Metabolic and Bariatric Endoscopy: A Mini-Review
Abstract
:1. Background
Search Strategy
2. The Pathophysiology of Obesity and Weight Regain
3. Treatment Strategies for Obesity
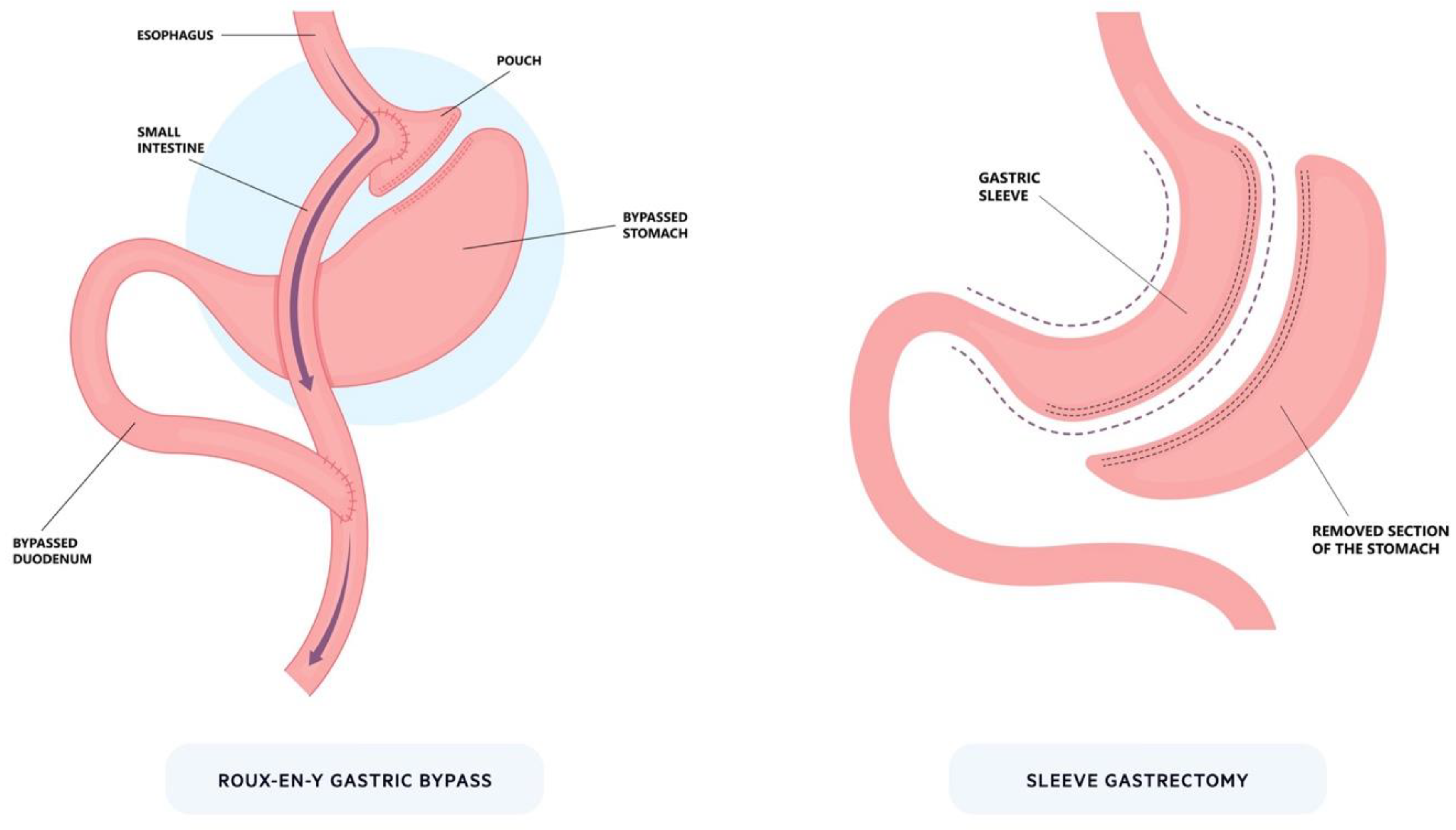
3.1. Surgery
3.2. Pharmacological
3.3. Endoscopy
4. Gastric Balloons
4.1. Effect on Weight Loss
4.2. Effect on Obesity-Related Complications
4.3. Combination Therapy
5. Gastric Remodelling
5.1. Endoscopy Devices
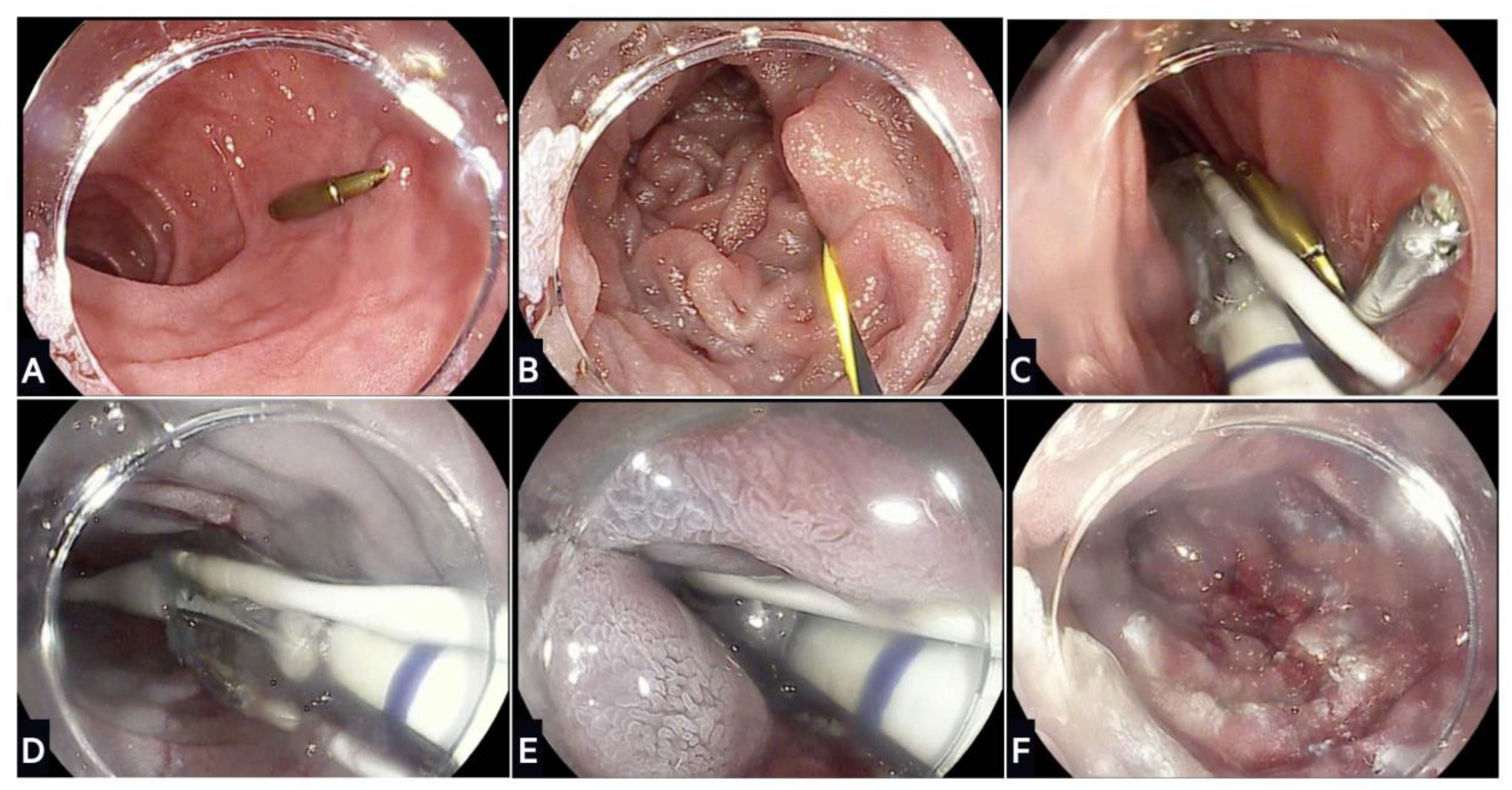
5.1.1. Apollo Overstitch
5.1.2. Primary Obesity Surgery Endoluminal
5.2. Effect on Weight Loss
5.3. Effect on Obesity-Related Complications
5.4. Gastric Remodelling Mechanisms
5.5. Combination Therapy
6. Duodenal Mucosal Resurfacing
6.1. REVITA System
6.2. Effect on Diabetes Mellitus
6.3. Experience beyond Diabetes
6.4. Combination Therapy
7. Transoral Outlet Reduction
7.1. Effect on Weight Loss
7.2. Effect on Dumping Syndrome
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Obesity and Overweight (WHO Fact Sheets); World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- NHS Digital. Statistics on Obesity, Physical Activity and Diet; NHS: Leeds, UK, 2021. [Google Scholar]
- Bjerregaard, L.G.; Jensen, B.W.; Angquist, L.; Osler, M.; Sorensen, T.I.A.; Baker, J.L. Change in Overweight from Childhood to Early Adulthood and Risk of Type 2 Diabetes. N. Engl. J. Med. 2018, 378, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Yaniv, G.; Levine, H.; Leiba, A.; Goldberger, N.; Derazne, E.; Ben-Ami Shor, D.; Tzur, D.; Afek, A.; Shamiss, A.; et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N. Engl. J. Med. 2016, 374, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E. Obesity and cancer. BMJ 2007, 335, 1107–1108. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Challenge of Obesity in the WHO European Region and the Strategies for Response; World Health Organisation: Geneva, Switzerland, 2012. [Google Scholar]
- Public Health England. Health Matters: Obesity and the Food Environment; Public Health England: London, UK, 2021.
