Validating the Nutraceutical Significance of Minor Millets by Employing Nutritional–Antinutritional Profiling
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Antinutritional Parameters
2.2.1. Determination of Tannins
2.2.2. Determination of Phytic Acid
2.2.3. Estimation of Total Phenolic Content (TPC)
2.2.4. Estimation of Total Flavonoid Content (TFC)
2.2.5. Determination of Proline Content
2.3. Nutritional Parameters
2.3.1. Extraction of Protein
2.3.2. Estimation of Total Amino Acids (TAA)
2.3.3. Estimation of Carbohydrates
2.3.4. Insoluble, Soluble and Total Dietary Fibers
2.3.5. Determination of Total Starch
2.3.6. Atomic Absorption Spectrophotometric (AAS) Analysis
2.4. Antioxidant Activity
DPPH Radical Scavenging Activity (DPPH)
2.5. In Vitro Anti-Diabetes Assay
2.5.1. α-Amylase Inhibition Assay
2.5.2. α-Glucosidase Inhibition Assay
2.6. Characterization of Proteins
2.7. Purification of the Peptides from Foxtail Millet
2.8. Sulphorhodamine B (SRB) Assay for Cytotoxicity Assessment
2.9. Statistical Analysis
3. Results and Discussion
3.1. Total Protein and Amino Acid Content
3.2. Total Sugars and Starch
3.3. Dietary Fibers
3.4. Determination of Minerals: Fe and Zn Contents
3.5. Tannic and Phytic Acid Content
3.6. Total Phenolic Content and Total Flavonoid Content
3.7. Proline
3.8. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
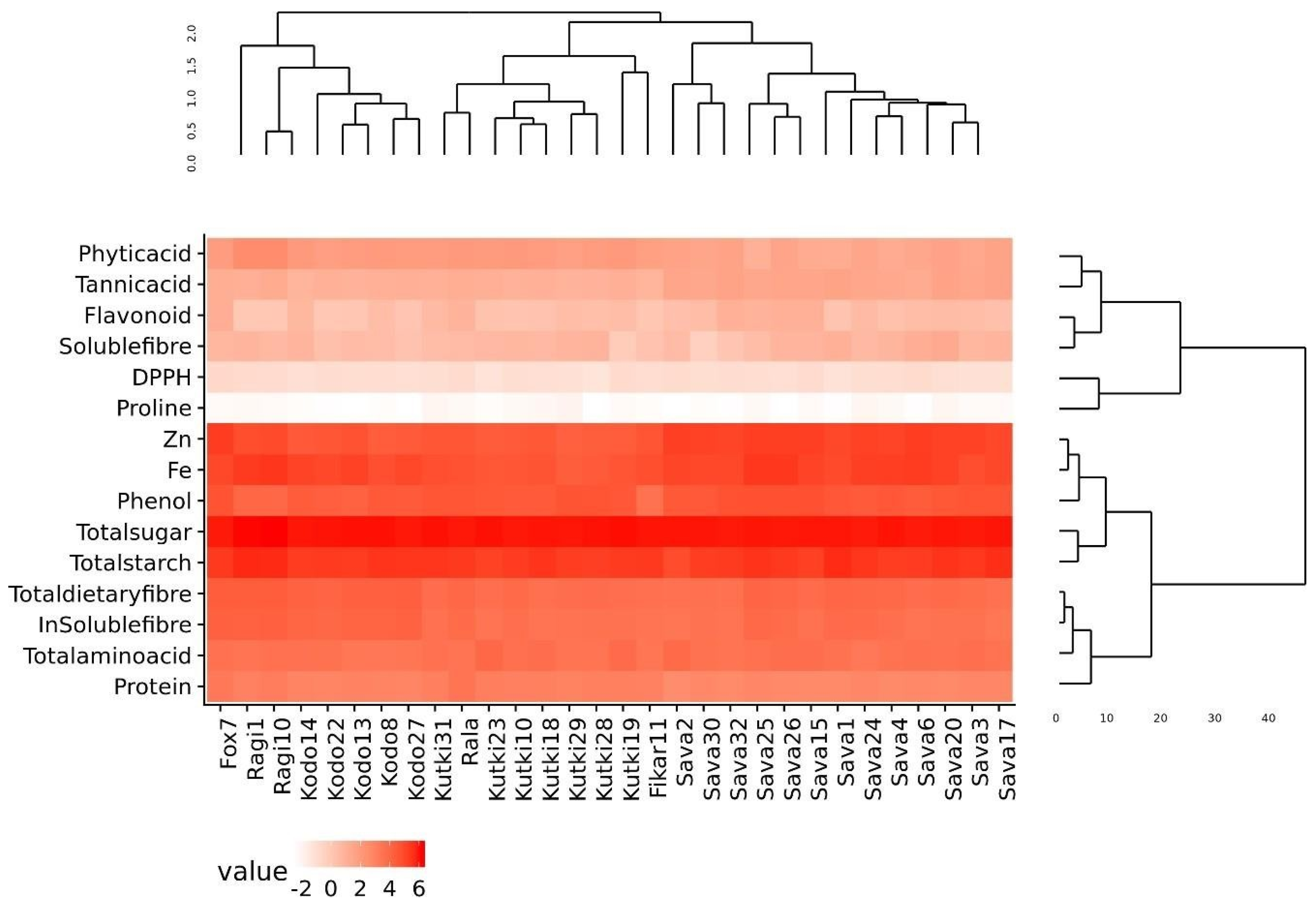
3.9. Correlation Coefficient, Diversity Assessment and Expression Analysis among Nutritional and Antinutritional Parameters
4. In Vitro Anti-Diabetes Assay
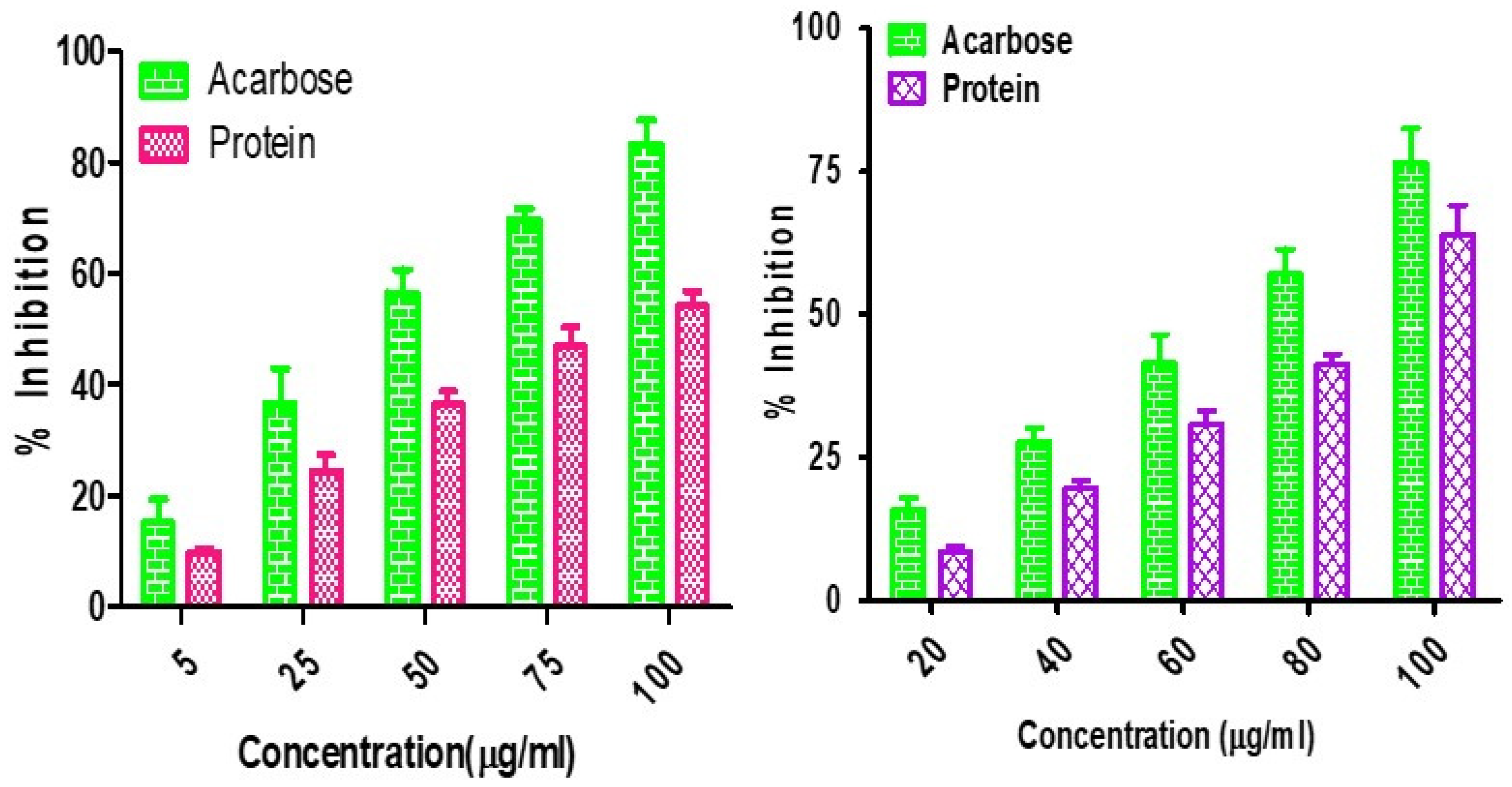
4.1. α-Amylase and α-Glucosidase Inhibition Assay
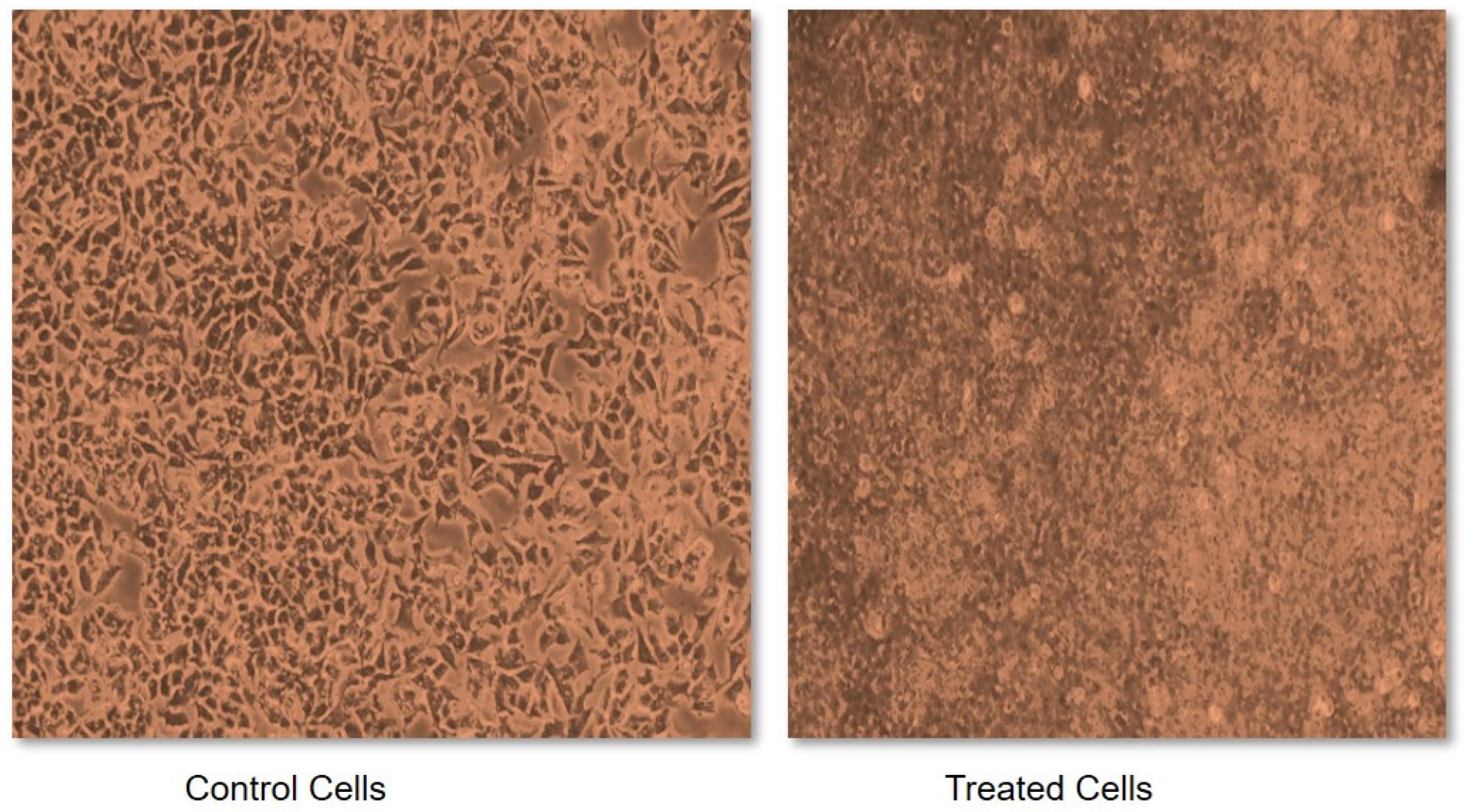
4.2. Sulforhodamine B (SRB) Assay for Cytotoxicity Assessment
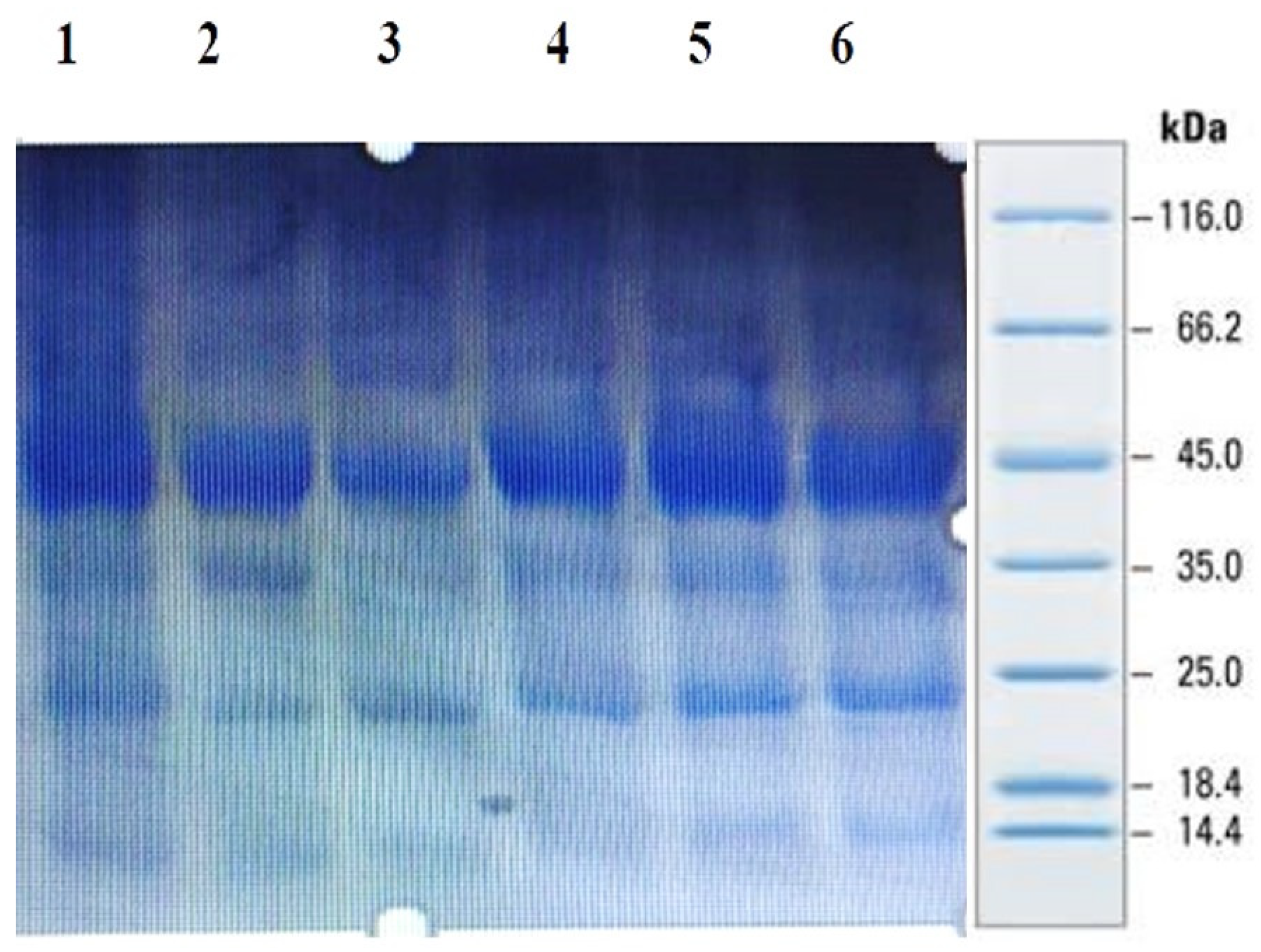
4.3. Purification of the Peptides from Foxtail Millet
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinoth, A.; Ravindhran, R. Biofortification in Millets: A Sustainable Approach for Nutritional Security. Front. Plant Sci. 2017, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The nutritional use of millet grain for food and feed: A review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Makwana, K.; Tiwari, S.; Tripathi, M.K.; Sharma, A.K.; Pandya, R.K.; Singh, A.K. Morphological characterization and DNA finger printing of pearl millet [Pennisetum glaucum (L.)] germplasms. Range Mang. Agrofor. 2021, 42, 205–211. [Google Scholar]
- Gowda, N.A.N.; Siliveru, K.; Prasad, P.V.V.; Bhatt, Y.; Netravati, B.P.; Gurikar, C. Modern Processing of Indian Millets: A Perspective on Changes in Nutritional Properties. Foods 2022, 11, 499. [Google Scholar] [CrossRef]
- Makwana, K.; Tiwari, S.; Tripathi, M.K.; Patel, V. Selection of blast resistant lines from diverse germplasm set of foxtail millet. Biol. Forum—Int. J. 2023, 15, 1–6. [Google Scholar]
