Induction of Arterial Inflammation by Immune Checkpoint Inhibitor Therapy in Lung Cancer Patients as Measured by 2-[18F]FDG Positron Emission Tomography/Computed Tomography Depends on Pre-Existing Vascular Inflammation
Abstract
:1. Introduction
2. Materials and Methods
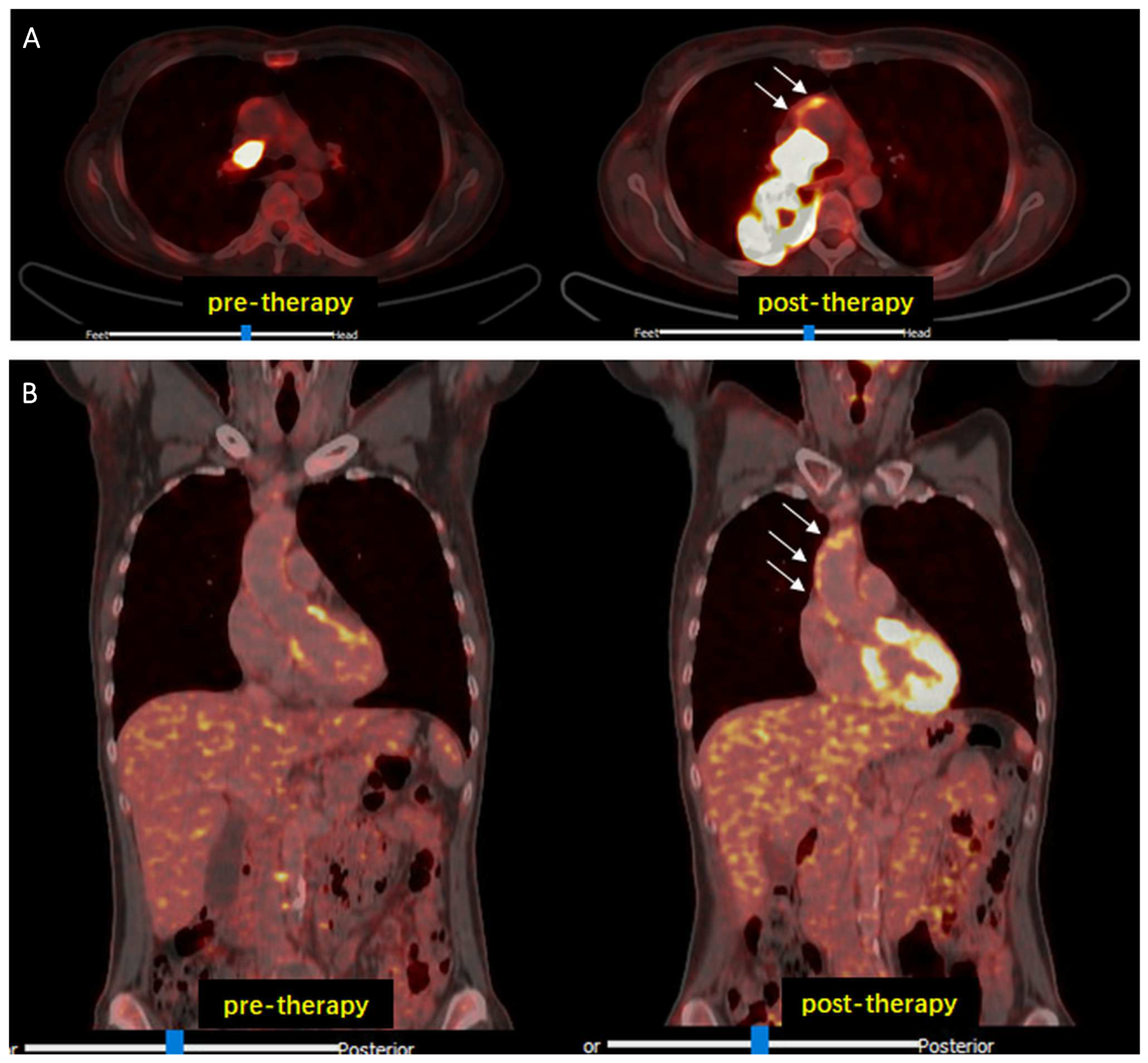
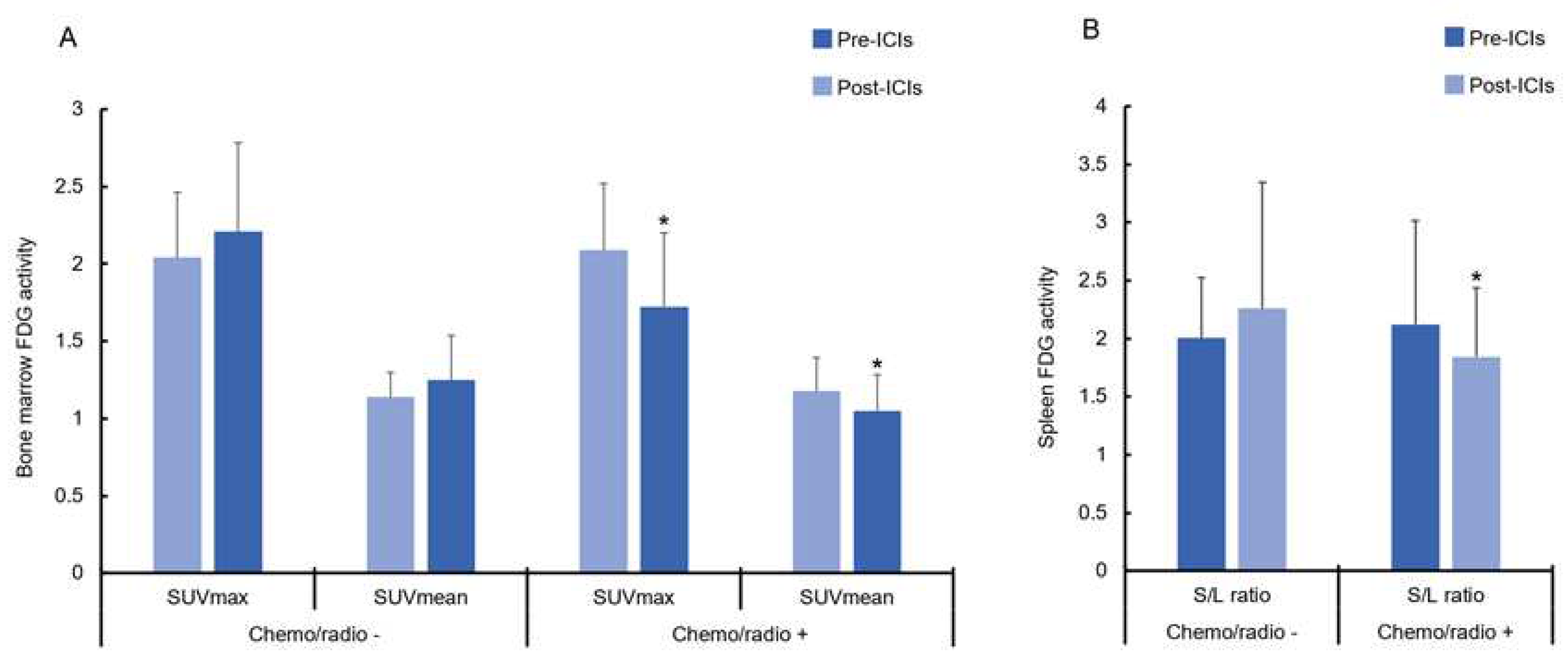
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, M.J.; Foy, K.C.; Kaumaya, P.T. Cancer immunotherapy: Present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov. Med. 2013, 15, 166–176. [Google Scholar] [PubMed]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr. Med. Chem. 2018, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Widakowich, C.; de Castro, G., Jr.; De Azambuja, E.; Dinh, P.; Awada, A. Review: Side effects of approved molecular targeted therapies in solid cancers. Oncologist 2007, 12, 1443–1455. [Google Scholar] [CrossRef]
- Zuppinger, C.; Suter, T.M. Cancer therapy-associated cardiotoxicity and signaling in the myocardium. J. Cardiovasc. Pharmacol. 2010, 56, 141–146. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Poels, K.; van Leent, M.M.; Boutros, C.; Tissot, H.; Roy, S.; Meerwaldt, A.E.; Toner, Y.C.; Reiche, M.E.; Kusters, P.J.; Malinova, T.; et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell–Driven Plaque Inflammation in Atherosclerosis. JACC CardioOncology 2020, 2, 599–610. [Google Scholar] [CrossRef]
- Lutgens, E.; Seijkens, T.T.P. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J. Immunother. Cancer 2020, 8, e000300. [Google Scholar] [CrossRef]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef]
- Inno, A.; Chiampan, A.; Lanzoni, L.; Verzè, M.; Molon, G.; Gori, S. Immune Checkpoint Inhibitors and Atherosclerotic Vascular Events in Cancer Patients. Front. Cardiovasc. Med. 2021, 8, 652186. [Google Scholar] [CrossRef]
- Flores-Gomez, D.; Bekkering, S.; Netea, M.G.; Riksen, N.P. Trained Immunity in Atherosclerotic Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 62–69. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs); Fact Sheet No. 317; World Health Organization: Geneva, Switzerland, 2015; Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 11 June 2021).