- NICE. Obesity: Identification, Assessment and Management; CG189; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Abu Dayyeh, B.K.; Bazerbachi, F.; Vargas, E.J.; Sharaiha, R.Z.; Thompson, C.C.; Thaemert, B.C.; Teixeira, A.F.; Chapman, C.G.; Kumbhari, V.; Ujiki, M.B.; et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): A prospective, multicentre, randomised trial. Lancet 2022, 400, 441–451. [Google Scholar] [CrossRef]
- Miller, K.; Turro, R.; Greve, J.W.; Bakker, C.M.; Buchwald, J.N.; Espinos, J.C. MILEPOST Multicenter Randomized Controlled Trial: 12-Month Weight Loss and Satiety Outcomes After pose (SM) vs. Medical Therapy. Obes. Surg. 2017, 27, 310–322. [Google Scholar] [CrossRef]
- Sullivan, S.; Swain, J.M.; Woodman, G.; Antonetti, M.; De La Cruz-Munoz, N.; Jonnalagadda, S.S.; Ujiki, M.; Ikramuddin, S.; Ponce, J.; Ryou, M.; et al. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: The ESSENTIAL trial. Obesity 2017, 25, 294–301. [Google Scholar] [CrossRef]
- Lopez Nava, G.; Arau, R.T.; Asokkumar, R.; Maselli, D.B.; Rapaka, B.; Matar, R.; Bautista, I.; Espinos Perez, J.C.; Bilbao, A.M.; Jaruvongvanich, V.; et al. Prospective Multicenter Study of the Primary Obesity Surgery Endoluminal (POSE 2.0) Procedure for Treatment of Obesity. Clin. Gastroenterol. Hepatol. 2023, 21, 81–89.e4. [Google Scholar] [CrossRef]
- Huberty, V.; Boskoski, I.; Bove, V.; Van Ouytsel, P.; Costamagna, G.; Barthet, M.A.; Deviere, J. Endoscopic sutured gastroplasty in addition to lifestyle modification: Short-term efficacy in a controlled randomised trial. Gut 2021, 70, 1479–1485. [Google Scholar] [CrossRef]
- Kumar, N.; Bazerbachi, F.; Rustagi, T.; McCarty, T.R.; Thompson, C.C.; Galvao Neto, M.P.; Zundel, N.; Wilson, E.B.; Gostout, C.J.; Abu Dayyeh, B.K. The Influence of the Orbera Intragastric Balloon Filling Volumes on Weight Loss, Tolerability, and Adverse Events: A Systematic Review and Meta-Analysis. Obes. Surg. 2017, 27, 2272–2278. [Google Scholar] [CrossRef]
- ASGE Bariatric Endoscopy Task Force; ASGE Technology Committee; Abu Dayyeh, B.K.; Kumar, N.; Edmundowicz, S.A.; Jonnalagadda, S.; Larsen, M.; Sullivan, S.; Thompson, C.C.; Banerjee, S. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest. Endosc. 2015, 82, 425–438.e5. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Maselli, D.B.; Rapaka, B.; Lavin, T.; Noar, M.; Hussan, H.; Chapman, C.G.; Popov, V.; Jirapinyo, P.; Acosta, A.; et al. Adjustable intragastric balloon for treatment of obesity: A multicentre, open-label, randomised clinical trial. Lancet 2021, 398, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Ramai, D.; Singh, J.; Mohan, B.P.; Madedor, O.; Brooks, O.W.; Barakat, M.; Ofosu, A.; Khan, S.R.; Chandan, S.; Dhindsa, B.; et al. Influence of the Elipse Intragastric Balloon on Obesity and Metabolic Profile: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2021, 55, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.H.; Almutairi, R.; Elabd, R.; AlSabah, S.K.; Alqattan, H.; Altaweel, T. The Safety and Efficacy of Procedureless Gastric Balloon: A Study Examining the Effect of Elipse Intragastric Balloon Safety, Short and Medium Term Effects on Weight Loss with 1-Year Follow-Up Post-removal. Obes. Surg. 2019, 29, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, E.; Krekshi, W.; Matia, P.; Hermida, C.; de la Torre, N.G.; Cabrerizo, L.; Rubio, M.A. Effectiveness and safety of air-filled balloon Heliosphere BAG(R) in 82 consecutive obese patients. Obes. Surg. 2011, 21, 1508–1512. [Google Scholar] [CrossRef]
- Sullivan, S.; Swain, J.; Woodman, G.; Edmundowicz, S.; Hassanein, T.; Shayani, V.; Fang, J.C.; Noar, M.; Eid, G.; English, W.J.; et al. Randomized sham-controlled trial of the 6-month swallowable gas-filled intragastric balloon system for weight loss. Surg. Obes. Relat. Dis. 2018, 14, 1876–1889. [Google Scholar] [CrossRef]
- Ponce, J.; Woodman, G.; Swain, J.; Wilson, E.; English, W.; Ikramuddin, S.; Bour, E.; Edmundowicz, S.; Snyder, B.; Soto, F.; et al. The REDUCE pivotal trial: A prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg. Obes. Relat. Dis. 2015, 11, 874–881. [Google Scholar] [CrossRef]
- ENDObesity® II Study: TransPyloric Shuttle® System for Weight Loss. (NCT02518685). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02518685 (accessed on 7 September 2023).
