Radioreparative Effect of Diode Laser on Leukopoiesis Recovery: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Groups
2.3. Source of Gamma Rays
2.4. Gamma Irradiation
2.5. Laser Irradiation
2.6. Peripheral Blood
- PE Rat Anti-Mouse CD3 Molecular Complex—for the labeling of T-lymphocytes;
- PerCP Rat Anti-Mouse CD45R/B220—for the labeling of B-lymphocytes;
- PE-Cy™7 Mouse Anti-Mouse NK-1.1—for the labeling of NK cells.
2.7. Data Analysis
3. Results
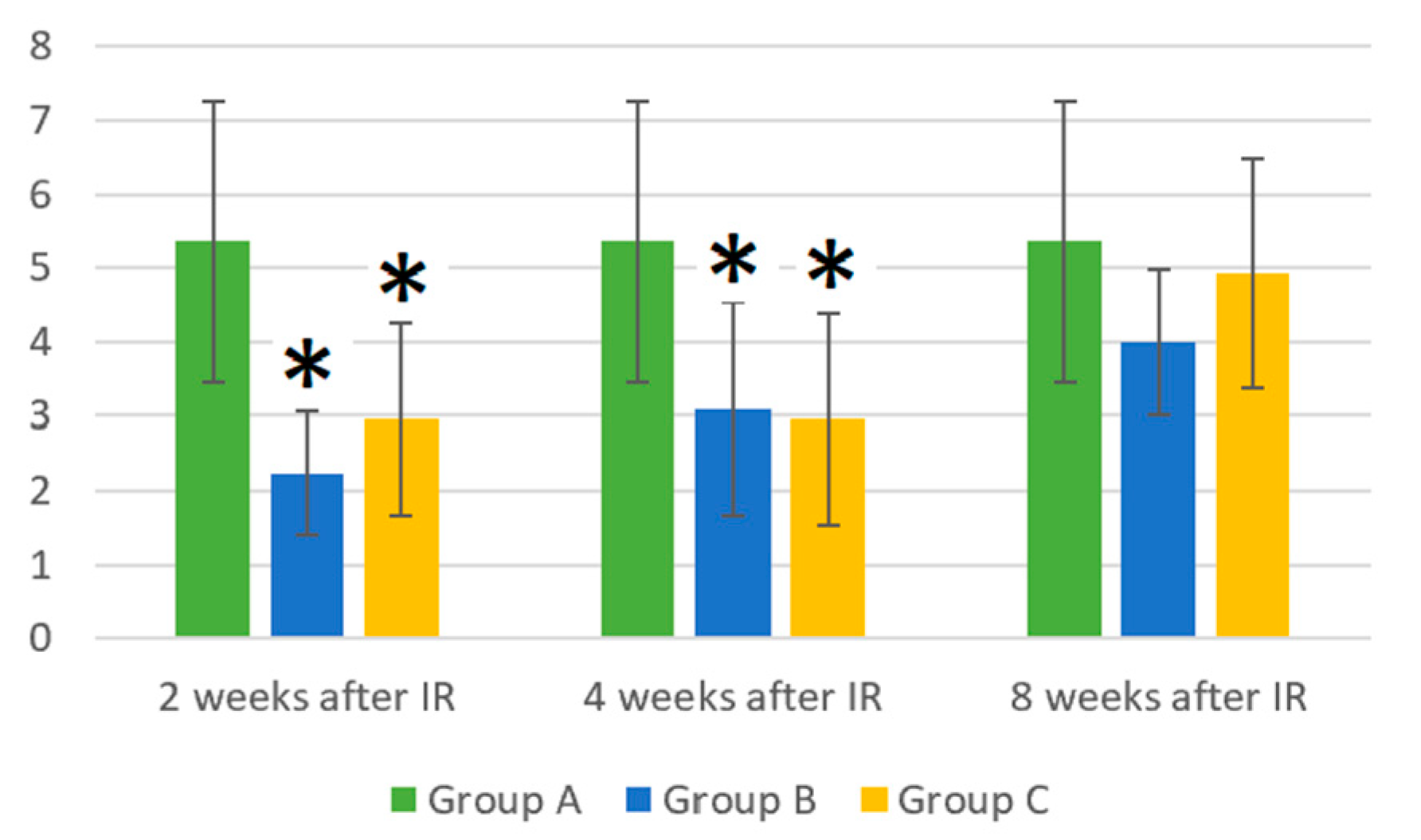
3.1. Absolute WBC Count in Control Mice (Group A), Mice Treated with Gamma Radiation (Group B), and Irradiation Group with Additional Laser Treatment (Group C)
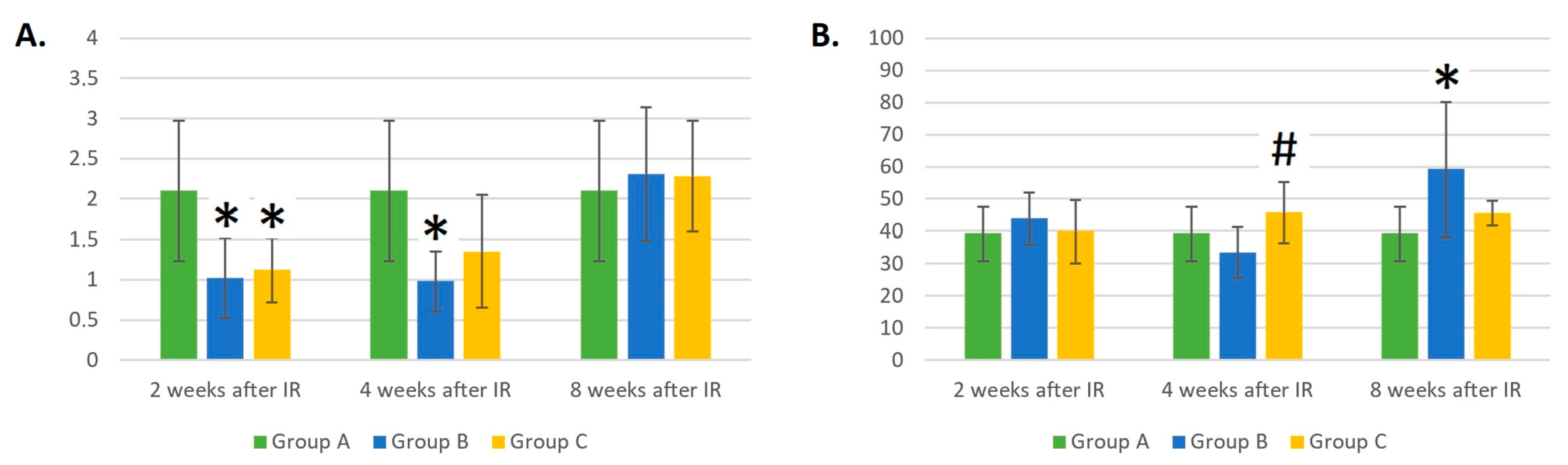
3.2. Dynamic Changes in the LYM Count of Control Mice (Group A), Mice Treated with Gamma Radiation (Group B), and the Irradiation Group with Additional Laser Treatment (Group C)
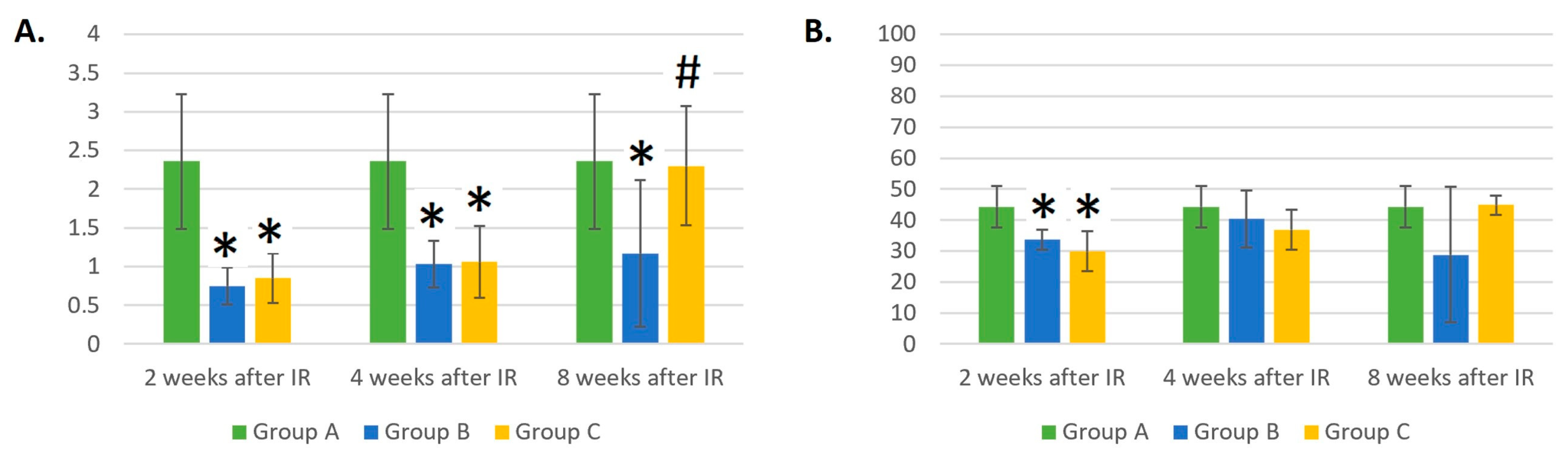
3.3. Dynamic Changes in B- and T-Cell and NK Counts in Control Mice (Group A), Mice Treated with Gamma Radiation (Group B), and the Irradiation Group with Additional Laser Treatment (Group C)
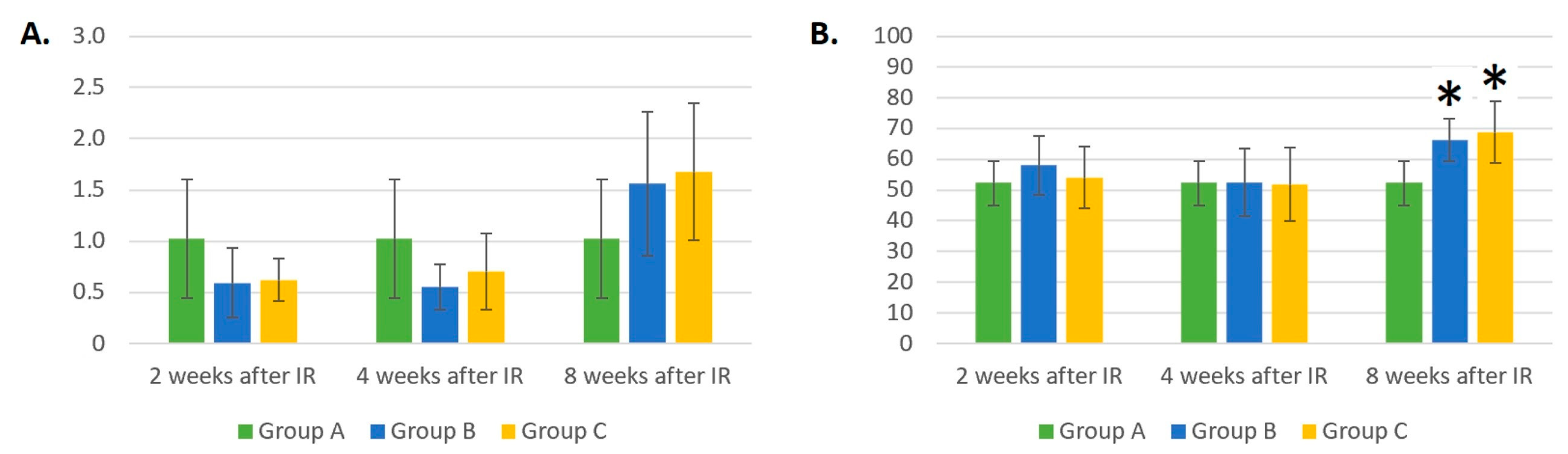
