rochester and Olmsted county, Minnesota, 1960 to 1984. Arch. Neurol. 1990, 47, 1233–1234. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, S.; Park, S.-K.; Lee, J.-A.; Park, K. Facial motor evoked potential with paired transcranial magnetic stimulation: Prognostic value following microvascular decompression for hemifacial spasm. J. Neurosurg. 2018, 131, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Rand, R.W. Gardner’s neurovascular decompression for hemifacial spasm. Arch. Neurol. 1982, 39, 510–511. [Google Scholar] [CrossRef]
- Bremond, G.; Garcin, M.; Magnan, J.; Bonnaud, G. L’abord a minima de l’espace pontocerebelleux. Cah ORL 1974, 19, 443–460. [Google Scholar]
- Jannetta, P. Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Surge Forum 1975, 26, 467–469. [Google Scholar]
- Park, J.S.; Kong, D.-S.; Lee, J.-A.; Park, K. Chronologic analysis of symptomatic change following microvascular decompression for hemifacial spasm: Value for predicting midterm outcome. Neurosurg. Rev. 2008, 31, 413–419. [Google Scholar] [CrossRef]
- Campos-Benitez, M.; Kaufmann, A.M. Neurovascular compression findings in hemifacial spasm. J. Neurosurg. 2008, 109, 416–420. [Google Scholar] [CrossRef]
- Dutton, J.J.; Buckley, E.G. Long-term Results and Complications of Botulinum A Toxin in the Treatment of Blepharospasm. Ophthalmology 1988, 95, 1529–1534. [Google Scholar] [CrossRef]
- Dutton, J.J.; Fowler, A.M. Botulinum toxin in ophthalmology. Surv. Ophthalmol. 2007, 52, 13–31. [Google Scholar] [CrossRef]
- Ababneh, O.H.; Cetinkaya, A.; Kulwin, D.R. Long-term efficacy and safety of botulinum toxin A injections to treat blepharospasm and hemifacial spasm. Clin. Exp. Ophthalmol. 2014, 42, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Defazio, G.; Abbruzzese, G.; Girlanda, P.; Vacca, L.; Curra, A.; De Salvia, R.; Marchese, R.; Raineri, R.; Roselli, F.; Livrea, P.; et al. Botulinum toxin A treatment for primary hemifacial spasm: A 10-year multicenter study. Arch. Neurol. 2002, 59, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-S.; Park, K.; Shin, B.-G.; Lee, J.A.; Eum, D.-O. Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J. Neurosurg. 2007, 106, 384–387. [Google Scholar] [CrossRef]
- Czyz, C.N.; Burns, J.A.; Petrie, T.P.; Watkins, J.R.; Cahill, K.V.; Foster, J.A. Long-term Botulinum Toxin Treatment of Benign Essential Blepharospasm, Hemifacial Spasm, and Meige Syndrome. Am. J. Ophthalmol. 2013, 156, 173–177.e172. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Miller, V.M. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: A systematic review. J. Neurosurg. 2012, 26, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Maeda, Y.; Toyooka, T.; Inaba, K.-I. Microvascular decompression for hemifacial spasm caused by the vertebral artery: A simple and effective transposition method using surgical glue. Surg. Neurol. 2004, 61, 398–403. [Google Scholar] [CrossRef]
- Masuoka, J.; Matsushima, T.; Kawashima, M.; Nakahara, Y.; Funaki, T.; Mineta, T. Stitched sling retraction technique for microvascular decompression: Procedures and techniques based on an anatomical viewpoint. Neurosurg. Rev. 2011, 34, 373–380. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.S.; Ahn, Y.H. Bioglue-Coated Teflon Sling Technique in Microvascular Decompression for Hemifacial Spasm Involving the Vertebral Artery. J. Korean Neurosurg. Soc. 2016, 59, 505–511. [Google Scholar] [CrossRef]
- Møller, A.R.; Jannetta, P.J. Physiological abnormalities in hemifacial spasm studied during microvascular decompression operations. Exp. Neurol. 1986, 93, 584–600. [Google Scholar] [CrossRef]
- Von Eckardstein, K.; Harper, C.; Castner, M.; Link, M. The significance of intraoperative electromyographic “lateral spread” in predicting outcome of microvascular decompression for hemifacial spasm. J. Neurol. Surg. Part B Skull Base 2014, 75, 198–203. [Google Scholar] [CrossRef]