- Choudhary, P.; Shukla, P.; Muthamilarasan, M. Genetic enhancement of climate-resilient traits in small millets: A review. Heliyon 2023, 9, e14502. [Google Scholar] [CrossRef]
- Makwana, K.; Tiwari, S.; Tripathi, M.K.; Patel, V. Morphological diversity analysis of foxtail millet (Sateria Italica (L.) Beauv.) using qualitative and quantitative traits. Scientist 2023, 2, 319–326. [Google Scholar] [CrossRef]
- Chinchole, M.; Pathak, R.K.; Singh, U.M.; Kumar, A. Molecular characterization of EcCIPK24 gene of finger millet (Eleusine coracana) for investigating its regulatory role in calcium transport. 3 Biotech 2017, 7, 17267. [Google Scholar] [CrossRef]
- Tiwari, S.; Yadav, S.K.; Sahu, V.K.; Tripathi, M.K. Current status and future prospects of marker assistedbreeding for genetic improvement of minor millet. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2587–2590. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Schneider, R.G. The Role of Amaranth, Quinoa, and Millets for the Development of Healthy, Sustainable Food Products—A Concise Review. Foods 2022, 11, 2442. [Google Scholar] [CrossRef]
- Kumar, A.; Tomer, V.; Kaur, A.; Kumar, V.; Gupta, K. Millets: A solution to agrarian and nutritional challenges. Agric. Food Secur. 2018, 7, 31. [Google Scholar] [CrossRef]
- Sangappa; Rafi, D.; Babu, K.S. A study on area-production-productivity of minor millets in India. Biol. Forum—Int. J. 2023, 15, 275–280. [Google Scholar]
- Upadhyaya, H.D.; Vetriventhan, M. Underutilized Climate-Smart Nutrient Rich Small Millets for Food and Nutritional Security; International Crops Research Institute for the Semi-Arid Tropics (ICRISAT): Patancheru, India, 2018. [Google Scholar]
- Lydia, P.J.; Ganesan, J.; Francis, N.; Rajasekharan, R.; Thinakaran, J. Revitalization of small millets for nutritional and food security by advanced genetics and genomics approaches. Front. Genet. 2023, 13, 1007552. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Chandra, S.; Pallavi; Sharma, A.K. Review of Finger millet (Eleusine coracana (L.) Gaertn): A power house of health benefiting nutrients. Food Sci. Hum. Wellness 2016, 5, 149–155. [Google Scholar] [CrossRef]
- Guo, X.; Sha, X.; Rahman, E.; Wang, Y.; Ji, B.; Wu, W.; Zhou, F. Antioxidant capacity and amino acid profile of millet branwine and the synergistic interaction between major polyphenols. J. Food Sci. Technol. 2018, 55, 1010–1020. [Google Scholar] [CrossRef]
- Antony, U.; Sripriya, G.; Chandra, T.S. Effect of fermentation on the primary nutrients in finger millet (Eleusine coracana). J. Agric. Food Chem. 1996, 44, 2616–2618. [Google Scholar] [CrossRef]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef]
- Panwar, P.; Dubey, A.; Verma, A.K. Evaluation of nutraceutical and antinutritional properties in barnyard and finger millet varieties grown in Himalayan region. J. Food Sci. Technol. 2016, 53, 779–787. [Google Scholar] [CrossRef]
