Chronic Kidney Disease Interplay with Comorbidities and Carbohydrate Metabolism: A Review
Abstract
1. Introduction
2. Carbohydrate Metabolism and Its Impairment in CKD Patients
3. CKD Controls Cellular Growth Homeostasis
- It is worth noting that the GH/IGF system is partially influenced by calorie and protein intake, and inadequate nutrition can therefore result in compromised growth [34]. In CKD, a wasting or cachectic syndrome is observed, and while the mechanisms behind cachexia are intricate, they encompass factors such as anorexia, nausea, vomiting, increased basal metabolic rate, loss of lean body mass, and reductions in serum proteins like albumin, transferrin, and prealbumin [17]. Other factor such as genetic factors also play a role in determining a child’s height potential even if they may inherit height traits from their parents [35]. Gender can influence growth patterns, with boys typically experiencing growth spurts during adolescence, while girls tend to grow earlier and at a steadier pace [36]. Certain genetic syndromes like syndromic kidney diseases affecting the kidneys can have a direct impact on growth due to the underlying genetic mutations [37]. Birth-related factors including premature birth may lead to delayed development and lower birth weights in infants [38]. Those born small for their gestational age could face growth restrictions due to intrauterine factors. Infants requiring intensive care after birth may experience growth challenges from medical interventions and associated stress. Prematurity can result in delayed development and lower birth weights, impacting infant growth [38]. Growth restrictions may affect infants born small for gestational age due to intrauterine factors, influencing their overall development. Other comorbid health conditions involving the central nervous system, liver, or heart can impact a child’s overall health and growth [39]. The age at which CKD begins can affect growth patterns, as children who develop CKD at a younger age may experience more significant growth issues [40]. The extent of kidney damage and the level of residual renal function can influence growth in children undergoing dialysis treatments. Metabolic disturbances such as salt and water metabolism imbalance can lead to fluid retention or dehydration that affect growth [41]. Metabolic acidosis, characterized by acidic blood pH, can affect both bone health and growth. In CKD-Mineral and Bone Disorder (MBD), disruptions in mineral and bone metabolism may contribute to issues with growth [42]. Anemia, a common complication of CKD, can lead to fatigue and reduced physical activity, potentially affecting growth [43]. Various factors can contribute to malnutrition in CKD, including altered taste sensation, anorexia (loss of appetite), vomiting, dietary restrictions, and nutrient losses during dialysis [44]. Protein-energy wasting is a complex issue involving factors such as infections, inflammation, uremic toxins, oxidative stress, and inflammatory cytokines [45]. These can collectively impair growth and nutritional status.
4. Hormonal Disturbances
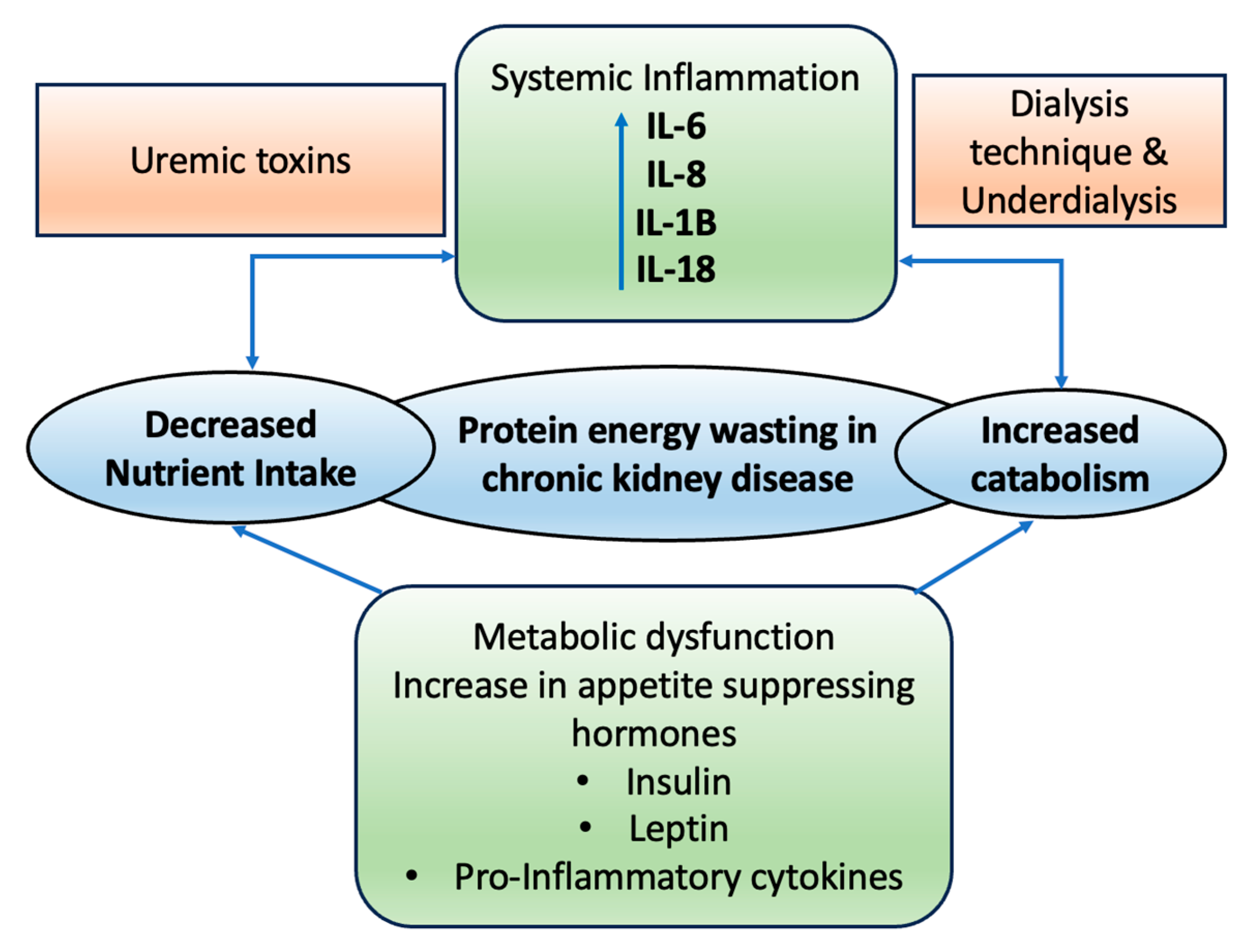
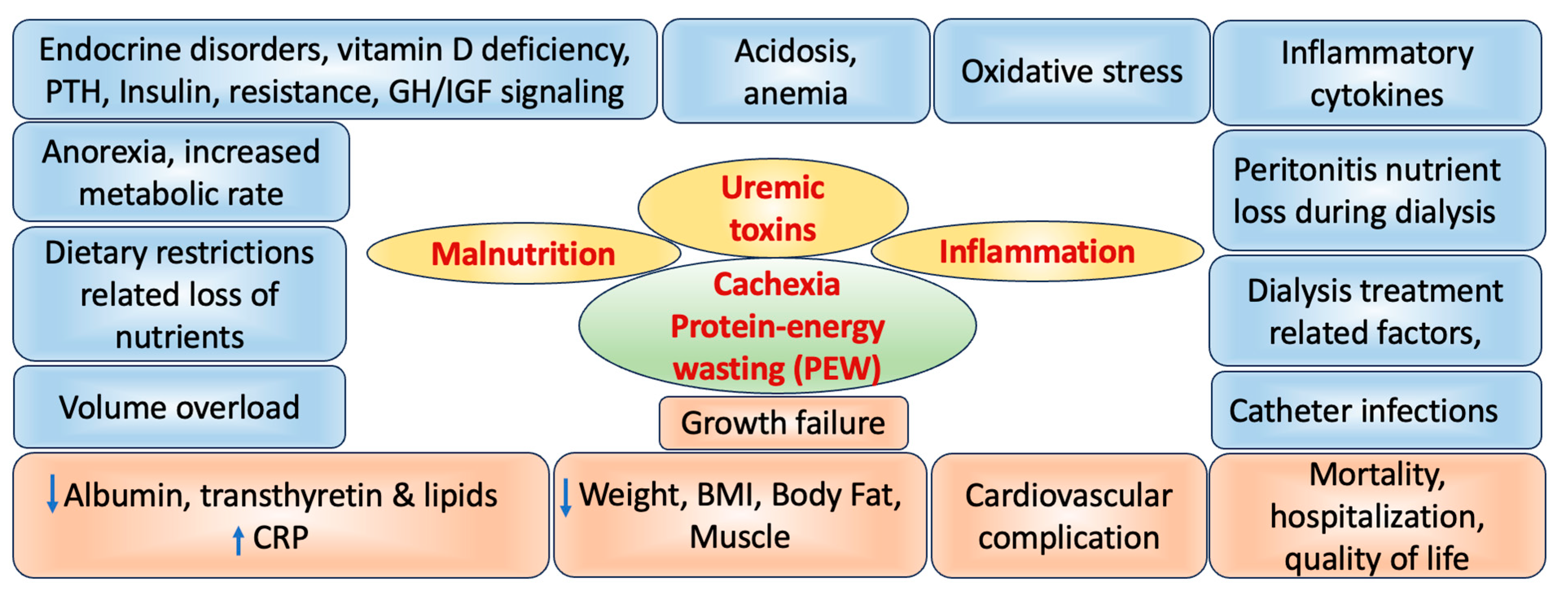
5. Role of CKD on Wasting and Malnutrition
6. Association of CKD Inflammation, Oxidative Stress and Cardiovascular Diseases
7. Effect of CKD on Functioning
7.1. Cognitive Dysfunction
7.2. On Emotional Functioning
7.3. On Taste Perception
8. Carbohydrate Diet in CKD
9. Future Recommendation