3.4. Dynamic Changes in MON Count in Control Mice (Group A), Mice Treated with Gamma Radiation (Group B), and the Irradiation Group with Additional Laser Treatment (Group C)
3.5. Dynamic Changes in the NEU Count in Control Mice (Group A), Mice Treated with Gamma Radiation (Group B), and the Irradiation Group with Additional Laser Treatment (Group C)
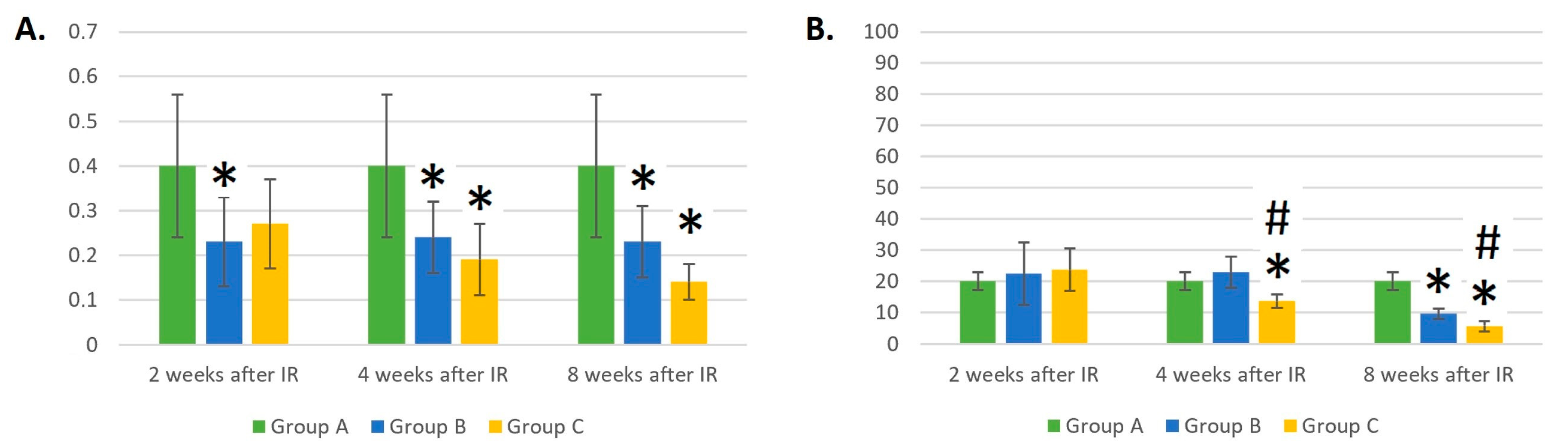
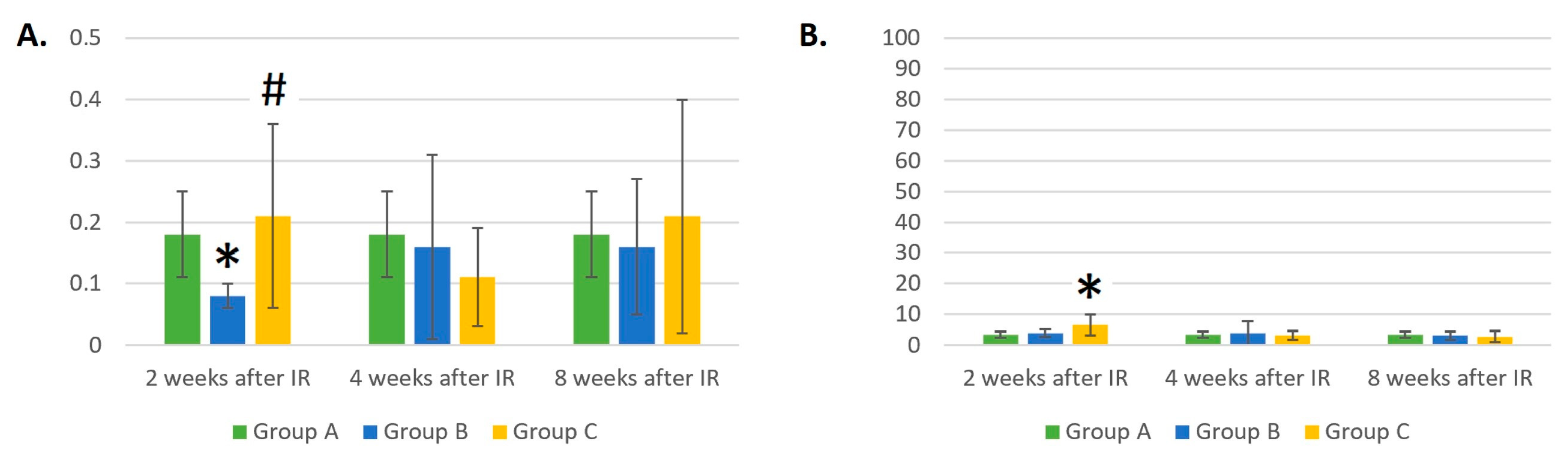
3.6. Dynamic Changes in the Eosinophil and Basophil Counts in Control Mice (Group A), Mice Treated with Gamma Radiation (Group B), and the Irradiation Group with Additional Laser Treatment (Group C)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Li, Y.; Xue, Z.; Yin, H.; Ma, H. Antiinflammatory Effect of Low-Level Laser Therapy on Staphylococcus Epidermidis Endophthalmitis in Rabbits. Lasers Med. Sci. 2012, 27, 585–591. [Google Scholar] [CrossRef]
- Assis, L.; Moretti, A.I.S.; Abrahão, T.B.; Cury, V.; Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low-Level Laser Therapy (808 Nm) Reduces Inflammatory Response and Oxidative Stress in Rat Tibialis Anterior Muscle after Cryolesion. Lasers Surg. Med. 2012, 44, 726–735. [Google Scholar] [CrossRef]