- 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yun, M.; Fernandez, C.; Xu, J.; Srinivasan, S.R.; Chen, W.; Berenson, G.S. Cigarette smoking exacerbates the adverse effects of age and metabolic syndrome on subclinical atherosclerosis: The Bogalusa Heart Study. PLoS ONE 2014, 9, e96368. [Google Scholar] [CrossRef] [PubMed]
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.; Lindner, O.; Sciagra, R.; Agostini, D.; Uebleis, C.; Gimelli, A.; Hacker, M.; et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef]
- Rominger, A.; Saam, T.; Wolpers, S.; Cyran, C.C.; Schmidt, M.; Foerster, S.; Nikolaou, K.; Reiser, M.F.; Bartenstein, P.; Hacker, M. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J. Nucl. Med. 2009, 50, 1611–1620. [Google Scholar] [CrossRef]
- Calabretta, R.; Hoeller, C.; Pichler, V.; Mitterhauser, M.; Karanikas, G.; Haug, A.; Li, X.; Hacker, M. Immune Checkpoint Inhibitor Therapy Induces Inflammatory Activity in Large Arteries. Circulation 2020, 142, 2396–2398. [Google Scholar] [CrossRef]
- Calabretta, R.; Staber, P.B.; Kornauth, C.; Lu, X.; Binder, P.; Pichler, V.; Mitterhauser, M.; Haug, A.; Li, X.; Hacker, M. Immune Checkpoint Inhibitor Therapy Induces Inflammatory Activity in the Large Arteries of Lymphoma Patients under 50 Years of Age. Biology 2021, 10, 1206. [Google Scholar] [CrossRef]
- Humbert, O.; Bauckneht, M.; Gal, J.; Paquet, M.; Chardin, D.; Rener, D.; Schiazza, A.; Genova, C.; Schiappa, R.; Zullo, L.; et al. Prognostic value of immunotherapy-induced organ inflammation assessed on 18FDG PET in patients with metastatic non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3878–3891. [Google Scholar] [CrossRef]
- Prigent, K.; Lasnon, C.; Ezine, E.; Janson, M.; Coudrais, N.; Joly, E.; Césaire, L.; Stefan, A.; Depontville, M.; Aide, N. Assessing immune organs on 18F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: Inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2573–2585. [Google Scholar] [CrossRef]
- Beer, L.; Hochmair, M.; Haug, A.R.; Schwabel, B.; Kifjak, D.; Wadsak, W.; Fuereder, T.; Fabikan, H.; Fazekas, A.; Schwab, S.; et al. Comparison of RECIST, iRECIST, and PERCIST for the Evaluation of Response to PD-1/PD-L1 Blockade Therapy in Patients with Non-Small Cell Lung Cancer. Clin. Nucl. Med. 2019, 44, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Heber, D.; Gonzalez, J.C.; Karanikas, G.; Mayerhoefer, M.E.; Rasul, S.; Beitzke, D.; Zhang, X.; Agis, H.; Mitterhauser, M.; et al. Association between Osteogenesis and Inflammation during the Progression of Calcified Plaque Evaluated by 18F-Fluoride and 18F-FDG. J. Nucl. Med. 2017, 58, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Emami, H.; Singh, P.; MacNabb, M.; Vucic, E.; Lavender, Z.; Rudd, J.H.; Fayad, Z.A.; Lehrer-Graiwer, J.; Korsgren, M.; Figueroa, A.L.; et al. Splenic metabolic activity predicts risk of future cardiovascular events: Demonstration of a cardiosplenic axis in humans. JACC Cardiovasc. Imaging 2015, 8, 121–130. [Google Scholar] [CrossRef]