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kopyt, N.P. Slowing progression along the renal disease continuum. J. Am. Osteopath. Assoc. 2005, 105, 207–215. [Google Scholar] [PubMed]
- Kundhal, K.; Lok, C.E. Clinical epidemiology of cardiovascular disease in chronic kidney disease. Nephron Clin. Pract. 2005, 101, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, D.; Lawlor, D.A.; Patel, R.; Carson, C.; Shah Ebrahim, S. The association of renal impairment and all-cause and cardiovascular disease mortality. Nephrol. Dial. Transplant. 2010, 25, 1191–1199. [Google Scholar] [CrossRef]
- Zoccali, C.; Kramer, A.; Jager, K.J. Chronic kidney disease and end-stage renal disease—A review produced to contribute to the report ‘the status of health in the European union: Towards a healthier Europe. NDT Plus 2010, 3, 213–224. [Google Scholar] [CrossRef]
- Locatelli, F.; Pozzoni, P.; Tentori, F.; Del Vecchio, L. Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2003, 18 (Suppl. S7), vii2–vii9. [Google Scholar] [CrossRef]
- Adams, G.R.; Vaziri, N.D. Skeletal muscle dysfunction in chronic renal failure: Effects of exercise. Am. J. Physiol. Renal. Physiol. 2006, 290, 753–761. [Google Scholar] [CrossRef]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from kidney disease: Improving global outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef]
- Vaziri, N.D. Dyslipidemia of chronic renal failure: The nature, mechanisms, and potential consequences. Am. J. Physiol. Renal. Physiol. 2006, 290, 262–272. [Google Scholar] [CrossRef]
- Siew, E.D.; Ikizler, T.A. Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin. Dial. 2010, 23, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Apovian, C.M.; Aronne, L.J.; Astrup, A.; Cantley, L.C.; Ebbeling, C.B.; Heymsfield, S.B.; Johnson, J.D.; King, J.C.; Krauss, R.M.; et al. Competing paradigms of obesity pathogenesis: Energy balance versus carbohydrate-insulin models. Eur. J. Clin. Nutr. 2022, 76, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L. Insulin resistance and muscle metabolism in chronic kidney disease. Int. Scholarly Res. Notices 2013, 2013, 329606. [Google Scholar] [CrossRef] [PubMed]
- Wahba, I.; Mak, R.H. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H. Insulin and its role in chronic kidney disease. Pediatr. Nephrol. 2008, 23, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Kaskel, F. Growth hormone axis in chronic kidney disease. Pediatr. Nephrol. 2008, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [CrossRef]
- Aveles, P.R.; Criminácio, C.R.; Gonçalves, S.; Bignelli, A.T.; Claro, L.M.; Siqueira, S.S.; Nakao, L.S.; Pecoits-Filho, R. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin. Pract. 2010, 116, c294–c299. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Kuchta, A.; Pacanis, A.; Kortas-Stempak, B.; Çwiklińska, A.; Ziętkiewicz, M.; Renke, M.; Rutkowski, B. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press Res. 2011, 34, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.; McCommis, K.S.; Finck, B.N. Carbohydrate metabolism. Surgery 2009, 27, 6–10. [Google Scholar]
- McCommis, K.S.; Finck, B.N. Mitochondrial pyruvate transport: A historical perspective and future research directions. Biochem. J. 2015, 466, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Exton, J.H.; Park, C.R. Control of gluconeogenesis in liver: I. General features of gluconeogenesis in the perfused livers of rats. J. Biol. Chem. 1967, 242, 2622–2636. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflügers Archiv Eur. J. Physiol. 2020, 472, 1345–1370. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic kidney disease and its complications. Prim. Care 2008, 35, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Yuen, D.A.; Stead, B.E.; Zhang, Y.; White, K.E.; Kabir, M.G.; Thai, K.; Advani, S.L.; Connelly, K.A.; Takano, T.; Zhu, L.; et al. eNOS deficiency predisposes podocytes to injury in diabetes. J. Am. Soc. Nephrol. 2012, 23, 1810–1823. [Google Scholar] [CrossRef]