- Yamaura, M.; Yao, M.; Yaroslavsky, I.; Cohen, R.; Smotrich, M.; Kochevar, I.E. Low Level Light Effects on Inflammatory Cytokine Production by Rheumatoid Arthritis Synoviocytes. Lasers Surg. Med. 2009, 41, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Chow, R. Low Level Laser Therapy-Mechanism of Action. In Lasers in Dentistry; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2015; pp. 34–39. [Google Scholar]
- Ferreira, D.M.; Zângaro, R.A.; Villaverde, A.B.; Cury, Y.; Frigo, L.; Picolo, G.; Longo, I.; Barbosa, D.G. Analgesic Effect of He-Ne (632.8 Nm) Low-Level Laser Therapy on Acute Inflammatory Pain. Photomed. Laser Surg. 2005, 23, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Posten, W.; Wrone, D.A.; Dover, J.S.; Arndt, K.A.; Silapunt, S.; Alam, M. Low-Level Laser Therapy for Wound Healing: Mechanism and Efficacy. Dermatol. Surg. 2006, 31, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Karu, T. Primary and Secondary Mechanisms of Action of Visible to Near-IR Radiation on Cells. J. Photochem. Photobiol. B 1999, 49, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jawad, M.M.; Husein, A.; Azlina, A.; Alam, M.K.; Hassan, R.; Shaari, R. Effect of 940 Nm Low-Level Laser Therapy on Osteogenesis in Vitro. J. Biomed. Opt. 2013, 18, 128001. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kharkwal, G.B.; Sajo, M.; Huang, Y.-Y.; De Taboada, L.; McCarthy, T.; Hamblin, M.R. Dose Response Effects of 810 Nm Laser Light on Mouse Primary Cortical Neurons. Lasers Surg. Med. 2011, 43, 851–859. [Google Scholar] [CrossRef]
- Silveira, P.C.L.; Silva, L.A.; Freitas, T.P.; Latini, A.; Pinho, R.A. Effects of Low-Power Laser Irradiation (LPLI) at Different Wavelengths and Doses on Oxidative Stress and Fibrogenesis Parameters in an Animal Model of Wound Healing. Lasers Med. Sci. 2011, 26, 125–131. [Google Scholar] [CrossRef]
- Silveira, P.; Silva, L.; Tuon, T.; Freitas, T.; Streck, E.; Pinho, R. Efeitos Da Laserterapia de Baixa Potência Na Reposta Oxidativa Epidérmica Induzida Pela Cicatrização de Feridas. Braz. J. Phys. Ther. 2009, 13, 281–287. [Google Scholar] [CrossRef][Green Version]
- Gonçalves, R.V.; Novaes, R.D.; do Carmo Cupertino, M.; Moraes, B.; Leite, J.P.V.; do Carmo Gouveia Peluzio, M.; de Mello Pinto, M.V.; da Matta, S.L.P. Time-Dependent Effects of Low-Level Laser Therapy on the Morphology and Oxidative Response in the Skin