- Thompson, C.C.; Abu Dayyeh, B.K.; Kushner, R.; Sullivan, S.; Schorr, A.B.; Amaro, A.; Apovian, C.M.; Fullum, T.; Zarrinpar, A.; Jensen, M.D.; et al. Percutaneous Gastrostomy Device for the Treatment of Class II and Class III Obesity: Results of a Randomized Controlled Trial. Am. J. Gastroenterol. 2017, 112, 447–457. [Google Scholar] [CrossRef]
- Van Baar, A.C.G.; Haidry, R.; Rodriguez Grunert, L.; Galvao, M.P.N.; Bisschops, R.; Hayee, B.H.; Costamagna, G.; Deviere, J.; Bergman, J. Duodenal mucosal resurfacing: Multicenter experience implementing a minimally invasive endoscopic procedure for treatment of type 2 diabetes mellitus. Endosc. Int. Open 2020, 8, E1683–E1689. [Google Scholar] [CrossRef]
- Mingrone, G.; van Baar, A.C.; Deviere, J.; Hopkins, D.; Moura, E.; Cercato, C.; Rajagopalan, H.; Lopez-Talavera, J.C.; White, K.; Bhambhani, V.; et al. Safety and efficacy of hydrothermal duodenal mucosal resurfacing in patients with type 2 diabetes: The randomised, double-blind, sham-controlled, multicentre REVITA-2 feasibility trial. Gut 2022, 71, 254–264. [Google Scholar] [CrossRef]
- Busch, C.M.; Van Baar, A.S.; Holleman, F.; Nieuwdorp, M.; Bergman, J. Re-Cellularization via Electroporation Therapy (RECET) Combined with GLP-1RA to Replace Insulin Therapy in Patients with Type 2 Diabetes 6 Months Results of the Eminent Study. Gastrointest. Endosc. 2023, 97, AB298. [Google Scholar] [CrossRef]
- Koehestanie, P.; de Jonge, C.; Berends, F.J.; Janssen, I.M.; Bouvy, N.D.; Greve, J.W. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann. Surg. 2014, 260, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Jirapinyo, P.; Haas, A.V.; Thompson, C.C. Effect of the Duodenal-Jejunal Bypass Liner on Glycemic Control in Patients With Type 2 Diabetes With Obesity: A Meta-analysis With Secondary Analysis on Weight Loss and Hormonal Changes. Diabetes Care 2018, 41, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Machytka, E.; Buzga, M.; Zonca, P.; Lautz, D.B.; Ryou, M.; Simonson, D.C.; Thompson, C.C. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest. Endosc. 2017, 86, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Chand, B.; Chen, Y.K.; DeMarco, D.C.; Miller, L.; Schweitzer, M.; Rothstein, R.I.; Lautz, D.B.; Slattery, J.; Ryan, M.B.; et al. Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology 2013, 145, 129–137.e3. [Google Scholar] [CrossRef]
- Vargas, E.J.; Bazerbachi, F.; Rizk, M.; Rustagi, T.; Acosta, A.; Wilson, E.B.; Wilson, T.; Neto, M.G.; Zundel, N.; Mundi, M.S.; et al. Transoral outlet reduction with full thickness endoscopic suturing for weight regain after gastric bypass: A large multicenter international experience and meta-analysis. Surg. Endosc. 2018, 32, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Gurian, G.C.; Watanabe, L.M.; Nonino, C.B.; Barato, M.; Ferreira-Julio, M.A.; Arantes, F.A.; Sivieri, T.; Noronha, N.Y.; Souza, D.S.R.; Fernandes-Ferreira, R.; et al. Efficacy of the argon plasma coagulation in patients with weight regain after gastric bypass: A randomized control trial. Endosc. Int. Open 2023, 11, E43–E51. [Google Scholar] [CrossRef]
- Maselli, D.B.; Alqahtani, A.R.; Abu Dayyeh, B.K.; Elahmedi, M.; Storm, A.C.; Matar, R.; Nieto, J.; Teixeira, A.; Al Khatry, M.; Neto, M.G.; et al. Revisional endoscopic sleeve gastroplasty of laparoscopic sleeve gastrectomy: An international, multicenter study. Gastrointest. Endosc. 2021, 93, 122–130. [Google Scholar] [CrossRef]
- Busetto, L.; Bettini, S.; Makaronidis, J.; Roberts, C.A.; Halford, J.C.G.; Batterham, R.L. Mechanisms of weight regain. Eur. J. Intern. Med. 2021, 93, 3–7. [Google Scholar] [CrossRef]
- Duca, F.A.; Waise, T.M.Z.; Peppler, W.T.; Lam, T.K.T. The metabolic impact of small intestinal nutrient sensing. Nat. Commun. 2021, 12, 903. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Vilarino-Garcia, T.; Fernandez-Riejos, P.; Martin-Gonzalez, J.; Segura-Egea, J.J.; Sanchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Rubino, F.; Forgione, A.; Cummings, D.E.; Vix, M.; Gnuli, D.; Mingrone, G.; Castagneto, M.; Marescaux, J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann. Surg. 2006, 244, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, V.; Dirinck, E.; Fandriks, L.; Maleckas, A.; le Roux, C.W.; Thorell, A. Glycemic Control after Sleeve Gastrectomy and Roux-En-Y Gastric Bypass in Obese Subjects with Type 2 Diabetes Mellitus. Obes. Surg. 2018, 28, 1461–1472. [Google Scholar] [CrossRef]
- Peterli, R.; Wolnerhanssen, B.; Peters, T.; Devaux, N.; Kern, B.; Christoffel-Courtin, C.; Drewe, J.; von Flue, M.; Beglinger, C. Improvement in glucose metabolism after bariatric surgery: Comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: A prospective randomized trial. Ann. Surg. 2009, 250, 234–241. [Google Scholar] [CrossRef]
- Stimac, D.; Klobucar Majanovic, S.; Belancic, A. Endoscopic Treatment of Obesity: From Past to Future. Dig. Dis. 2020, 38, 150–162. [Google Scholar] [CrossRef]
- Wadden, T.A.; McGuckin, B.G.; Rothman, R.A.; Sargent, S.L. Lifestyle modification in the management of obesity. J. Gastrointest. Surg. 2003, 7, 452–463. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef]
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Shoar, S.; Saber, A.A. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: A systematic review and meta-analysis of comparative studies. Surg. Obes. Relat. Dis. 2017, 13, 170–180. [Google Scholar] [CrossRef]