- Basturk, T.; Unsal, A. What is the frequency of carbohydrate metabolism disorder in CKD? J. Ren. Care 2012, 38, 15–21. [Google Scholar] [CrossRef]
- Seikaly, M.G.; Salhab, N.; Gipson, D.; Yiu, V.; Stablein, D. Stature in children with chronic kidney disease: Analysis of NAPRTCS database. Pediatr. Nephrol. 2006, 21, 793–799. [Google Scholar] [CrossRef]
- Seikaly, M.G.; Salhab, N.; Warady, B.A.; Stablein, D. Use of rhGH in children with chronic kidney disease: Lessons from NAPRTCS. Pediatr. Nephrol. 2007, 22, 1195–1204. [Google Scholar] [CrossRef]
- Smith, J.M.; Stablein, D.M.; Munoz, R.; Munoz, R.; Hebert, D.; McDonald, R.A. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr. Transplant. 2007, 11, 366–373. [Google Scholar] [CrossRef]
- Mak, R.H.; Cheung, W.; Cone, R.D.; Marks, D.L. Orexigenic and anorexigenic mechanisms in the control of nutrition in chronic kidney disease. Pediatr. Nephrol. 2005, 20, 427–431. [Google Scholar] [CrossRef]
- Rees, L.; Mak, R.H. Nutrition and growth in children with chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 615–623. [Google Scholar] [CrossRef]
- Brook, C.G. Normal growth. In Clinical Paediatric Endocrinology; Brook, C.G., Ed.; Blackwell: Oxford, UK, 1981; pp. 63–73. [Google Scholar]
- Friedman, D.J. Genes and environment in chronic kidney disease hotspots. Curr. Opin. Nephrol. Hypertens. 2019, 28, 87–96. [Google Scholar] [CrossRef]
- Soliman, A.S.; Kamal, N.M.; Abukhatwah, M.W.; El Mashad, G.M.; Abd El Gowaad, I.R.; Halabi, Y.A.; Alalyani, S.A.; Qari, S.A.; Afifi, W.E. Sexual maturity of children on regular hemodialysis: Role of testosterone and estradiol, a tertiary multicenter experience. Medicine 2022, 101, e28689. [Google Scholar] [CrossRef]
- Hildebrandt, F. Genetic kidney diseases. Lancet 2010, 375, 1287–1295. [Google Scholar] [CrossRef]
- Grillo, M.A.; Mariani, G.; Ferraris, J.R. Prematurity and low birth weight in neonates as a risk factor for obesity, hypertension, and chronic kidney disease in pediatric and adult age. Front. Med. 2022, 8, 769734. [Google Scholar] [CrossRef]
- Haffner, D. Strategies for optimizing growth in children with chronic kidney disease. Front. Pediatr. 2020, 8, 399. [Google Scholar] [CrossRef]
- Rodig, N.M.; McDermott, K.C.; Schneider, M.F.; Hotchkiss, H.M.; Yadin, O.; Seikaly, M.G.; Furth, S.L.; Warady, B.A. Growth in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children Study. Pediatr. Nephrol. 2014, 29, 1987–1995. [Google Scholar] [CrossRef]
- Dhondup, T.; Qian, Q. Acid-base and electrolyte disorders in patients with and without chronic kidney disease: An update. Kidney Dis. 2017, 3, 136–148. [Google Scholar] [CrossRef]
- Shroff, R.; Wesseling-Perry, K.; Bacchetta, J. Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Pediatr. Nephrol. 2022, 2, 1751–1778. [Google Scholar]
- Mathias, S.D.; Blum, S.I.; Sikirica, V.; Johansen, K.L.; Colwell, H.H.; Okoro, T. Symptoms and impacts in anemia of chronic kidney disease. J. Patient Rep. Outcomes 2020, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Iorember, F.M. Malnutrition in chronic kidney disease. Front. Pediatr. 2018, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein energy-wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254. [Google Scholar] [CrossRef]