Wound Healing in Rats. Lasers Med. Sci. 2013, 28, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.C.L.; da Silva, L.A.; Pinho, C.A.; De Souza, P.S.; Ronsani, M.M.; da Luz Scheffer, D.; Pinho, R.A. Effects of Low-Level Laser Therapy (GaAs) in an Animal Model of Muscular Damage Induced by Trauma. Lasers Med. Sci. 2013, 28, 431–436. [Google Scholar] [CrossRef]
- Abdul-Aziz, K.K.; Tuorkey, M.J. Argon Laser Phototherapy Could Eliminate the Damage Effects Induced by the Ionizing Radiation “Gamma Radiation” in Irradiated Rabbits. J. Photochem. Photobiol. B 2010, 99, 29–35. [Google Scholar] [CrossRef] [PubMed]
- de Lima, F.M.; Albertini, R.; Dantas, Y.; Maia-Filho, A.L.; de Loura Santana, C.; Castro-Faria-Neto, H.C.; França, C.; Villaverde, A.B.; Aimbire, F. Low-Level Laser Therapy Restores the Oxidative Stress Balance in Acute Lung Injury Induced by Gut Ischemia and Reperfusion. Photochem. Photobiol. 2013, 89, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-G.; Zhou, Y.-J.; Liu, T.C.-Y.; Yuan, J.-Q. Effects of Low-Level Laser Irradiation on Rat Skeletal Muscle Injury after Eccentric Exercise. Photomed. Laser Surg. 2009, 27, 863–869. [Google Scholar] [CrossRef]