- Aminian, A.; Kashyap, S.R.; Wolski, K.E.; Brethauer, S.A.; Kirwan, J.P.; Nissen, S.E.; Bhatt, D.L.; Schauer, P.R. Patient-reported Outcomes After Metabolic Surgery Versus Medical Therapy for Diabetes: Insights from the STAMPEDE Randomized Trial. Ann. Surg. 2021, 274, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Mulla, C.M.; Middelbeek, R.J.W.; Patti, M.E. Mechanisms of weight loss and improved metabolism following bariatric surgery. Ann. N. Y. Acad. Sci. 2018, 1411, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Haluzik, M.; Kratochvilova, H.; Haluzikova, D.; Mraz, M. Gut as an emerging organ for the treatment of diabetes: Focus on mechanism of action of bariatric and endoscopic interventions. J. Endocrinol. 2018, 237, R1–R17. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Bode, B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res. Clin. Pract. 2012, 97, 27–42. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Wadden, T.A.; Tronieri, J.S.; Sugimoto, D.; Lund, M.T.; Auerbach, P.; Jensen, C.; Rubino, D. Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: The SCALE IBT Randomized Controlled Trial. Obesity 2020, 28, 529–536. [Google Scholar] [CrossRef]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjoth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; Group, N.N.S. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA 2015, 314, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Hollander, P.; Klein, S.; Niswender, K.; Woo, V.; Hale, P.M.; Aronne, L.; Investigators, N.N. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int. J. Obes. 2013, 37, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Christou, G.A.; Katsiki, N.; Blundell, J.; Fruhbeck, G.; Kiortsis, D.N. Semaglutide as a promising antiobesity drug. Obes. Rev. 2019, 20, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Faerch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sorrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; Investigators, S. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef]
- Weiss, T.; Yang, L.; Carr, R.D.; Pal, S.; Sawhney, B.; Boggs, R.; Rajpathak, S.; Iglay, K. Real-world weight change, adherence, and discontinuation among patients with type 2 diabetes initiating glucagon-like peptide-1 receptor agonists in the UK. BMJ Open Diabetes Res. Care 2022, 10, e002517. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef]
- Kelly, J.; Menon, V.; O’Neill, F.; Elliot, L.; Combe, E.; Drinkwater, W.; Abbott, S.; Hayee, B. UK cost-effectiveness analysis of endoscopic sleeve gastroplasty versus lifestyle modification alone for adults with class II obesity. Int. J. Obes. 2023. [Google Scholar] [CrossRef]
- Saumoy, M.; Schneider, Y.; Novikov, A.A.; Afaneh, C.; Shukla, A.; Tyberg, A.; Aronne, L.J.; Kahaleh, M.; Sharaiha, R.Z. A cost-utility analysis comparing endoscopic, surgical and lifestyle management of obesity. Gastroenterology 2017, 152, S831–S832. [Google Scholar] [CrossRef]
- Hogan, R.B.; Johnston, J.H.; Long, B.W.; Sones, J.Q.; Hinton, L.A.; Bunge, J.; Corrigan, S.A. A double-blind, randomized, sham-controlled trial of the gastric bubble for obesity. Gastrointest. Endosc. 1989, 35, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.B.; Maher, K.A.; Cattau, E.L., Jr.; Collen, M.J.; Fleischer, D.E.; Lewis, J.H.; Ciarleglio, C.A.; Earll, J.M.; Schaffer, S.; Mirkin, K.; et al. Double-blind controlled trial of the Garren-Edwards gastric bubble: An adjunctive treatment for exogenous obesity. Gastroenterology 1988, 95, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Hughes, R.W., Jr.; Ilstrup, D.M.; Jensen, M.D. Intragastric balloons in comparison with standard therapy for obesity--a randomized, double-blind trial. Mayo Clin. Proc. 1987, 62, 992–996. [Google Scholar] [CrossRef]
- Benjamin, S.B. Small bowel obstruction and the Garren-Edwards gastric bubble: An iatrogenic bezoar. Gastrointest. Endosc. 1988, 34, 463–467. [Google Scholar] [CrossRef]
- Schapiro, M.; Benjamin, S.; Blackburn, G.; Frank, B.; Heber, D.; Kozarek, R.; Randall, S.; Stern, W. Obesity and the gastric balloon: A comprehensive workshop. Tarpon Springs, Florida, March 19-21, 1987. Gastrointest. Endosc. 1987, 33, 323–327. [Google Scholar] [CrossRef]
- Saber, A.A.; Shoar, S.; Almadani, M.W.; Zundel, N.; Alkuwari, M.J.; Bashah, M.M.; Rosenthal, R.J. Efficacy of First-Time Intragastric Balloon in Weight Loss: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2017, 27, 277–287. [Google Scholar] [CrossRef]
- Bazerbachi, F.; Haffar, S.; Sawas, T.; Vargas, E.J.; Kaur, R.J.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Abu Dayyeh, B.K. Fluid-Filled Versus Gas-Filled Intragastric Balloons as Obesity Interventions: A Network Meta-analysis of Randomized Trials. Obes. Surg. 2018, 28, 2617–2625. [Google Scholar] [CrossRef]
- Layec, S.; Val-Laillet, D.; Heresbach, D.; Malbert, C.H. Gastric tone, volume and emptying after implantation of an intragastric balloon for weight control. Neurogastroenterol. Motil. 2010, 22, 1016-e266. [Google Scholar] [CrossRef]
- Mion, F.; Napoleon, B.; Roman, S.; Malvoisin, E.; Trepo, F.; Pujol, B.; Lefort, C.; Bory, R.M. Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. Obes. Surg. 2005, 15, 510–516. [Google Scholar] [CrossRef]
- Hussain, S.S.; Bloom, S.R. The regulation of food intake by the gut-brain axis: Implications for obesity. Int. J. Obes. 2013, 37, 625–633. [Google Scholar] [CrossRef]
- Muniraj, T.; Day, L.W.; Teigen, L.M.; Ho, E.Y.; Sultan, S.; Davitkov, P.; Shah, R.; Murad, M.H. AGA Clinical Practice Guidelines on Intragastric Balloons in the Management of Obesity. Gastroenterology 2021, 160, 1799–1808. [Google Scholar] [CrossRef]
- Neto, M.G.; Silva, L.B.; Grecco, E.; de Quadros, L.G.; Teixeira, A.; Souza, T.; Scarparo, J.; Parada, A.A.; Dib, R.; Moon, R.; et al. Brazilian Intragastric Balloon Consensus Statement (BIBC): Practical guidelines based on experience of over 40,000 cases. Surg. Obes. Relat. Dis. 2018, 14, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Espinet Coll, E.; Del Pozo Garcia, A.J.; Turro Arau, R.; Nebreda Duran, J.; Cortes Rizo, X.; Serrano Jimenez, A.; Escarti Uso, M.A.; Munoz Tornero, M.; Carral Martinez, D.; Bernabeu Lopez, J.; et al. Spanish Intragastric Balloon Consensus Statement (SIBC): Practical guidelines based on experience of over 20,000 cases. Rev. Esp. Enferm. Dig. 2023, 115, 22–34. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Swallowable Gastric Balloon Capsule for Weight Loss; NICE Guidelines, Interventional Procedures Guidance [IPG684]; NICE: London, UK, 2020. [Google Scholar]
- Kotzampassi, K.; Grosomanidis, V.; Papakostas, P.; Penna, S.; Eleftheriadis, E. 500 intragastric balloons: What happens 5 years thereafter? Obes. Surg. 2012, 22, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Cruz, J.R.; Mui, W.L.; Wong, S.K.H.; Ng, E.K.W. Outcomes with Intra-gastric Balloon Therapy in BMI < 35 Non-morbid Obesity: 10-Year Follow-Up Study of an RCT. Obes. Surg. 2021, 31, 781–786. [Google Scholar]
- Moore, R.L.; Eaton, L.; Ellner, J. Safety and Effectiveness of an Intragastric Balloon as an Adjunct to Weight Reduction in a Post-Marketing Clinical Setting. Obes. Surg. 2020, 30, 4267–4274. [Google Scholar] [CrossRef]