- Tönshoff, B.; Powell, D.R.; Zhao, D.; Durham, S.K.; Coleman, M.E.; Domene, H.M.; Blum, W.F.; Moore, L.C.; Kaskel, F.J. Decreased hepatic insulin-like growth factor (IGF)-I and increased IGF binding protein-1 and -2 gene expression in experimental uremia. Endocrinology 1997, 138, 938–946. [Google Scholar] [CrossRef]
- Eknoyan, G.; Levin, N.W. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification: Kidney Disease Outcome Quality Initiative. Am. J. Kidney Dis. 2002, 39, S14–S214. [Google Scholar]
- Zivicnjak, M.; Franke, D.; Filler, G.; Haffner, D.; Froede, K.; Nissel, R.; Haase, S.; Offner, G.; Ehrich, J.H.; Querfeld, U. Growth impairment shows an age-dependent pattern in boys with chronic kidney disease. Pediatr. Nephrol. 2007, 22, 420–429. [Google Scholar] [CrossRef]
- Domrongkitchaiporn, S.; Pongskul, C.; Sirikulchayanonta, V.; Stitchantrakul, W.; Leeprasert, V.; Ongphiphadhanakul, B.; Radinahamed, P.; Rajatanavin, R. Bone histology and bone mineral density after correction of acidosis in distal renal tubular acidosis. Kidney Int. 2002, 62, 2160–2166. [Google Scholar] [CrossRef]
- Fine, R.N.; Stablein, D. Long-term use of recombinant human growth hormone in pediatric allograft recipients: A report of the NAPRTCS transplant registry. Pediatr. Nephrol. 2005, 20, 404–408. [Google Scholar] [CrossRef]
- Gil, S.; Vaiani, E.; Guercio, G.; Ciaccio, M.; Turconi, A.; Delgado, N.; Rivarola, M.A.; Belgorosky, A. Effectiveness of rhGH treatment on final height of renal-transplant recipients in childhood. Pediatr. Nephrol. 2012, 27, 1005–1009. [Google Scholar] [CrossRef]
- Hodson, E.M.; Willis, N.S.; Craig, J.C. Growth hormone for children with chronic kidney disease. Cochrane Database Syst. Rev. 2012, 2, CD003264. [Google Scholar] [CrossRef] [PubMed]
- Rees, L. Growth hormone therapy in children with CKD after more than two decades of practice. Pediatr. Nephrol. 2016, 31, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Mahan, J.D.; Warady, B.A.; Consensus Committee. Assessment and treatment of short stature in pediatric patients with chronic kidney disease: A consensus statement. Pediatr. Nephrol. 2006, 21, 917–930. [Google Scholar] [CrossRef]
- Tonshoff, B.; Kiepe, D.; Ciarmatori, S. Growth hormone/insulin-like growth factor system in children with chronic renal failure. Pediatr. Nephrol. 2005, 20, 279–289. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.K.; Kam, K.K.; Yan, B.P.; Lam, Y.Y. Renin–angiotensin–aldosterone system blockade for cardiovascular diseases: Current status. Br. J. Pharmacol. 2010, 160, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 319–330. [Google Scholar] [CrossRef]
- Shih, H.M.; Wu, C.J.; Lin, S.L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Cooper, A.C. Erythropoietin resistance: The role of inflammation and pro-inflammatory cytokines. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S11), 39–43. [Google Scholar] [CrossRef]
- Selamet, U.; Katz, R.; Ginsberg, C.; Rifkin, D.E.; Fried, L.F.; Kritchevsky, S.B.; Hoofnagle, A.N.; Bibbins-Domingo, K.; Drew, D.; Harris, T.; et al. Serum calcitriol concentrations and kidney function decline, heart failure, and mortality in elderly community-living adults: The health, aging, and body composition study. Am. J. Kidney Dis. 2018, 72, 419–428. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, J.M. Klotho and postmenopausal hormone replacement therapy in women with chronic kidney disease. J. Menopausal Med. 2018, 24, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, M.J.; Kim, S.J.; Oh, H.J.; Kim, H.R.; Han, J.H.; Koo, H.M.; Doh, F.M.; Park, J.T.; Han, S.H.; et al. Preservation of renal function by thyroid hormone replacement therapy in chronic kidney disease patients with subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2012, 97, 2732–2740. [Google Scholar] [CrossRef]