- Schandrei, S.H. Method in Food Analysis; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Wilcox, J.R.; Premachandra, G.S.; Young, K.A.; Raboy, V. Isolation of high seed inorganic P, low-phytate soybean mutants. Crop. Sci. 2000, 40, 1601–1605. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; de Mello, J.C.P. Application and analysis of the folinciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hassan, A.B.; Osman, G.A.; Babiker, E.E. Effect of chymotrypsin digestion followed by polysaccharide conjugation or transglutaminase treatment on functional properties of millet proteins. Food Chem. 2007, 102, 257–262. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Dubois, M.; Gillea, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Calorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W.; Furda, I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: Interlaboratory study. J. Assoc. Off. Anal. Chem. 1988, 71, 1017–1023. [Google Scholar] [CrossRef]
- Annor, G.A.; Marcone, M.; Bertoft, E.; Seetharaman, K. In vitro starch digestibility and expected glycemic index of Kodo millet (Paspalum scrobiculatum) as affected by starch-protein-lipid interactions. Cereal Chem. J. 2013, 90, 211–217. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm. Wiss. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012, 135, 2078–2085. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Amadou, I.; Gounga, M.E.; Le, G.W. Millets: Nutritional composition, some health benefits and processing e a review. Emir. J. Food Agric. 2013, 25, 501–508. [Google Scholar] [CrossRef]
- Cordelino, I.; Tyl, C.; Inamdar, L.; Vickers, Z.; Marti, A.; Ismail, B. Cooking quality, digestibility, and sensory properties of proso millet pasta as impacted by amylose content and prolamin profile. LWT Food Sci. Technol. 2019, 99, 1–7. [Google Scholar] [CrossRef]
- Pramitha, L.; Choudhary, P.; Das, P.; Sharma, S.; Karthi, V.; Vemuri, H.; Muthamilarasan, M. Integrating Genomics and Phenomics Tools to Dissect Climate Resilience Traits in Small Millets. In Omics of Climate Resilient Small Millets; Pudake, R.N., Solanke, A.U., Sevanthi, A.M., Rajendrakumar, P., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Wafula, W.N.; Ojulong, H.F.; Siamb, M.I.; Gweyi-Onyango, J.P. Protein, Calcium, Zinc, and iron contents of finger millet grain response to varietal differences and phosphorus application in Kenya. Agronomy 2018, 8, 24. [Google Scholar] [CrossRef]
- Nazim, M.U.; Mitra, K.; Rahman, M.M.; Abdullah, A.T.M.; Parveen, S. Evaluation of the nutritional quality and microbiological analysis of newly developed soya cheese. Int. Food Res. J. 2013, 20, 3373–3380. [Google Scholar]
- Bhagyawant, S.S.; Gautam, A.K.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Srivastava, N.; Upadhyaya, H.D. Biochemical diversity evaluation in chickpea accessions employing mini-core collection. Physiol. Mol. Biol. Plants 2018, 24, 1165–1183. [Google Scholar] [CrossRef]