- Voskanyan, K.; Vorozhtsova, S.; Abrosimova, A.; Mitsyn, G.; Gaevsky, V. Laser Device for the Protection of Biological Objects from the Damaging Action of Ionizing Radiation. In Proceedings of the Nanotechnologies and Biomedical Engineering, Chișinău, Republic of Moldova, 18–20 April 2013; pp. 519–521. [Google Scholar]
- Voskanyan, K.; Vorozhtsova, S.; Abrosimova, A.; Mitsyn, G.; Gaevsky, V. The Effectiveness of Radiation Damage Reduction in Mice by Laser Light in Dependence of the Time Interval between Exposures. J. Phys. Sci. Appl. 2015, 5, 291–295. [Google Scholar] [CrossRef]
- Efremova, Y.; Sinkorova, Z.; Navratil, L. Protective Effect of 940 Nm Laser on Gamma-Irradiated Mice. Photomed. Laser Surg. 2015, 33, 82–91. [Google Scholar] [CrossRef]
- Fakulta Biomedicínského Inženýrství ČVUT v Praze. Available online: https://intranet.fbmi.cvut.cz/index.php/s/FaosLzpvs7MwJNf/download?path=%2FStudent%2FDoktorsk%C3%A9%20studium%2Fbmkt%2FDiserta%C4%8Dn%C3%AD%20pr%C3%A1ce%2Fefremova&files=Yulia%20Efremova%20(2017)%20Interakce%20ionizuj%C3%ADc%C3%ADho%20a%20neionizuj%C3%ADc%C3%ADho%20z%C3%A1%C5%99en%C3%AD.%20Diserta%C4%8Dn%C3%AD%20pr%C3%A1ce.pdf (accessed on 9 January 2024).