- Snyder, S.; Pendergraph, B. Detection and evaluation of chronic kidney disease. Am. Fam. Physician 2005, 72, 1723–1732. [Google Scholar]
- Stevens, P.E.; O’Donoghue, D.J.; De Lusignan, S.; Van Vlymen, J.; Klebe, B.; Middleton, R.; Hague, N.; New, J.; Farmer, C.K. Chronic kidney disease management in the United Kingdom: NE-OERICA project results. Kidney Int. 2007, 72, 92–99. [Google Scholar] [CrossRef]
- Pupim, L.B.; Cuppari, L.; Ikizler, T.A. Nutrition and metabolism in kidney disease. Semin. Nephrol. 2006, 26, 134–157. [Google Scholar] [CrossRef]
- Brem, A.S.; Lambert, C.; Hill, C.; Kitsen, J.; Shemin, D.G. Prevalence of protein malnutrition in children maintained on peritoneal dialysis. Pediatr. Nephrol. 2002, 17, 527–530. [Google Scholar] [CrossRef]
- Sozeri, B.; Mir, S.; Kara, O.D.; Dincel, N. Growth impairment and nutritional status in children with chronic kidney disease. Iran J. Pediatr. 2011, 21, 271–277. [Google Scholar]
- Apostolou, A.; Printza, N.; Karagiozoglou-Lampoudi, T.; Dotis, J.; Papachristou, F. Nutrition assessment of children with advanced stages of chronic kidney disease-A single center study. Hippokratia 2014, 18, 212–216. [Google Scholar]
- Dai, L.; Mukai, H.; Lindholm, B.; Heimbürger, O.; Barany, P.; Stenvinkel, P.; Qureshi, A.R. Clinical global assessment of nutritional status as predictor of mortality in chronic kidney disease patients. PLoS ONE 2017, 12, e0186659. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Cheung, W.W.; Zhan, J.Y.; Shen, Q.; Foster, B.J. Cachexia and protein-energy wasting in children with chronic kidney disease. Pediatr. Nephrol. 2012, 27, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E. Malnutrition: A frequent misdiagnosis for hemodialysis patients. J. Clin. Investig. 2002, 110, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E. Insights into the abnormalities of chronic renal disease attributed to malnutrition. J. Am. Soc. Nephrol. 2002, 13 (Suppl. S1), S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Cheung, W.; Cone, R.D.; Marks, D.L. Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney Int. 2006, 69, 794–797. [Google Scholar] [CrossRef]
- Ingulli, E.G.; Mak, R.H. Growth in children with chronic kidney disease: Role of nutrition, growth hormone, dialysis, and steroids. Curr. Opin. Pediatr. 2014, 26, 187–192. [Google Scholar] [CrossRef]
- Mak, R.H. Cachexia in children with chronic kidney disease: Challenges in diagnosis and treatment. Curr. Opin. Support Palliat. Care 2016, 10, 293–297. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef]
- Kopple, J.D.; Berg, R.; Houser, H.; Steinman, T.I.; Teschan, P. Nutritional status of patients with different levels of chronic renal insufficiency. Modification of diet in renal disease (MDRD) study group. Kidney Int. Suppl. 1989, 27, S184–S194. [Google Scholar] [PubMed]
- Hollinger, D.; Maroni, B.J.; Merrill, D.; Scherch, L.K.; Schulman, G.; Wang, S.R.; Zimmer, G.S. Relationship between nutritional status and the glomerular filtration rate: Results from the MDRD study. Kidney Int. 2000, 57, 1688–1703. [Google Scholar]