- Singh, P.; Raghuvanshi, S. Finger millet for food and nutritional security. Afr. J. Food Sci. 2012, 6, 77–84. [Google Scholar] [CrossRef]
- Obilana, A.B.; Manyasa, E. Millets. In Pseudo Cereals and Less Common Cereals: Grain Properties and Utilization Potential; Belton, P.S., Taylor, J.R.N., Eds.; Springer: New York, NY, USA, 2002; pp. 177–217. [Google Scholar]
- Shobana, S.; Krishnaswamy, K.; Sudha, V.; Malleshi, N.G.; Anjana, R.M.; Palaniappan, L.; Mohan, V. Finger Millet (Ragi, Eleusine coracana L.): A Review of Its Nutritional Properties, Processing and Plausible Health Benefits. Adv. Food Nutr. Res. 2013, 69, 1–39. [Google Scholar]
- Kumar, A.; Metwal, M.; Kaur, S.; Gupta, A.K.; Puranik, S.; Singh, S.; Singh, M.; Gupta, S.; Babu, B.K.; Sood, S.; et al. Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Front. Plant Sci. 2016, 7, 934. [Google Scholar] [CrossRef]
- Yadav, B.S.; Sharma, A.; Yadav, R.B. Resistant starch content of conventionally boiled and pressure- cooked cereals, legumes and tubers. J. Food Sci. Technol. 2010, 47, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Aspect. Med. 2020, 75, 100864. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, F.S.; Urooj, A. Antioxidant Activity in Two Pearl millet (Pennisetum typhoideum) Cultivars as Influenced by Processing. Antioxidants 2014, 3, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.Y.; Arntfield, S.D.; Nayacholi, C.M. Nutritional quality of legume seeds as affected by some physical treatment. Part 2: Antinutritional factors. LWT Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins a review of efficacy and mechanisms. LWT Food Sci. Technol. 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Pujol, A.; Sanchis, P.; Grases, F.; Masmiquel, L. Phytate Intake, Health and Disease: “Let Thy Food Be Thy Medicine and Medicine Be Thy Food”. Antioxidants 2023, 12, 146. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef]
- Sahu, V.K.; Tiwari, S.; Gupta, N.; Tripathi, M.K.; Yasin, M. Evaluation of Physiological and Biochemical Contents in Desi and Kabuli Chickpea. Legume Res. 2020, 45, 1197–1208. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R.N. Phenolic content and antioxidant properties of bran in 51 wheat cultivars. Cereal Chem. 2008, 85, 544–549. [Google Scholar] [CrossRef]
- Tiwari, P.N.; Tiwari, S.; Sapre, S.; Babbar, A.; Tripathi, N.; Tiwari, S.; Tripathi, M.K. Screening and Selection of Drought-Tolerant High-Yielding Chickpea Genotypes Based on Physio-Biochemical Selection Indices and Yield Trials. Life 2023, 13, 1405. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, M.; Grayer, R.J.; Simmonds, M.S.J.; Damak, M.; Sayadi, S. Identification and antioxidant potential of flavonoids and low molecular weight phenols in olive cultivar chemlali growing in Tunisia. J. Agric. Food Chem. 2005, 53, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Choudhary, M.L.; Tripathi, M.K.; Gupta, N.; Tiwari, S.; Tripathi, N.; Parihar, P.; Pandya, R.K. Screening of pearl millet [Pennisetum glaucum [L] R Br] germplasm lines against drought tolerance based on biochemical traits. Curr. J. Appl. Sci. Technol. 2021, 40, 46–63. [Google Scholar] [CrossRef]