- Stavrou, G.; Shrewsbury, A.; Kotzampassi, K. Six intragastric balloons: Which to choose? World J. Gastrointest. Endosc. 2021, 13, 238–259. [Google Scholar] [CrossRef]
- Fittipaldi-Fernandez, R.J.; Zotarelli-Filho, I.J.; Diestel, C.F.; Klein, M.; de Santana, M.F.; de Lima, J.H.F.; Bastos, F.S.S.; Dos Santos, N.T. Randomized Prospective Clinical Study of Spatz3(R) Adjustable Intragastric Balloon Treatment with a Control Group: A Large-Scale Brazilian Experiment. Obes. Surg. 2021, 31, 787–796. [Google Scholar] [CrossRef]
- NICE. Swallowable Gastric Balloon Capsule for Weight Loss; ([IPG684]); National Institute for Health and Care Excellence: London, UK, 2020. [Google Scholar]
- Mion, F.; Ibrahim, M.; Marjoux, S.; Ponchon, T.; Dugardeyn, S.; Roman, S.; Deviere, J. Swallowable Obalon(R) gastric balloons as an aid for weight loss: A pilot feasibility study. Obes. Surg. 2013, 23, 730–733. [Google Scholar] [CrossRef]
- De Castro, M.L.; Morales, M.J.; Del Campo, V.; Pineda, J.R.; Pena, E.; Sierra, J.M.; Arbones, M.J.; Prada, I.R. Efficacy, safety, and tolerance of two types of intragastric balloons placed in obese subjects: A double-blind comparative study. Obes. Surg. 2010, 20, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, C.; Borrelli, A.; Silvestri, E.; Antognozzi, V.; Iodice, G.; Lorenzo, M. Air-filled vs water-filled intragastric balloon: A prospective randomized study. Obes. Surg. 2012, 22, 1916–1919. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.B.; Thompson, C.C.; Kumar, N.; Ciarleglio, M.M.; Deng, Y.; Laine, L. Effect of Intragastric Balloons on Liver Enzymes: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2016, 61, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Low, H.C.; Lim, L.G.; Dan, Y.Y.; Aung, M.O.; Cheng, C.L.; Wee, A.; Lim, S.G.; Ho, K.Y. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: A pilot study. Gastrointest. Endosc. 2012, 76, 756–760. [Google Scholar] [CrossRef]
- Bazerbachi, F.; Vargas, E.J.; Rizk, M.; Maselli, D.B.; Mounajjed, T.; Venkatesh, S.K.; Watt, K.D.; Port, J.D.; Basu, R.; Acosta, A.; et al. Intragastric Balloon Placement Induces Significant Metabolic and Histologic Improvement in Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2021, 19, 146–154.e144. [Google Scholar] [CrossRef]
- Chandan, S.; Mohan, B.P.; Khan, S.R.; Facciorusso, A.; Ramai, D.; Kassab, L.L.; Bhogal, N.; Asokkumar, R.; Lopez-Nava, G.; McDonough, S.; et al. Efficacy and Safety of Intragastric Balloon (IGB) in Non-alcoholic Fatty Liver Disease (NAFLD): A Comprehensive Review and Meta-analysis. Obes. Surg. 2021, 31, 1271–1279. [Google Scholar] [CrossRef]
- Alami, R.S.; Morton, J.M.; Schuster, R.; Lie, J.; Sanchez, B.R.; Peters, A.; Curet, M.J. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg. Obes. Relat. Dis. 2007, 3, 141–145, discussion 145–146. [Google Scholar] [CrossRef]
- Van Nieuwenhove, Y.; Dambrauskas, Z.; Campillo-Soto, A.; van Dielen, F.; Wiezer, R.; Janssen, I.; Kramer, M.; Thorell, A. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study. Arch. Surg. 2011, 146, 1300–1305. [Google Scholar] [CrossRef]
- Coffin, B.; Maunoury, V.; Pattou, F.; Hebuterne, X.; Schneider, S.; Coupaye, M.; Ledoux, S.; Iglicki, F.; Mion, F.; Robert, M.; et al. Impact of Intragastric Balloon Before Laparoscopic Gastric Bypass on Patients with Super Obesity: A Randomized Multicenter Study. Obes. Surg. 2017, 27, 902–909. [Google Scholar] [CrossRef]
- Loo, J.H.; Lim, Y.H.; Seah, H.L.; Chong, A.Z.Q.; Tay, K.V. Intragastric Balloon as Bridging Therapy Prior to Bariatric Surgery for Patients with Severe Obesity (BMI >/= 50 kg/m2): A Systematic Review and Meta-analysis. Obes. Surg. 2022, 32, 489–502. [Google Scholar] [CrossRef]
- Mehta, A.; Shah, S.; Dawod, E.; Hajifathalian, K.; Kumar, R.; Igel, L.I.; Saunders, K.H.; Kumbhari, V.; Farha, J.; Badurdeen, D.; et al. Impact of Adjunctive Pharmacotherapy With Intragastric Balloons for the Treatment of Obesity. Am. Surg. 2023, 89, 707–713. [Google Scholar] [CrossRef]
- Mosli, M.M.; Elyas, M. Does combining liraglutide with intragastric balloon insertion improve sustained weight reduction? Saudi J. Gastroenterol. 2017, 23, 117–122. [Google Scholar] [CrossRef]
- Saunders, K.H.; Igel, L.I.; Saumoy, M.; Sharaiha, R.Z.; Aronne, L.J. Devices and Endoscopic Bariatric Therapies for Obesity. Curr. Obes. Rep. 2018, 7, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Nicolle, H.; Sahdala, P.a.; Shaikh, S.; Wilson, E.B.; Manoel, G.N.; Zundel, N.; Thompson, C.C. Mo1155 Endoscopic Sleeve Gastroplasty for Primary Therapy of Obesity: Initial Human Cases. Gastroenterology 2014, 146, S-571–S-572. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Rajan, E.; Gostout, C.J. Endoscopic sleeve gastroplasty: A potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest. Endosc. 2013, 78, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Espinet-Coll, E.; Nebreda-Duran, J.; Galvao-Neto, M.; Bautista-Altamirano, C.; Diaz-Galan, P.; Gomez-Valero, J.A.; Vila-Lolo, C.; Guirola-Puche, M.A.; Fernandez-Huelamo, A.; Bargallo-Carulla, D.; et al. Suture pattern does not influence outcomes of endoscopic sleeve gastroplasty in obese patients. Endosc. Int. Open 2020, 8, E1349–E1358. [Google Scholar] [CrossRef] [PubMed]
- Maselli, R.; Palma, R.; Traina, M.; Granata, A.; Juzgado, D.; Bisello, M.; Neuhaus, H.; Beyna, T.; Bansi, D.; Flor, L.; et al. Endoscopic suturing for GI applications: Initial results from a prospective multicenter European registry. Gastrointest. Endosc. 2022, 96, 780–786. [Google Scholar] [CrossRef]
- Espinos, J.C.; Turro, R.; Mata, A.; Cruz, M.; da Costa, M.; Villa, V.; Buchwald, J.N.; Turro, J. Early experience with the Incisionless Operating Platform (IOP) for the treatment of obesity: The Primary Obesity Surgery Endolumenal (POSE) procedure. Obes. Surg. 2013, 23, 1375–1383. [Google Scholar] [CrossRef]
- Dayyeh, B.K.A.; Lopez-Nava, G.; Neto, M.G.; Vargas, E.J.; Saleh, T. Greater gastric curvature endoscopic tubularization using snowshoe anchors for weight loss: A first inhuman pilot prospective feasibility study of the POSE 2.0 procedure. Gastrointest. Endosc. 2019, 89, AB266. [Google Scholar] [CrossRef]