- Costelli, P.; Reffo, P.; Penna, F.; Autelli, R.; Bonelli, G.; Baccino, F.M. Ca-dependent proteolysis in muscle wasting. Int. J. Biochem. Cell Biol. 2005, 37, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Poulianiti, K.P.; Kaltsatou, A.; Mitrou, G.I.; Jamurtas, A.Z.; Koutedakis, Y.; Maridaki, M.; Stefanidis, I.; Sakkas, G.K.; Karatzaferi, C. Systemic redox imbalance in chronic kidney disease. Oxid. Med. Cell. Longev. 2016, 2016, 8598253. [Google Scholar] [CrossRef]
- Ling, X.C.; Kuo, K. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Descamps-Latscha, B.; Drüeke, T.; Witko-Sarsat, V. Dialysis-induced oxidative stress: Biological aspects, clinical consequences, and therapy. Semin. Dial. 2001, 14, 193–199. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, J.S.; Wu, W.M.; Liao, T.N.; Chu, P.; Lin, S.H.; Chuang, C.H.; Lin, Y.F. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible dialysis membranes. Nephrol. Dial. Transplant. 2005, 20, 1134–1139. [Google Scholar] [CrossRef]
- Miranda-Díaz, A.G.; Pazarín-Villaseñor, L.; Yanowsky-Escatell, F.G.; Andrade-Sierra, J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J. Diabetes Res. 2016, 2016, 7047238. [Google Scholar] [CrossRef]
- Mahmoodpoor, F.; Saadat, Y.R.; Barzegari, A.; Ardalan, M.; Vahed, S.Z. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef]
- San, A.; Fahim, M.; Campbell, K.; Hawley, C.M.; Johnson, D.W. The role of oxidative stress and systemic inflammation in kidney disease and its associated cardiovascular risk. Nov. Prospect. Oxidative Nitrosative Stress 2018, 8, 3. [Google Scholar]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive oxygen species in macrophages: Sources and targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Heimburger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Fried, L.F.; Crump, C.; Bleyer, A.J.; Manolio, T.A.; Tracy, R.P.; Furberg, C.D.; Psaty, B.M. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2002, 107, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between albuminuria, kidney function and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Clapp, B.R.; Hingorani, A.D.; Kharbanda, R.K.; Mohamed-Ali, V.; Stephens, J.W.; Vallance, P.; MacAllister, R.J. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc. Res. 2004, 64, 172–178. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C.; Muros de Fuentes, M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, T.; Nakano, K.; Hashiguchi, T.; Iwamoto, H.; Miura, K.; Yoshimura, Y.; Hanyu, N.; Hirata, K.; Imakuma, M.; Motomiya, Y.; et al. Elevation of N-(carboxymethyl) valine residue in hemoglobin of diabetic patients: Its role in the development of diabetic nephropathy. Diabetes Care 2001, 24, 891–896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santhanam, A.V.; d’Uscio, L.V.; Katusic, Z.S. Erythropoietin increases bioavailability of tetrahydrobiopterin and protects cerebral microvasculature against oxidative stress induced by eNOS uncoupling. J. Neurochem. 2014, 131, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, G.; Reboldi, G.; Verdecchia, P. High-normal serum creatinine concentration is a predictor of cardiovascular risk in essential hypertension. Arch. Intern. Med. 2001, 161, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Hailpern, S.M.; Melamed, M.L.; Cohen, H.W.; Hostetter, T.H. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J. Am. Soc. Nephrol. 2007, 18, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Ackerson, L.; Tamura, M.K.; Le Blanc, P.; Kusek, J.W.; Sehgal, A.R.; Cohen, D.; Anderson, C.; Appel, L.; DeSalvo, K.; et al. Chronic Renal Insufficiency Cohort Investigators. Chronic kidney disease and cognitive function in older adults: Findings from the Chronic Renal Insufficiency Cohort Cognitive Study. J. Am. Geriatr. Soc. 2010, 58, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kurella, M.; Mapes, D.L.; Port, F.K.; Chertow, G.M. Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2006, 21, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Griva, K.; Stygall, J.; Hankins, M.; Davenport, A.; Harrison, M.; Newman, S.P. Cognitive impairment and 7-year mortality in dialysis patients. Am. J. Kidney Dis. 2010, 56, 693–703. [Google Scholar] [CrossRef]