- Mishra, N.; Tripathi, M.K.; Tripathi, N.; Tiwari, S.; Gupta, N.; Sharma, A.; Shrivastav, M.K. Changes in biochemical and antioxidant enzymes activities play significant role in drought tolerance in soybean. Int. J. Agric. Technol. 2021, 17, 1425–1446. [Google Scholar]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant. 2019, 41, 23. [Google Scholar] [CrossRef]
- Sharma, A.; Tripathi, M.K.; Tiwari, S.; Gupta, N.; Tripathi, N.; Mishra, N. Evaluation of soybean (Glycine max L.) genotypes on the basis of biochemical contents and anti-oxidant enzyme activities. Legume Res. 2021, 44, 1419–1429. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Li-Chan, E.C. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: A natural approach to complement pharmacotherapy in the management of diabetes. Mol. Nutr. Food Res. 2014, 58, 61–78. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.; Sreerama, Y.N. Impact of processing on the phenolic profiles of small millets: Evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia. Food Chem. 2015, 169, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.M.; Chelliah, R.; Ham, H.J.; Kim, J.H.; Han, S.I.; Hur, J.H.; Oh, D.H. Phenolic Profile, Antioxidant, and Antidiabetic Potential Exerted by Millet Grain Varieties. Antioxidants 2020, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Niu, W. Phytochemical and antiproliferative activity of proso millet. PLoS ONE 2014, 9, e104058. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 2011, 3, 144–158. [Google Scholar] [CrossRef]
- Gupta, N.; Bhagyawant, S.S. Impact of hydrolysis on functional properties, antioxidant, ACE-I inhibitory and anti-proliferative activity of Cicer arietinum and Cicer reticulatum hydrolysates. Nutrire 2019, 44, 5. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]

| S.N. | Genotypes | Protein (g100 g−1) | Total Amino Acid (g) | Total Starch (g 100 g−1) | Total Sugar (g 100 g−1) | Insoluble Fiber (g 100 g−1) | Soluble Fiber (g 100 g−1) | Total Dietary Fiber (g 100 g−1) | Fe (ppm) | Zn (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sava-1 | 6.4 ± 0.1 | 12.4 ± 1.2 | 52.4 ± 0.9 | 64.2 ± 1.2 | 14.6 ± 2.1 | 2.1 ± 0.5 | 16.7 ± 1.4 | 31.2 ± 0.6 | 32.4 ± 0.4 |
| 2 | Sava-2 | 6.1 ± 0.3 | 14.8 ± 1.3 | 30.1 ± 0.3 | 65.1 ± 1.4 | 11.2 ± 1.1 | 1.5 ± 0.5 | 12.7 ± 1.5 | 34.6 ± 0.5 | 38.3 ± 0.3 |
| 3 | Sava-3 | 7.2 ± 1.1 | 13.2 ± 1.6 | 43.1 ± 0.4 | 62.8 ± 0.5 | 12.5 ± 3.1 | 1.7 ± 0.5 | 14.2 ± 1.6 | 28.9 ± 0.5 | 36.4 ± 0.3 |