- Singh, S.; Hourneaux de Moura, D.T.; Khan, A.; Bilal, M.; Ryan, M.B.; Thompson, C.C. Safety and efficacy of endoscopic sleeve gastroplasty worldwide for treatment of obesity: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2020, 16, 340–351. [Google Scholar] [CrossRef]
- Hedjoudje, A.; Abu Dayyeh, B.K.; Cheskin, L.J.; Adam, A.; Neto, M.G.; Badurdeen, D.; Morales, J.G.; Sartoretto, A.; Nava, G.L.; Vargas, E.; et al. Efficacy and Safety of Endoscopic Sleeve Gastroplasty: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 1043–1053.e4. [Google Scholar] [CrossRef] [PubMed]
- Due-Petersson, R.; Poulsen, I.M.; Hedback, N.; Karstensen, J.G. Effect and safety of endoscopic sleeve gastroplasty for treating obesity—A systematic review. Dan. Med. J. 2020, 67, A05200359. [Google Scholar]
- Sharaiha, R.Z.; Hajifathalian, K.; Kumar, R.; Saunders, K.; Mehta, A.; Ang, B.; Skaf, D.; Shah, S.; Herr, A.; Igel, L.; et al. Five-Year Outcomes of Endoscopic Sleeve Gastroplasty for the Treatment of Obesity. Clin. Gastroenterol. Hepatol. 2021, 19, 1051–1057.e2. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Kosta, S.; Reddy, M.; Mathur, W.; Neto, M.G.; Bhandari, M. Four-year outcomes for endoscopic sleeve gastroplasty from a single centre in India. J. Minim. Access Surg. 2023, 19, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; de Moura, D.T.H.; Khan, A.; Bilal, M.; Chowdhry, M.; Ryan, M.B.; Bazarbashi, A.N.; Thompson, C.C. Intragastric Balloon Versus Endoscopic Sleeve Gastroplasty for the Treatment of Obesity: A Systematic Review and Meta-analysis. Obes. Surg. 2020, 30, 3010–3029. [Google Scholar] [CrossRef] [PubMed]
- Marincola, G.; Gallo, C.; Hassan, C.; Raffaelli, M.; Costamagna, G.; Bove, V.; Pontecorvi, V.; Orlandini, B.; Boskoski, I. Laparoscopic sleeve gastrectomy versus endoscopic sleeve gastroplasty: A systematic review and meta-analysis. Endosc. Int. Open 2021, 9, E87–E95. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.R.; Elahmedi, M.; Aldarwish, A.; Abdurabu, H.Y.; Alqahtani, S. Endoscopic gastroplasty versus laparoscopic sleeve gastrectomy: A noninferiority propensity score-matched comparative study. Gastrointest. Endosc. 2022, 96, 44–50. [Google Scholar] [CrossRef]
- Lopez Nava, G.; Asokkumar, R.; Laster, J.; Negi, A.; Normand, E.; Fook-Chong, S.; Bautista-Castano, I. Primary obesity surgery endoluminal (POSE-2) procedure for treatment of obesity in clinical practice. Endoscopy 2021, 53, 1169–1173. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Kumta, N.A.; Saumoy, M.; Desai, A.P.; Sarkisian, A.M.; Benevenuto, A.; Tyberg, A.; Kumar, R.; Igel, L.; Verna, E.C.; et al. Endoscopic Sleeve Gastroplasty Significantly Reduces Body Mass Index and Metabolic Complications in Obese Patients. Clin. Gastroenterol. Hepatol. 2017, 15, 504–510. [Google Scholar] [CrossRef]
- Alqahtani, A.; Al-Darwish, A.; Mahmoud, A.E.; Alqahtani, Y.A.; Elahmedi, M. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest. Endosc. 2019, 89, 1132–1138. [Google Scholar] [CrossRef]
- Jagtap, N.; Kalapala, R.; Katakwar, A.; Sharma, M.; Aslam, M.; Gupta, R.; Rao, P.N.; Goud, R.; Tandan, M.; Kanakagiri, H.; et al. Endoscopic sleeve gastroplasty—Minimally invasive treatment for non-alcoholic fatty liver disease and obesity. Indian J. Gastroenterol. 2021, 40, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Hajifathalian, K.; Mehta, A.; Ang, B.; Skaf, D.; Shah, S.L.; Saumoy, M.; Dawod, Q.; Dawod, E.; Shukla, A.; Aronne, L.; et al. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest. Endosc. 2021, 93, 1110–1118. [Google Scholar] [CrossRef]
- Lavin-Alconero, L.; Fernandez-Lanas, T.; Iruzubieta-Coz, P.; Arias-Loste, M.T.; Rodriguez-Duque, J.C.; Rivas, C.; Cagigal, M.L.; Montalban, C.; Useros, A.L.; Alvarez-Cancelo, A.; et al. Efficacy and safety of endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy in obese subjects with Non-Alcoholic SteatoHepatitis (NASH): Study protocol for a randomized controlled trial (TESLA-NASH study). Trials 2021, 22, 756. [Google Scholar] [CrossRef] [PubMed]
- Abu Dayyeh, B.K.; Acosta, A.; Camilleri, M.; Mundi, M.S.; Rajan, E.; Topazian, M.D.; Gostout, C.J. Endoscopic Sleeve Gastroplasty Alters Gastric Physiology and Induces Loss of Body Weight in Obese Individuals. Clin. Gastroenterol. Hepatol. 2017, 15, 37–43 e31. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.J.; Rizk, M.; Gomez-Villa, J.; Edwards, P.K.; Jaruvongvanich, V.; Storm, A.C.; Acosta, A.; Lake, D.; Fidler, J.; Bharucha, A.E.; et al. Effect of endoscopic sleeve gastroplasty on gastric emptying, motility and hormones: A comparative prospective study. Gut 2023, 72, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ding, X.; Fu, H.; Cai, Q. Potential mechanisms of sleeve gastrectomy for reducing weight and improving metabolism in patients with obesity. Surg. Obes. Relat. Dis. 2019, 15, 1861–1871. [Google Scholar] [CrossRef]
- Kalinowski, P.; Paluszkiewicz, R.; Wroblewski, T.; Remiszewski, P.; Grodzicki, M.; Bartoszewicz, Z.; Krawczyk, M. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass-results of a randomized clinical trial. Surg. Obes. Relat. Dis. 2017, 13, 181–188. [Google Scholar] [CrossRef]
- Li, G.; Ji, G.; Hu, Y.; Liu, L.; Jin, Q.; Zhang, W.; Liu, L.; Wang, Y.; Zhao, J.; von Deneen, K.M.; et al. Reduced plasma ghrelin concentrations are associated with decreased brain reactivity to food cues after laparoscopic sleeve gastrectomy. Psychoneuroendocrinology 2019, 100, 229–236. [Google Scholar] [CrossRef]
- Tsoli, M.; Chronaiou, A.; Kehagias, I.; Kalfarentzos, F.; Alexandrides, T.K. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: A comparative prospective study. Surg. Obes. Relat. Dis. 2013, 9, 667–677. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Daskalakis, M.; Kampa, M.; Peppe, A.; Papadakis, J.A.; Melissas, J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: A prospective clinical and laboratory investigational study. Ann. Surg. 2013, 257, 647–654. [Google Scholar] [CrossRef]
- Vargas, E.J.; Bazerbachi, F.; Calderon, G.; Prokop, L.J.; Gomez, V.; Murad, M.H.; Acosta, A.; Camilleri, M.; Abu Dayyeh, B.K. Changes in Time of Gastric Emptying After Surgical and Endoscopic Bariatrics and Weight Loss: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 57–68 e55. [Google Scholar] [CrossRef]