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Adamstein, N.H.; Ridker, P.M. The neutrophil-lymphocyte ratio: Considerations for clinical application. Eur. Heart J. 2021, 42, 2216–2217. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Joshi, F.R.; Rudd, J.H. PET imaging of inflammation in atherosclerosis. Nat. Rev. Cardiol. 2014, 11, 443–457. [Google Scholar] [CrossRef]
- Cai, J.M.; Hatsukami, T.S.; Ferguson, M.S.; Small, R.; Polissar, N.L.; Yuan, C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002, 106, 1368–1373. [Google Scholar] [CrossRef]
- Li, X.; Heber, D.; Rausch, I.; Beitzke, D.; Mayerhoefer, M.E.; Rasul, S.; Kreissl, M.; Mitthauser, M.; Wadsak, W.; Hartenbach, M.; et al. Quantitative assessment of atherosclerotic plaques on (18)F-FDG PET/MRI: Comparison with a PET/CT hybrid system. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1503–1512. [Google Scholar] [CrossRef]
- Walker, A.K.; Chan, R.J.; Vardy, J.L. Sustained Mild Inflammation in Cancer Survivors: Where to from Here? JNCI Cancer Spectr. 2022, 6, pkac054. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Millon, A.; Fayad, Z.A. Molecular imaging in atherosclerosis: FDG PET. Curr. Atheroscler. Rep. 2012, 14, 429–437. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Sekine, I. Atherosclerotic cardiovascular events associated with immune checkpoint inhibitors in cancer patients. Jpn. J. Clin. Oncol. 2022, 52, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Gómez, V.; Mustapha, R.; Ng, K.; Ng, T. Radiation therapy and the innate immune response: Clinical implications for immunotherapy approaches. Br. J. Clin. Pharmacol. 2020, 86, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.H.; Devaud, C.; John, L.B.; Westwood, J.A.; Darcy, P.K. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. Oncoimmunology 2013, 2, e25962. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Gao, M.; Yun, M.; Meng, J.; Yu, W.; Zhu, Z.; Tian, Y.; Mou, T.; Zhang, Y.; Hacker, M.; et al. In Vivo Coronary 18F-Sodium Fluoride Activity: Correlations with Coronary Plaque Histological Vulnerability and Physiological Environment. JACC Cardiovasc. Imaging 2023, 16, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.A.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef]
- Denegri, A.; Boriani, G. High Sensitivity C-reactive Protein (hsCRP) and its Implications in Cardiovascular Outcomes. Curr. Pharm. Des. 2021, 27, 263–275. [Google Scholar] [CrossRef]
- Soeki, T.; Sata, M. Inflammatory Biomarkers and Atherosclerosis. Int. Heart J. 2016, 57, 134–139. [Google Scholar] [CrossRef]



| Gender (males/females) | 28/19 |
| Age (years, mean ± SD) | 61 ± 9 |
| BMI (kg/m2, mean ± SD) | 26 ± 3.7 |
| Smoking, N (%) | 31 (70) |
| Hypertension, N (%) | 16 (34) |
| Dyslipidemia, N (%) | 4 (8.5) |
| Diabetes, N (%) | 4 (8.5) |
| COPD, N (%) | 16 (34) |
| Prior myocardial infarction, N (%) | 4 (8.5) |
| Prior TIA/Stroke, N (%) | 4 (8.5) |