- Kurella, M.C.; Glenn, M.; Luan, J.; Yaffe, K. Cognitive impairment in chronic kidney disease. J. Am. Geriatr. Soc. 2004, 52, 1863–1869. [Google Scholar] [CrossRef]
- Murray, A.M.; Knopman, D.S. Cognitive impairment in CKD: No longer an occult burden. Am. J. Kidney Dis. 2010, 56, 615–618. [Google Scholar] [CrossRef]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Tabatabai, S.; Tighiouart, H.; Elsayed, E.; Bansal, N.; Griffith, J.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Cardiovascular outcomes and all-cause mortality: Exploring the interaction between CKD and cardiovascular disease. Am. J. Kidney Dis. 2006, 48, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Moreira, J.M.; Kummer, A.M.; Barbosa, I.G.; Teixeira, A.L.; Silva, A.C.S. Cognitive alterations in chronic kidney disease: An update. J. Bras. Nefrol. 2014, 36, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Bhadelia, R.; Tighiouart, H.; Bovak, V.; Scott, T.M.; Lou, K.V.; Shaffi, K.; Weiner, D.E.; Sarnak, M.J. Anatomic brain disease in hemodialysis patients: A cross-sectional study. Am. J. Kidney Dis. 2013, 61, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Pringle, E.; Phillips, C.; Thijs, L.; Davidson, C.; Staessen, J.A.; de Leeuw, P.W.; Jaaskivi, M.; Nachev, C.; Parati, G.; T O’Brien, E.; et al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J. Hypertens. 2003, 21, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Flythe, J.E.; Inrig, J.K.; Shafi, T.; Chang, T.I.; Cape, K.; Dinesh, K.; Kunaparaju, S.; Brunelli, S.M. Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am. J. Kidney Dis. 2013, 61, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.C.; Hsieh, T.J.; Lin, Y.L.; Hsieh, Y.T.; Li, W.Z.; Chang, J.M.; Ko, C.H.; Kao, E.F.; Jaw, T.S.; Liu, G.C. Widespread white matter alterations in patients with end-stage renal disease: A voxelwise diffusion tensor imaging study. Am. J. Neuroradiol. 2013, 34, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L. Depression in patients with chronic renal disease: What we know and what we need to know. J. Psychosom. Res. 2002, 53, 951–956. [Google Scholar] [CrossRef]
- Davison, S.N.; Jhangri, G.S. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J. Pain Symptom Manag. 2010, 39, 477–485. [Google Scholar] [CrossRef]
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013, 84, 179–191. [Google Scholar] [CrossRef]
- Hedayati, S.S.; Minhajuddin, A.T.; Afshar, M.; Toto, R.D.; Trivedi, M.H.; Rush, A.J. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA 2010, 303, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Cukor, D.; Fruchter, Y.; Ver Halen, N.; Naidoo, S.; Patel, A.; Saggi, S.J. A preliminary investigation of depression and kidney functioning in patients with chronic kidney disease. Nephron Clin. Pract. 2012, 122, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.H.; Guo, H.R.; Livneh, H.; Lu, M.C.; Yen, M.L.; Tsai, T.Y. Increased risk of progression to dialysis or death in CKD patients with depressive symptoms: A prospective 3-year follow-up cohort study. J. Psychosom. Res. 2015, 79, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Kittiskulnam, P.; Sheshadri, A.; Johansen, K.L. Consequences of CKD on functioning. Semin. Nephrol. 