| 4 | Sava-4 | 6.4 ± 1.6 | 12.1 ± 0.7 | 39.9 ± 0.4 | 65.7 ± 1.4 | 13.6 ± 2.1 | 1.8 ± 0.5 | 15.4 ± 1.7 | 39.5 ± 0.7 | 35.2 ± 0.3 |
| 5 | Sava-6 | 6.8 ± 0.2 | 12.9 ± 0.9 | 40.3 ± 0.5 | 62.4 ± 1.2 | 11.7 ± 1.1 | 2.2 ± 0.8 | 13.9 ± 1.8 | 40.8 ± 0.8 | 39.8 ± 0.3 |
| 6 | Sava-17 | 7.0 ± 0.5 | 12.1 ± 0.4 | 49.5 ± 0.5 | 64.6 ± 1.5 | 10.9 ± 1.3 | 1.8 ± 0.9 | 12.7 ± 1.5 | 32.4 ± 0.8 | 33.5 ± 0.3 |
| 7 | Sava-20 | 6.4 ± 0.8 | 12.7 ± 0.5 | 46.8 ± 0.6 | 64.1 ± 1.6 | 12.4 ± 2.3 | 2.5 ± 0.7 | 14.9 ± 0.9 | 36.4 ± 0.7 | 36.7 ± 0.3 |
| 8 | Sava-24 | 6.9 ± 0.9 | 10.5 ± 0.6 | 44.4 ± 0.6 | 62.5 ± 1.4 | 14.5 ± 0.9 | 1.6 ± 0.5 | 16.1 ± 1.0 | 38.2 ± 0.6 | 37.2 ± 0.7 |
| 9 | Sava-25 | 7.2 ± 1.4 | 12.7 ± 0.7 | 46.8 ± 0.8 | 64.8 ± 0.8 | 15.7 ± 2.6 | 1.4 ± 0.5 | 17.1 ± 1.6 | 44.2 ± 0.6 | 39.1 ± 0.8 |
| 10 | Sava-26 | 6.6 ± 1.7 | 13.8 ± 0.8 | 42.8 ± 0.9 | 63.3 ± 1.6 | 14.5 ± 1.1 | 1.8 ± 0.7 | 16.3 ± 1.9 | 43.2 ± 0.6 | 38.7 ± 0.9 |
| 11 | Sava-30 | 6.8 ± 0.2 | 12.5 ± 0.9 | 39.9 ± 0.2 | 64.6 ± 1.9 | 12.2 ± 1.1 | 0.8 ± 0.9 | 13.0 ± 1.4 | 32.5 ± 0.1 | 36.6 ± 0.4 |
| 12 | Sava-32 | 6.3 ± 0.4 | 11.4 ± 0.7 | 41.5 ± 0.6 | 62.8 ± 1.7 | 11.6 ± 2.1 | 1.1 ± 0.5 | 12.7 ± 1.6 | 33.1 ± 0.4 | 34.8 ± 0.7 |
| 13 | Sava-15 | 6.5 ± 0.8 | 13.8 ± 1.3 | 38.1 ± 0.7 | 64.0 ± 1.7 | 12.5 ± 2.1 | 1.8 ± 0.6 | 14.3 ± 1.7 | 35.6 ± 0.4 | 38.2 ± 0.4 |
| 14 | Kutki-10 | 8.4 ± 0.9 | 12.9 ± 0.4 | 40.3 ± 0.3 | 63.9 ± 1.8 | 13.6 ± 1.1 | 1.7 ± 0.7 | 15.3 ± 1.8 | 24.5 ± 0.4 | 22.4 ± 0.4 |
| 15 | Kutki-18 | 8.6 ± 0.1 | 13.7 ± 1.7 | 45.3 ± 0.5 | 65.3 ± 0.9 | 11.3 ± 2.1 | 1.6 ± 0.9 | 12.9 ± 1.1 | 25.7 ± 0.8 | 23.5 ± 0.4 |
| 16 | Kutki-31 | 8.8 ± 0.6 | 12.6 ± 1.1 | 44.5 ± 0.6 | 66.7 ± 1.2 | 12.6 ± 1.8 | 1.4 ± 0.4 | 14.0 ± 1.4 | 28.2 ± 0.9 | 24.6 ± 0.4 |
| 17 | Kutki-29 | 8.1 ± 1.1 | 11.3 ± 1.7 | 39.6 ± 0.7 | 64.1 ± 1.3 | 11.9 ± 0.9 | 1.8 ± 0.4 | 13.7 ± 1.6 | 20.5 ± 0.2 | 19.2 ± 0.4 |
| 18 | Kutki-28 | 8.6 ± 1.4 | 11.7 ± 1.9 | 38.4 ± 0.8 | 65.8 ± 1.4 | 12.4 ± 2.2 | 1.9 ± 0.4 | 14.3 ± 1.7 | 21.6 ± 0.4 | 20.1 ± 0.4 |
| 19 | Kutki-23 | 8.9 ± 1.7 | 15.8 ± 2.1 | 37.1 ± 0.2 | 66.7 ± 1.4 | 11.6 ± 2.6 | 1.6 ± 0.4 | 13.2 ± 1.7 | 23.4 ± 0.5 | 21.4 ± 0.8 |
| 20 | Kutki-19 | 8.5 ± 2.1 | 14.7 ± 2.4 | 41.5 ± 0.3 | 68.2 ± 1.2 | 12.4 ± 1.7 | 0.9 ± 0.2 | 13.3 ± 1.7 | 24.8 ± 0.7 | 20.8 ± 0.9 |
| 21 | Kodo-8 | 7.7 ± 2.3 | 10.8 ± 2.3 | 46.4 ± 0.5 | 67.2 ± 1.5 | 17.6 ± 1.4 | 1.4 ± 0.4 | 19.0 ± 1.8 | 28.4 ± 0.8 | 20.6 ± 0.9 |
| 22 | Kodo-27 | 7.4 ± 1.2 | 11.2 ± 2.4 | 44.7 ± 0.7 | 63.4 ± 1.6 | 18.6 ± 1.6 | 1.2 ± 0.5 | 19.8 ± 1.3 | 32.4 ± 0.9 | 22.4 ± 0.3 |
| 23 | Kodo-22 | 7.3 ± 1.1 | 12.5 ± 2.6 | 42.2 ± 0.6 | 65.6 ± 0.9 | 15.8 ± 1.8 | 1.3 ± 0.8 | 17.1 ± 1.3 | 33.4 ± 0.2 | 24.1 ± 0.4 |
| 24 | Kodo-13 | 7.8 ± 1.4 | 10.9 ± 2.7 | 40.8 ± 0.8 | 67.1 ± 1.6 | 17.5 ± 1.9 | 1.5 ± 0.9 | 19.0 ± 1.3 | 36.7 ± 0.3 | 26.5 ± 0.6 |
| 25 | Kodo-14 | 7.6 ± 1.5 | 12.6 ± 2.9 | 41.3 ± 0.9 | 64.2 ± 0.8 | 16.8 ± 1.5 | 1.8 ± 0.4 | 18.6 ± 1.4 | 35.6 ± 0.4 | 22.6 ± 0.7 |