- Lee, J.-A.; Park, K. Short-term versus long-term outcomes of microvascular decompression for hemifacial spasm. Acta Neurochir. 2019, 161, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Oishi, M.; Hiraishi, T.; Fujii, Y. Facial nerve motor-evoked potential monitoring during microvascular decompression for hemifacial spasm. J. Neurol. Neurosurg. Psychiatry 2010, 81, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Pan, L.; Li, B.; Zhou, Y.; Pan, Y.; Zhang, X.; Hu, Y.; Dressler, D.; Jin, L. Botulinum toxin therapy of hemifacial spasm: Bilateral injections can reduce facial asymmetry. J. Neurol. 2018, 265, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Cavus, H.; İşeri, P.; Öztürk, O.; Anık, Y. Evaluation of MR-Tractography Findings in Hemifacial Spasm Patients Injected with Botulinum Neurotoxin. Neurol. India 2022, 70, 543. [Google Scholar] [CrossRef]
- Lolekha, P.; Choolam, A.; Kulkantrakorn, K. A comparative crossover study on the treatment of hemifacial spasm and blepharospasm: Preseptal and pretarsal botulinum toxin injection techniques. Neurol. Sci. 2017, 38, 2031–2036. [Google Scholar] [CrossRef]
- Park, J.S.; Ahn, Y.H. Glossopharyngeal Neuralgia. J. Korean Neurosurg. Soc. 2022, 66, 12–23. [Google Scholar] [CrossRef]
- Zhao, Z.; Chai, S.; Xiao, D.; Zhou, Y.; Gan, J.; Jiang, X.; Zhao, H. Microscopic versus endoscopic microvascular decompression for the treatment of hemifacial spasm in China: A meta-analysis and systematic review. J. Clin. Neurosci. 2021, 91, 23–31. [Google Scholar] [CrossRef]
- Flanders, T.M.; Blue, R.; Roberts, S.; McShane, B.J.; Wilent, B.; Tambi, V.; Petrov, D.; Lee, J.Y.K. Fully endoscopic microvascular decompression for hemifacial spasm. J. Neurosurg. 2018, 131, 813–819. [Google Scholar] [CrossRef]
- Thirumala, P.D.; Altibi, A.M.; Chang, R.; Saca, E.E.; Iyengar, P.; Reddy, R.; Anetakis, K.; Crammond, D.J.; Balzer, J.R.; Sekula, R.F., Jr. The utility of intraoperative lateral spread recording in microvascular decompression for hemifacial spasm: A systematic review and meta-analysis. Neurosurgery 2020, 87, E473–E484. [Google Scholar] [CrossRef]

| Distinctive Feature | Tips for Operation | |
|---|---|---|
| Classic compression patterns | ||
| Loop type | Compression most commonly by PICA ¶ | Technically least challenging |
| Arachnoid type | Thickened arachnoid membrane |
|
| Perforator type | Tight perforators | Do not attempt to move the vessel off the REZ forcefully or excessively |
| Branch type | Caught between branches | Make sure the main trunk is moved off the REZ |
| Sandwich type | Two independent arteries from each side | After a successful decompression, always consider another possible source of compression |
| Tandem type | Larger vessel (most commonly VA β) compressing a smaller one | Trajectory of the larger vessel must be altered to accomplish a full decompression |
| Challenging patterns | ||
| Perforator type | Written above | Written above |
| Tandem type | Written above | Written above |
| Cisternal type | Compression on a cisternal portion of the facial nerve, instead of the REZ δ | Be careful not to excessively retract the cerebellum |
| Encircling type | More than 270° contact with the nerve | Decompression process should be carried out from medial to lateral side of the REZ |
| Penetrating type | Most rare (0.13%) | Extra care must be taken not to injure the facial nerve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Park, K. Operative Findings of over 5000 Microvascular Decompression Surgeries for Hemifacial Spasm: Our Perspective and Current Updates. Life 2023, 13, 1904. https://doi.org/10.3390/life13091904
Park JS, Park K. Operative Findings of over 5000 Microvascular Decompression Surgeries for Hemifacial Spasm: Our Perspective and Current Updates. Life. 2023; 13(9):1904. https://doi.org/10.3390/life13091904
Chicago/Turabian StylePark, Jae Sung, and Kwan Park. 2023. "Operative Findings of over 5000 Microvascular Decompression Surgeries for Hemifacial Spasm: Our Perspective and Current Updates" Life 13, no. 9: 1904. https://doi.org/10.3390/life13091904