- Rhee, N.A.; Vilsboll, T.; Knop, F.K. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Negi, A.; Bautista-Castano, I.; Rubio, M.A.; Asokkumar, R. Gut and Metabolic Hormones Changes After Endoscopic Sleeve Gastroplasty (ESG) Vs. Laparoscopic Sleeve Gastrectomy (LSG). Obes. Surg. 2020, 30, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Badurdeen, D.; Hoff, A.C.; Hedjoudje, A.; Adam, A.; Itani, M.I.; Farha, J.; Abbarh, S.; Kalloo, A.N.; Khashab, M.A.; Singh, V.K.; et al. Endoscopic sleeve gastroplasty plus liraglutide versus endoscopic sleeve gastroplasty alone for weight loss. Gastrointest. Endosc. 2021, 93, 1316–1324 e1311. [Google Scholar] [CrossRef]
- Alqahtani, A.R.; Elahmedi, M.; Alqahtani, Y.A.; Al-Darwish, A. Laparoscopic Sleeve Gastrectomy After Endoscopic Sleeve Gastroplasty: Technical Aspects and Short-Term Outcomes. Obes. Surg. 2019, 29, 3547–3552. [Google Scholar] [CrossRef]
- Pories, W.J.; Caro, J.F.; Flickinger, E.G.; Meelheim, H.D.; Swanson, M.S. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann. Surg. 1987, 206, 316–323. [Google Scholar] [CrossRef]
- Herbst, C.A.; Hughes, T.A.; Gwynne, J.T.; Buckwalter, J.A. Gastric bariatric operation in insulin-treated adults. Surgery 1984, 95, 209–214. [Google Scholar] [PubMed]
- Courcoulas, A.P.; Belle, S.H.; Neiberg, R.H.; Pierson, S.K.; Eagleton, J.K.; Kalarchian, M.A.; DeLany, J.P.; Lang, W.; Jakicic, J.M. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015, 150, 931–940. [Google Scholar] [CrossRef]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Madsen, L.R.; Baggesen, L.M.; Richelsen, B.; Thomsen, R.W. Effect of Roux-en-Y gastric bypass surgery on diabetes remission and complications in individuals with type 2 diabetes: A Danish population-based matched cohort study. Diabetologia 2019, 62, 611–620. [Google Scholar] [CrossRef]
- Shimizu, H.; Eldar, S.; Heneghan, H.M.; Schauer, P.R.; Kirwan, J.P.; Brethauer, S.A. The effect of selective gut stimulation on glucose metabolism after gastric bypass in the Zucker diabetic fatty rat model. Surg. Obes. Relat. Dis. 2014, 10, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Haidry, R.J.; van Baar, A.C.; Galvao Neto, M.P.; Rajagopalan, H.; Caplan, J.; Levin, P.S.; Bergman, J.J.; Rodriguez, L.; Deviere, J.; Thompson, C.C. Duodenal mucosal resurfacing: Proof-of-concept, procedural development, and initial implementation in the clinical setting. Gastrointest. Endosc. 2019, 90, 673–681 e672. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, H.; Cherrington, A.D.; Thompson, C.C.; Kaplan, L.M.; Rubino, F.; Mingrone, G.; Becerra, P.; Rodriguez, P.; Vignolo, P.; Caplan, J.; et al. Endoscopic Duodenal Mucosal Resurfacing for the Treatment of Type 2 Diabetes: 6-Month Interim Analysis From the First-in-Human Proof-of-Concept Study. Diabetes Care 2016, 39, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Van Baar, A.C.G.; Devière, J.; Hopkins, D.; Crenier, L.; Holleman, F.; Galvão Neto, M.P.; Becerra, P.; Vignolo, P.; Rodriguez Grunert, L.; Mingrone, G.; et al. Durable metabolic improvements 2 years after duodenal mucosal resurfacing (DMR) in patients with type 2 diabetes (REVITA-1 Study). Diabetes Res. Clin. Pract. 2022, 184, 109194. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Dimitriadis, G.K.; Perez-Pevida, B.; Bansi, D.S.; Jayasena, C.; Bate, D.; Houghton, R.; Fielding, B.A.; Balfoussia, D.; Webber, L.; et al. Mechanisms of action of duodenal mucosal resurfacing in insulin resistant women with polycystic ovary syndrome. Metabolism 2021, 125, 154908. [Google Scholar] [CrossRef]
- Van Baar, A.C.G.; Meiring, S.; Smeele, P.; Vriend, T.; Holleman, F.; Barlag, M.; Mostafavi, N.; Tijssen, J.G.P.; Soeters, M.R.; Nieuwdorp, M.; et al. Duodenal mucosal resurfacing combined with glucagon-like peptide-1 receptor agonism to discontinue insulin in type 2 diabetes: A feasibility study. Gastrointest. Endosc. 2021, 94, 111–120 e113. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Lautz, D.B.; Thompson, C.C. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin. Gastroenterol. Hepatol. 2011, 9, 228–233. [Google Scholar] [CrossRef]
- Ramadan, M.; Loureiro, M.; Laughlan, K.; Caiazzo, R.; Iannelli, A.; Brunaud, L.; Czernichow, S.; Nedelcu, M.; Nocca, D. Risk of Dumping Syndrome after Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: Early Results of a Multicentre Prospective Study. Gastroenterol. Res. Pract. 2016, 2016, 2570237. [Google Scholar] [CrossRef]
- Coakley, B.A.; Deveney, C.W.; Spight, D.H.; Thompson, S.K.; Le, D.; Jobe, B.A.; Wolfe, B.M.; McConnell, D.B.; O’Rourke, R.W. Revisional bariatric surgery for failed restrictive procedures. Surg. Obes. Relat. Dis. 2008, 4, 581–586. [Google Scholar] [CrossRef]
- Schulman, A.R.; Kumar, N.; Thompson, C.C. Transoral outlet reduction: A comparison of purse-string with interrupted stitch technique. Gastrointest. Endosc. 2018, 87, 1222–1228. [Google Scholar] [CrossRef]
- Jirapinyo, P.; Kumar, N.; AlSamman, M.A.; Thompson, C.C. Five-year outcomes of transoral outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Gastrointest. Endosc. 2020, 91, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvi, V.; Matteo, M.V.; Bove, V.; De Siena, M.; Giannetti, G.; Carlino, G.; Polidori, G.; Vinti, L.; Angelini, G.; Iaconelli, A.; et al. Long-term Outcomes of Transoral Outlet Reduction (TORe) for Dumping Syndrome and Weight Regain After Roux-en-Y Gastric Bypass. Obes. Surg. 2023, 33, 1032–1039. [Google Scholar] [CrossRef]
- Dolan, R.D.; Jirapinyo, P.; Thompson, C.C. Endoscopic versus surgical gastrojejunal revision for weight regain in Roux-en-Y gastric bypass patients: 5-year safety and efficacy comparison. Gastrointest. Endosc. 2021, 94, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.J.; Abu Dayyeh, B.K.; Storm, A.C.; Bazerbachi, F.; Matar, R.; Vella, A.; Kellogg, T.; Stier, C. Endoscopic management of dumping syndrome after Roux-en-Y gastric bypass: A large international series and proposed management strategy. Gastrointest. Endosc. 2020, 92, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Bazarbashi, A.N.; Dolan, R.D.; McCarty, T.R.; Jirapinyo, P.; Thompson, C.C. Endoscopic revision of gastrojejunal anastomosis for the treatment of dumping syndrome in patients with Roux-en-Y gastric bypass: A systematic review and meta-analysis. Surg. Endosc. 2022, 36, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- Fildes, A.; Charlton, J.; Rudisill, C.; Littlejohns, P.; Prevost, A.T.; Gulliford, M.C. Probability of an Obese Person Attaining Normal Body Weight: Cohort Study Using Electronic Health Records. Am. J. Public Health 2015, 105, e54–e59. [Google Scholar] [CrossRef]