| 26 | Fox-7 | 10.5 ± 1.6 | 12.7 ± 1.1 | 42.8 ± 0.9 | 62.6 ± 1.4 | 19.2 ± 0.6 | 1.7 ± 0.3 | 20.9 ± 1.2 | 32.4 ± 0.4 | 40.5 ± 0.8 |
| 27 | Fox-6 | 11.4 ± 1.7 | 11.6 ± 1.1 | 42.9 ± 0.4 | 63.7 ± 0.6 | 14.5 ± 0.7 | 1.5 ± 0.2 | 16.0 ± 1.4 | 26.6 ± 0.4 | 24.2 ± 0.9 |
| 28 | Ragi-1 | 8.2 ± 1.8 | 11.8 ± 1.6 | 53.3 ± 0.7 | 70.2 ± 1.7 | 18.6 ± 0.8 | 1.8 ± 0.3 | 20.4 ± 1.6 | 41.5 ± 0.7 | 29.2 ± 0.2 |
| 29 | Ragi-10 | 9.1 ± 0.9 | 12.6 ± 1.1 | 52.8 ± 0.8 | 71.6 ± 1.3 | 19.4 ± 0.9 | 1.6 ± 0.4 | 21.0 ± 1.7 | 44.6 ± 0.8 | 30.7 ± 0.4 |
| 30 | Fox-5 | 8.2 ± 1.4 | 11.2 ± 1.7 | 42.9 ± 0.9 | 65.1 ± 0.4 | 11.8 ± 1.4 | 1.2 ± 0.7 | 13.0 ± 1.8 | 28.6 ± 0.9 | 24.8 ± 0.5 |
| S.N. | Genotypes | Tannic Acid (mg g−1) | Phytic Acid (mg g−1) | Phenol (mg g−1) | Flavonoid (mg g−1) | Proline (mg g−1) | DPPH (mg g−1) |
|---|---|---|---|---|---|---|---|
| 1 | Sava-1 | 3.11 ± 1.4 | 2.34 ± 1.7 | 23.6 ± 0.9 | 1.15 ± 2.2 | 0.19 ± 1.8 | 0.45 ± 1.6 |
| 2 | Sava-2 | 2.93 ± 1.6 | 3.18 ± 0.1 | 22.8 ± 0.7 | 1.32 ± 0.4 | 0.18 ± 1.4 | 0.56 ± 2.4 |
| 3 | Sava-3 | 2.84 ± 1.7 | 2.68 ± 1.1 | 24.8 ± 1.4 | 1.44 ± 0.9 | 0.21 ± 1.7 | 0.48 ± 2.7 |
| 4 | Sava-4 | 2.64 ± 1.5 | 2.45 ± 1.2 | 23.7 ± 1.3 | 1.26 ± 1.5 | 0.22 ± 1.2 | 0.52 ± 2.4 |
| 5 | Sava-6 | 2.46 ± 1.5 | 2.76 ± 1.3 | 21.4 ± 1.7 | 1.40 ± 1.5 | 0.19 ± 1.3 | 0.56 ± 2.7 |
| 6 | Sava-17 | 3.07 ± 1.1 | 3.11 ± 0.9 | 24.8 ± 1.0 | 1.32 ± 1.7 | 0.21 ± 1.3 | 0.48 ± 2.9 |
| 7 | Sava-20 | 3.18 ± 1.4 | 3.16 ± 1.4 | 23.4 ± 0.9 | 1.48 ± 1.7 | 0.24 ± 1.5 | 0.49 ± 2.7 |
| 8 | Sava-24 | 2.84 ± 1.4 | 2.94 ± 1.8 | 21.6 ± 1.9 | 1.54 ± 1.8 | 0.25 ± 1.6 | 0.51 ± 2.2 |
| 9 | Sava-25 | 2.77 ± 1.3 | 2.11 ± 1.9 | 26.9 ± 1.8 | 1.92 ± 1.7 | 0.22 ± 1.7 | 0.52 ± 2.1 |
| 10 | Sava-26 | 2.91 ± 1.3 | 2.98 ± 1.2 | 27.3 ± 0.4 | 2.10 ± 0.4 | 0.18 ± 1.8 | 0.50 ± 1.8 |
| 11 | Sava-30 | 2.78 ± 1.2 | 2.94 ± 0.4 | 22.7 ± 2.6 | 1.47 ± 0.9 | 0.20 ± 1.2 | 0.51 ± 1.9 |
| 12 | Sava-32 | 3.12 ± 1.6 | 3.21 ± 0.9 | 26.5 ± 1.8 | 2.10 ± 1.9 | 0.19 ± 1.3 | 0.54 ± 2.4 |
| 13 | Sava-15 | 2.97 ± 1.7 | 2.44 ± 1.5 | 27.4 ± 1.9 | 2.10 ± 1.8 | 0.21 ± 1.5 | 0.55 ± 2.1 |
| 14 | Kutki-10 | 2.01 ± 1.8 | 4.12 ± 1.7 | 21.6 ± 1.0 | 1.17 ± 0.4 | 0.22 ± 1.6 | 0.51 ± 1.5 |
| 15 | Kutki-18 | 2.11 ± 1.7 | 3.77 ± 1.8 | 22.3 ± 1.7 | 1.20 ± 0.1 | 0.23 ± 1.5 | 0.49 ± 1.7 |
| 16 | Kutki-31 | 2.13 ± 1.6 | 3.89 ± 1.9 | 24.5 ± 1.9 | 1.56 ± 1.6 | 0.25 ± 1.3 | 0.51 ± 1.4 |
| 17 | Kutki-29 | 1.90 ± 1.4 | 3.45 ± 1.3 | 25.6 ± 1.7 | 1.45 ± 1.7 | 0.26 ± 1.5 | 0.47 ± 1.2 |
| 18 | Kutki-28 | 1.97 ± 1.2 | 3.89 ± 1.7 | 24.8 ± 1.8 | 1.34 ± 1.2 | 0.18 ± 1.3 | 0.42 ± 1.1 |
| 19 | Kutki-23 | 2.16 ± 1.1 | 3.96 ± 1.8 | 22.9 ± 1.0 | 1.23 ± 1.2 | 0.20 ± 1.5 | 0.44 ± 1.6 |
| 20 | Kutki-19 | 2.18 ± 1.0 | 4.12 ± 0.4 | 23.6 ± 1.3 | 1.44 ± 1.1 | 0.21 ± 1.6 | 0.57 ± 1.8 |
| 21 | Kodo-8 | 2.10 ± 1.9 | 4.11 ± 1.0 | 22.7 ± 1.6 | 1.42 ± 0.8 | 0.20 ± 1.5 | 0.52 ± 2.2 |
| 22 | Kodo-27 | 2.16 ± 1.1 | 3.87 ± 1.8 | 21.9 ± 1.7 | 1.10 ± 1.9 | 0.18 ± 1.3 | 0.49 ± 2.9 |