| PAD, N (%) | 8 (17) |
| Histology (N; %) | Lung adenocarcinoma (33; 70.2) Lung squamous cells carcinoma (14; 29.8) |
| Tumor stadium (N; %) | I (0; 0) II (0; 0) IIIA/IIIB (6; 12.8) IV (41; 87.2) |
| ICI Therapy (N; %) | 47; 100 PD-1 Inhibitors (26; 55.3) Nivolumab (7; 14.9) Pembrolizumab (19; 40.2) PD-L1 Inhibitors (12; 25.5) Atezolizumab (8; 17) Durvalumab (4; 8.5) Combination of ICI + CHT (9; 19.1) |
| Previous ICI Therapy, N (%) | 1 (2.1) |
| CHT before ICI therapy, N (%) | 28 (59.6) |
| RT during ICI therapy, N (%) | 1 (2.1) |
| RT before ICI therapy, N (%) | 22 (46.8) |
| Previous surgery, N (%) | 16 (34) |
| PD-1 Expression > 50%, N (%) | 25 (53.2) |
| PD-1 Expression ≤ 50%, N (%) | 22 (46.8) |
| Pre | Post | p Value | |
|---|---|---|---|
| hsCRP | 22.04 ± 27.19 | 22.61 ± 6.75 | 0.887 |
| ALeC | 7.97 ± 3.22 | 7.94 ± 3.03 | 0.957 |
| AEC | 4.36 ± 0.56 | 4.36 ± 0.53 | 0.924 |
| APC | 285.20 ± 130.01 | 295.37 ± 111.93 | 0.550 |
| ANC | 5.49 ± 2.59 | 5.33 ± 2.72 | 0.700 |
| RNC | 66.37 ± 11.28 | 64.98 ± 11.24 | 0.338 |
| ALC | 1.66 ± 0.86 | 1.62 ± 0.78 | 0.676 |
| RLC | 21.32 ± 9.39 | 22.00+10.43 | 0.550 |
| NLR absolute | 4.32 ± 3.60 | 4.27 ± 3.47 | 0.921 |
| NLR relative | 4.32 ± 3.60 | 4.26 ± 3.46 | 0.911 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabretta, R.; Beer, L.; Prosch, H.; Kifjak, D.; Zisser, L.; Binder, P.; Grünert, S.; Langsteger, W.; Li, X.; Hacker, M. Induction of Arterial Inflammation by Immune Checkpoint Inhibitor Therapy in Lung Cancer Patients as Measured by 2-[18F]FDG Positron Emission Tomography/Computed Tomography Depends on Pre-Existing Vascular Inflammation. Life 2024, 14, 146. https://doi.org/10.3390/life14010146
Calabretta R, Beer L, Prosch H, Kifjak D, Zisser L, Binder P, Grünert S, Langsteger W, Li X, Hacker M. Induction of Arterial Inflammation by Immune Checkpoint Inhibitor Therapy in Lung Cancer Patients as Measured by 2-[18F]FDG Positron Emission Tomography/Computed Tomography Depends on Pre-Existing Vascular Inflammation. Life. 2024; 14(1):146. https://doi.org/10.3390/life14010146
Chicago/Turabian StyleCalabretta, Raffaella, Lucian Beer, Helmut Prosch, Daria Kifjak, Lucia Zisser, Patrick Binder, Stefan Grünert, Werner Langsteger, Xiang Li, and Marcus Hacker. 2024. "Induction of Arterial Inflammation by Immune Checkpoint Inhibitor Therapy in Lung Cancer Patients as Measured by 2-[18F]FDG Positron Emission Tomography/Computed Tomography Depends on Pre-Existing Vascular Inflammation" Life 14, no. 1: 146. https://doi.org/10.3390/life14010146
APA StyleCalabretta, R., Beer, L., Prosch, H., Kifjak, D., Zisser, L., Binder, P., Grünert, S., Langsteger, W., Li, X., & Hacker, M. (2024). Induction of Arterial Inflammation by Immune Checkpoint Inhibitor Therapy in Lung Cancer Patients as Measured by 2-[18F]FDG Positron Emission Tomography/Computed Tomography Depends on Pre-Existing Vascular Inflammation. Life, 14(1), 146. https://doi.org/10.3390/life14010146