- Huertas, R.; Luna-Bertos, E.; Ramos-Torrecillas, J.; Leyva, F.; Ruiz, C.; Garcia-Martinez, O. Effect and Clinical Implications of the Low-Energy Diode Laser on Bone Cell Proliferation. Biol. Res. Nurs. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Yu, A.; Merrill, K.; Truong, S.; Forward, K.; Morse, L.; Telander, D. The Comparative Histologic Effects of Subthreshold 532- and 810-nm Diode Micropulse Laser on the Retina. Investig. Opthalmol. 2013, 54, 2216–2224. [Google Scholar] [CrossRef]
- Saygun, I.; Karacay, S.; Serdar, M.; Ural, A.; Sencimen, M.; Kurtis, B. Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers Med. Sci. 2008, 23, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Lowell, C.A.; Schnoor, M.; Uribe-Querol, E. Neutrophils: Their Role in Innate and Adaptive Immunity 2017. J. Immunol. Res. 2017, 2017, 9748345. [Google Scholar] [CrossRef]
- Akuthota, P.; Wang, H.B.; Spencer, L.A.; Weller, P.F. Immunoregulatory Roles of Eosinophils: A New Look at a Familiar Cell. Clin. Exp. Allergy 2008, 38, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Karasuyama, H.; Miyake, K.; Yoshikawa, S.; Yamanishi, Y. Multifaceted Roles of Basophils in Health and Disease. J. Allergy Clin. Immunol. 2018, 142, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Z.-M.; Wang, X.-F. The Roles of Basophils in Mediating the Immune Responses. Eur. J. Inflamm. 2021, 19, 205873922110476. [Google Scholar] [CrossRef]
- de Sousa, A.P.C.; Paraguassú, G.M.; Silveira, N.T.T.; de Souza, J.; Cangussú, M.C.T.; dos Santos, J.N.; Pinheiro, A.L.B. Laser and LED phototherapies on angiogenesis. Lasers Med. Sci. 2013, 28, 981–987. [Google Scholar] [CrossRef]
- Mirsky, N.; Krispel, Y.; Shoshany, Y.; Maltz, L.; Oron, U. Promotion of Angiogenesis by Low Energy Laser Irradiation. Antioxid. Redox Signal. 2002, 4, 785–790. [Google Scholar] [CrossRef]
- Loinard, C.; Ribault, A.; Lhomme, B.; Benderitter, M.; Flamant, S.; Paul, S.; Dubois, V.; Lai, R.C.; Lim, S.K.; Tamarat, R. HuMSC-EV induce monocyte/macrophage mobilization to orchestrate neovascularization in wound healing process following radiation injury. Cell Death Discov. 2023, 9, 38. [Google Scholar] [CrossRef]
- Zalesskaya, G.A.; Batay, L.E.; Koshlan, I.V.; Nasek, V.M.; Zilberman, R.D.; Milevich, T.I.; Govorun, R.D.; Koshlan, N.A.; Blaga, P. Combined Impact of Gamma and Laser Radiation on Peripheral Blood of Rates in vivo. J. Appl. Spectrosc. 2017, 84, 796–803. [Google Scholar] [CrossRef]
- Zalesskaya, G.A.; Nasek, V.M.; Zilberman, R.D. The Radioprotective Effects of Low-Intensity Laser Radiation on Rat Peripheral Blood Cells. Biophysics 2019, 64, 463–469. [Google Scholar] [CrossRef]