| 23 | Kodo-22 | 2.11 ± 1.3 | 3.56 ± 1.6 | 19.6 ± 1.8 | 1.04 ± 1.6 | 0.19 ± 1.5 | 0.54 ± 2.7 |
| 24 | Kodo-13 | 1.97 ± 1.2 | 3.81 ± 1.2 | 18.6 ± 1.2 | 1.06 ± 0.9 | 0.18 ± 1.6 | 0.51 ± 2.3 |
| 25 | Kodo-14 | 1.88 ± 1.2 | 4.13 ± 1.3 | 21.4 ± 0.6 | 1.67 ± 1.1 | 0.20 ± 1.3 | 0.50 ± 1.9 |
| 26 | Fox-7 | 2.24 ± 1.3 | 3.91 ± 1.1 | 26.2 ± 1.7 | 2.23 ± 1.9 | 0.21 ± 1.5 | 0.62 ± 1.8 |
| 27 | Fox-6 | 2.32 ± 1.2 | 4.21 ± 1.6 | 24.1 ± 0.7 | 1.90 ± 1.6 | 0.22 ± 1.6 | 0.56 ± 1.9 |
| 28 | Ragi-1 | 2.19 ± 1.4 | 6.12 ± 1.7 | 16.5 ± 1.2 | 1.01 ± 1.7 | 0.22 ± 1.3 | 0.57 ± 1.7 |
| 29 | Ragi-10 | 2.47 ± 1.7 | 5.94 ± 1.8 | 15.8 ± 1.8 | 1.05 ± 1.8 | 0.21 ± 1.5 | 0.55 ± 1.6 |
| 30 | Fox-5 | 1.85 ± 1.6 | 3.42 ± 1.4 | 12.5 ± 1.1 | 1.08 ± 1.9 | 0.20 ± 1.6 | 0.52 ± 1.2 |
| Correlations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | AA | Starch | Sugar | IF | SF | DF | Fe | Zn | TA | PA | Phenol | |
| Protein | 1 | −0.025 | 0.107 | 0.261 | 0.296 | −0.148 | 0.275 | −0.409 * | −0.473 ** | −0.614 ** | 0.603 ** | −0.153 |
| AA | 1 | −0.360 | 0.088 | −0.360 | 0.047 | −0.353 | −0.074 | 0.135 | 0.163 | −0.081 | 0.256 | |
| Starch | 1 | 0.342 | 0.465 ** | 0.181 | 0.487 ** | 0.320 | 0.019 | 0.125 | 0.326 | −0.263 | ||
| Sugar | 1 | 0.339 | −0.180 | 0.313 | 0.102 | −0.379 * | −0.331 | 0.707 ** | −0.518 ** | |||
| IF | 1 | −0.041 | 0.991 ** | 0.421 * | −0.064 | −0.282 | 0.514 ** | −0.299 | ||||
| SF | 1 | 0.093 | 0.190 | 0.252 | 0.227 | −0.119 | 0.134 | |||||
| DF | 1 | 0.445 * | −0.030 | −0.250 | 0.496 ** | −0.280 | ||||||
| Fe | 1 | 0.681 ** | 0.448 * | −0.061 | −0.144 | |||||||
| Zn | 1 | 0.794 ** | −0.506 ** | 0.298 | ||||||||
| TA | 1 | −0.550 ** | 0.444 * | |||||||||
| PA | 1 | −0.542 ** | ||||||||||
| Phenol | 1 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, S.S.; Tiwari, S.; Gupta, N.; Tripathi, M.K.; Tripathi, N.; Singh, S.; Bhagyawant, S.S. Validating the Nutraceutical Significance of Minor Millets by Employing Nutritional–Antinutritional Profiling. Life 2023, 13, 1918. https://doi.org/10.3390/life13091918
Rana SS, Tiwari S, Gupta N, Tripathi MK, Tripathi N, Singh S, Bhagyawant SS. Validating the Nutraceutical Significance of Minor Millets by Employing Nutritional–Antinutritional Profiling. Life. 2023; 13(9):1918. https://doi.org/10.3390/life13091918
Chicago/Turabian StyleRana, Shivani Singh, Sushma Tiwari, Neha Gupta, Manoj Kumar Tripathi, Niraj Tripathi, Sangeeta Singh, and Sameer S. Bhagyawant. 2023. "Validating the Nutraceutical Significance of Minor Millets by Employing Nutritional–Antinutritional Profiling" Life 13, no. 9: 1918. https://doi.org/10.3390/life13091918
APA StyleRana, S. S., Tiwari, S., Gupta, N., Tripathi, M. K., Tripathi, N., Singh, S., & Bhagyawant, S. S. (2023). Validating the Nutraceutical Significance of Minor Millets by Employing Nutritional–Antinutritional Profiling. Life, 13(9), 1918. https://doi.org/10.3390/life13091918