2016, 36, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Xie, D.; Jordan, N.; Kop, W.J.; Krousel-Wood, M.; Tamura, M.K.; Kusek, J.W.; Ford, V.; Rosen, L.K.; Strauss, L.; et al. CRIC Study Group Investigators CRIC Study Group Investigators. Factors associated with depressive symptoms and use of antidepressant medications among participants in the Chronic Renal Insufficiency Cohort (CRIC) and Hispanic-CRIC Studies. Am. J. Kidney Dis. 2012, 60, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Middleton, R.A.; Allman-Farinelli, M.A. Taste sensitivity is altered in patients with chronic renal failure receiving continuous ambulatory peritoneal dialysis. J. Nutr. 1999, 129, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Mori, Y.; Masami, O.; Hiroko, N.; Adachi, T.; Sugishita, C.; Sonomura, K.; Kimura, T.; Kishimoto, N.; Nakagawa, H.; et al. Sodium restriction improves the gustatory threshold for salty taste in patients with chronic kidney disease. Kidney Int. 2009, 76, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Manley, K.J.; Haryono, R.Y.; Keast, R.S. Taste changes and saliva composition in chronic kidney disease. Ren. Soc. Australas J. 2012, 8, 56–60. [Google Scholar]
- van der Eijk, I.; Allman Farinelli, M.A. Taste testing in renal patients. J. Ren. Nutr. 1997, 7, 3–9. [Google Scholar] [CrossRef]
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D. Taste perception in kidney disease and relationship to dietary sodium intake. Appetite 2014, 83, 236–241. [Google Scholar] [CrossRef]
- Nam, K.H.; An, S.Y.; Joo, Y.S.; Lee, S.; Yun, H.R.; Jhee, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Carbohydrate-rich diet is associated with increased risk of incident chronic kidney disease in non-diabetic subjects. J. Clin. Med. 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.V.; Westerlund, P.; Bygren, P. A low-carbohydrate diet may prevent end-stage renal failure in type 2 diabetes. A case report. Nutr. Metab. 2006, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010, 120, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Abbott, K.C.; Salahudeen, A.K.; Kilpatrick, R.D.; Horwich, T.B. Survival advantages of obesity in dialysis patients. Am. J. Clin. Nutr. 2005, 81, 543–554. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Cupisti, A.; D’Alessandro, C.; Finato, V.; Del Corso, C.; Catania, B.; Caselli, G.M.; Egidi, M.F. Assessment of physical activity, capacity and nutritional status in elderly peritoneal dialysis patients. BMC Nephrol. 2017, 18, 180. [Google Scholar] [CrossRef]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37 (Suppl. S2), S66–S70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushwaha, R.; Vardhan, P.S.; Kushwaha, P.P. Chronic Kidney Disease Interplay with Comorbidities and Carbohydrate Metabolism: A Review. Life 2024, 14, 13. https://doi.org/10.3390/life14010013
Kushwaha R, Vardhan PS, Kushwaha PP. Chronic Kidney Disease Interplay with Comorbidities and Carbohydrate Metabolism: A Review. Life. 2024; 14(1):13. https://doi.org/10.3390/life14010013
Chicago/Turabian StyleKushwaha, Radha, Pothabathula Seshu Vardhan, and Prem Prakash Kushwaha. 2024. "Chronic Kidney Disease Interplay with Comorbidities and Carbohydrate Metabolism: A Review" Life 14, no. 1: 13. https://doi.org/10.3390/life14010013
APA StyleKushwaha, R., Vardhan, P. S., & Kushwaha, P. P. (2024). Chronic Kidney Disease Interplay with Comorbidities and Carbohydrate Metabolism: A Review. Life, 14(1), 13. https://doi.org/10.3390/life14010013