- Jackson, S.E.; Llewellyn, C.H.; Smith, L. The obesity epidemic—Nature via nurture: A narrative review of high-income countries. SAGE Open Med. 2020, 8, 2050312120918265. [Google Scholar] [CrossRef]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef]
- Koliaki, C.; Spinos, T.; Spinou, M.; Brinia Mu, E.; Mitsopoulou, D.; Katsilambros, N. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare 2018, 6, 73. [Google Scholar] [CrossRef]
- Wing, R.R.; Hill, J.O. Successful weight loss maintenance. Annu. Rev. Nutr. 2001, 21, 323–341. [Google Scholar] [CrossRef]
- Hill, A.J. Does dieting make you fat? Br. J. Nutr. 2004, 92 (Suppl. S1), S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Harp, J.B.; Reitman, M.L.; Beetsch, J.W.; Schoeller, D.A.; Erondu, N.; Pietrobelli, A. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am. J. Clin. Nutr. 2007, 85, 346–354. [Google Scholar] [CrossRef] [PubMed]

| Gastric | ||||||||
|---|---|---|---|---|---|---|---|---|
| Endoscopic Option | Procedure | Description | Key Trials | Trial Type | No. Patients | WL 4–6 Months (SD) | WL 12 Months (SD) | SAE |
| Gastric remodelling | ESG (Apollo Endosurgery) | Suturing to reduce stomach volume | MERIT [9] | RCT | 209 | - | TBWL 13.5% (±8.0) | 2.0% |
| POSE 1.0 (USGI medical) | Endoscopic plications to reduce stomach volume (fundus) | MILEPOST [10] ESSENTIAL [11] | RCT RCT | 44 332 | - - | TBWL 13.0% (±2.7) TBWL 4.95% (±7.0) | 0.0% 5.0% | |
| POSE 2.0 (USGI Medical) | Endoscopic plications to reduce stomach volume (body) | Lopez Nava et al. [12] | PRO | 44 | - | TBWL 15.7% (±6.8) | 0.0% | |
| Endomina (Endo Tools Therapeutics) | Endoscopic suturing to reduce stomach volume | Huberty et al. [13] | RCT | 71 | - | TBWL 11.9% (±2.6) | 0.0% | |
| Gastric balloons | Orbera (Apollo Endosurgery) | Fluid-filled balloon inserted by endoscopy | Kumar et al. [14] ASGE [15] | MA MA | 5549 1638 | TBWL 13.2% (±0.8) - | - EWL 25.4% (±4.0) | 1.5% |
| Spatz (Spatz Medical) | Fluid-filled balloon inserted by endoscopy | SABO [16] | RCT | 288 | TBWL 15.0% (±1.1) | - | 4.0% | |
| Elipse (Allurion Technologies) | Fluid-filled balloon inserted by swallowing | Ramai et al. [17] Jamal et al. [18] | MA PRO | 2152 112 | TBWL 12.0% (±1.2) - | - TBWL 7.9% (±6.7) | 0.5% 0.0% | |
| Heliosphere (Helioscopie) | Air-filled balloon inserted by endoscopy | Lecumberri et al. [19] | PRO | 84 | TBWL 13.4% (±7.0) | - | 0.0% | |
| Obalon (Obalon Therapeutics) | Air-filled balloon inserted by swallowing | SMART [20] | RCT | 387 | TBWL 7.1% (±5.0) | - | 0.4% | |
| Reshape Duo (Apollo Endosurgery) | Fluid-filled balloon inserted by endoscopy | REDUCE [21] | RCT | 326 | EWL 25.1% (±1.6) | - | 3.0% | |
| Transpyloric shuttle | Transpyloric shuttle (BAROnova) | Spherical bulb attached to a smaller bulb across the pylori | ENDObesity II [22] | RCT | 302 | - | TBWL 9.5% (±0.7) | 4.7% |
| Gastric aspiration | AspireAssist (Aspire Bariatrics) | Gastrostomy tube used to aspirate gastric content | PATHWAY [23] | RCT | 207 | - | TBWL 12.1 (±9.6) | 3.6% |
| Small intestinal | ||||||||
| Endoscopic option | Procedure | Description | Key trials | Trial type | No. patients | Mean fall in HbA1c (24–34 weeks) | SAE | |
| Duodenal mucosal resurfacing | Revita (Fractyl) | Hydrothermal ablation of the duodenal mucosa | REVITA-1 [24] REVITA-2 [25] | PRO RCT | 46 108 | −10.0 mmol/mol −10.4 mmol/mol | 2.2% 3.6% | |
| Electroporation Therapy (Endogenex) | Electroporation of the duodenal mucosa | EMINENT * [26] | PRO | 14 | −6.6 mmol/mol | 0.0% | ||
| Bypass liners | Endobarrier (GI Dynamics) | 60 cm Impermeable liner anchored in the duodenum | Koehestanie et al. [27] Jirapinyo et al. [28] | RCT MA | 73 412 | −13.3 mmol/mol −13.3 mmol/mol | 14.7% 15.7% | |
| Partial jejunal diversion | Incisionless anastomotic system (GI Windows) | Self-assembling magnets placed between jejunum and ileum | Machytka et al. [29] | PRO | 10 | −18.6 mmol/mol | 10.0% | |
| Revisional | ||||||||
| Endoscopic option | Procedure | Description | Key trials | Trial type | No. patients | WL 6 months (SD) | WL 12 months (SD) | SAE |
| Revision gastrojejunal anastomosis after RYGB | TORe (Apollo Endosurgery) | Endoscopic suturing to narrow the gastrojejunal anastomosis | RESTORe [30] Vargas et al. [31] | RCT MA | 77 330 | TBWL 3.5% (±1.7) - | - WL 8.4 kg (±2.5) | 0.0% 0.0% |
| APC (Olympus) | APC to narrow the gastrojejunal anastomosis | Gurian et al. [32] | RCT | 66 | TBWL 7.5% (±3.6) | TBWL 1.8% (±5.7) | 2.6% | |
| Revision sleeve gastrectomy | ESG: sleeve-in-sleeve (Apollo Endosurgery) | Endoscopic suturing to reduce stomach volume | Maselli et al. [33] | PRO | 82 | - | TBWL 15.7% (±7.6) | 1.2% |
| Balloon Type | Filling | Insertion | Removal | FDA Approval | CE Mark | Time In Situ (Marks) |
|---|---|---|---|---|---|---|
| Orbera (Apollo Endosurgery) | Liquid | Endoscopy | Endoscopy | + | + | 6 * |
| Spatz (Spatz Medical) | Liquid | Endoscopy | Endoscopy | + | + | 12 |
| Elipse (Allurion Technologies) | Liquid | Swallow | Excretion | - | + | 4 |
| Heliosphere (Helioscopie) | Gas | Endoscopy | Endoscopy | - | + | 6 |
| Obalon (Obalon Therapeutics) | Gas | Swallow | Endoscopy | + | + | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, B.C.; Telese, A.; Papaefthymiou, A.; Aslam, N.; Makaronidis, J.; Murray, C.; Haidry, R. Metabolic and Bariatric Endoscopy: A Mini-Review. Life 2023, 13, 1905. https://doi.org/10.3390/life13091905
Norton BC, Telese A, Papaefthymiou A, Aslam N, Makaronidis J, Murray C, Haidry R. Metabolic and Bariatric Endoscopy: A Mini-Review. Life. 2023; 13(9):1905. https://doi.org/10.3390/life13091905
Chicago/Turabian StyleNorton, Benjamin Charles, Andrea Telese, Apostolis Papaefthymiou, Nasar Aslam, Janine Makaronidis, Charles Murray, and Rehan Haidry. 2023. "Metabolic and Bariatric Endoscopy: A Mini-Review" Life 13, no. 9: 1905. https://doi.org/10.3390/life13091905
APA StyleNorton, B. C., Telese, A., Papaefthymiou, A., Aslam, N., Makaronidis, J., Murray, C., & Haidry, R. (2023). Metabolic and Bariatric Endoscopy: A Mini-Review. Life, 13(9), 1905. https://doi.org/10.3390/life13091905