- Servetto, N.; Cremonezzi, D.; Simes, J.; Moya, M.; Soriano, F.; Palma, J.; Campana, V. Evaluation of inflammatory biomarkers associated with oxidative stress and histological assessment of low-level laser therapy in experimental myopathy. Lasers Surg. Med. 2010, 42, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Sun, Z.; Zhang, L.; Li, X.; Dong, Y.; Liu, T. Effects of low-level laser therapy on ROS homeostasis and expression of IGF-1 and TGF-β1 in skeletal muscle during the repair process. Lasers Med. Sci. 2013, 28, 725–734. [Google Scholar] [CrossRef] [PubMed]



| Group A | Group B | Group C |
|---|---|---|
| Control group of mice (neither gamma nor laser irradiation)—11 mice. | Mice were irradiated with gamma irradiation a only. Tests were performed on days 14 (2 weeks), 28 (4 weeks), and 56 (8 weeks) after gamma radiation. Each group was composed of 9 mice, for a total of 27 animals. | Mice were irradiated with a laser b after gamma irradiation a. Tests were performed on days 14 (2 weeks) 28 (4 weeks), and 56 (8 weeks) after gamma radiation. Each group was composed of 9 mice, for a total of 27 animals. |
| Power, Radiant Flux in Pulse Amplitude | Energy Density in One Treatment (200 s) | Energy Density in the Series of 5 Procedures | |
|---|---|---|---|
| Initial (0.07 cm2) | 25 W | 856 J/cm2 | 4285 J/cm2 |
| Body surface (0.07 cm2) | 15 W | 514 J/cm2 | 2570 J/cm2 |
| Target organ (1 cm2) | 7.5 W | 18 J/cm2 | 90 J/cm2 |
| Antibody | Fluorochrome | Emission Maximum (nm) |
|---|---|---|
| anti-Mouse CD3 | phycoerythrin | 578 |
| anti-Mouse CD45R/B220 | peridinin chlorophyll | 678 |
| anti-Mouse NK—1.1 | phycoerythrin + cyanine dye | 785 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Průcha, J.; Šinkorová, Z.; Carrillo, A.; Burda, T.; Čuprová, J. Radioreparative Effect of Diode Laser on Leukopoiesis Recovery: A Pilot Study. Life 2024, 14, 123. https://doi.org/10.3390/life14010123
Průcha J, Šinkorová Z, Carrillo A, Burda T, Čuprová J. Radioreparative Effect of Diode Laser on Leukopoiesis Recovery: A Pilot Study. Life. 2024; 14(1):123. https://doi.org/10.3390/life14010123
Chicago/Turabian StylePrůcha, Jaroslav, Zuzana Šinkorová, Anna Carrillo, Tomáš Burda, and Julie Čuprová. 2024. "Radioreparative Effect of Diode Laser on Leukopoiesis Recovery: A Pilot Study" Life 14, no. 1: 123. https://doi.org/10.3390/life14010123
APA StylePrůcha, J., Šinkorová, Z., Carrillo, A., Burda, T., & Čuprová, J. (2024). Radioreparative Effect of Diode Laser on Leukopoiesis Recovery: A Pilot Study. Life, 14(1), 123. https://doi.org/10.3390/life14010123






