Loss of Human Epidermal Receptor 2 Expression in Formalin-Fixed Paraffin-Embedded Breast Cancer Samples and the Rescuing Effect of Enhanced Antigen Retrieval and Signal Amplification
Abstract
:1. Introduction
2. Methods
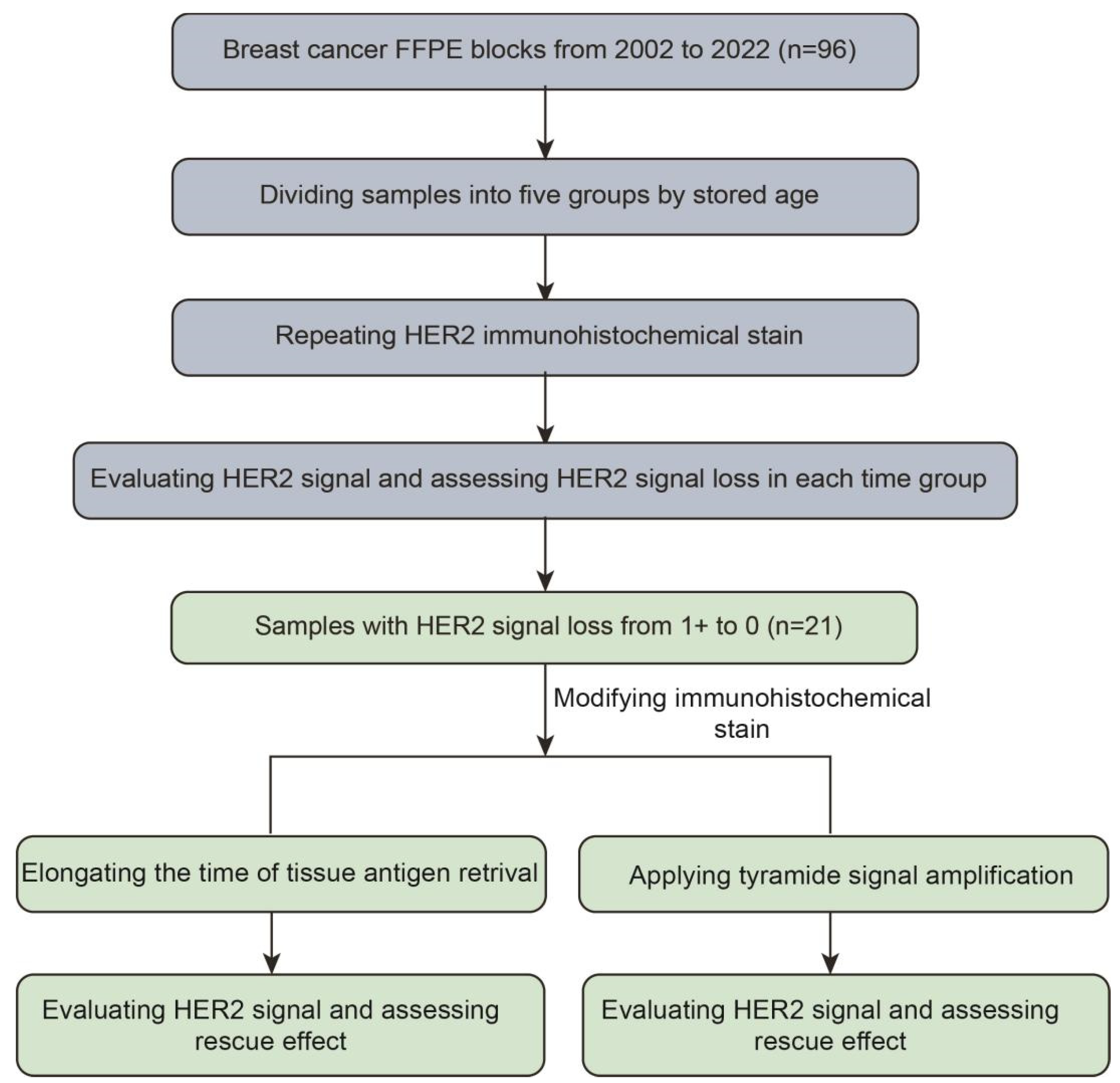
2.1. Tissue Selection
2.2. Immunohistochemical Staining
2.3. Pathology Evaluation
2.4. Data Analysis
2.5. Tissue Antigen Retrieval (TAR) Elongating and TSA Signal Amplification Applying
3. Results
3.1. Loss of HER2 Antigenicity in FFPE Blocks of Breast Cancer with Stored Age
3.2. The Rescuing Effect of Modified Immunohistochemical Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Mello-Thoms, C.; Brennan, P.C. Descriptive epidemiology of breast cancer in China: Incidence, mortality, survival and prevalence. Breast Cancer Res. Treat. 2016, 159, 395–406. [Google Scholar] [CrossRef]
- Libson, S.; Lippman, M. A review of clinical aspects of breast cancer. Int. Rev. Psychiatry 2014, 26, 4–15. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Zhang, H.; Moisini, I.; Ajabnoor, R.M.; Turner, B.M.; Hicks, D.G. Applying the New Guidelines of HER2 Testing in Breast Cancer. Curr. Oncol. Rep. 2020, 22, 51. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Ponde, N.; Aftimos, P.; Piccart, M. Antibody-Drug Conjugates in Breast Cancer: A Comprehensive Review. Curr. Treat. Options Oncol. 2019, 20, 37. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab vedotin: A novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022, 29, 1335–1344. [Google Scholar] [CrossRef]
- Zhang, H.; Katerji, H.; Turner, B.M.; Hicks, D.G. HER2-Low Breast Cancers. Am. J. Clin. Pathol. 2022, 157, 328–336. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Eng. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Zhang, H.; Karakas, C.; Tyburski, H.; Turner, B.M.; Peng, Y.; Wang, X.; Katerji, H.; Schiffhauer, L.; Hicks, D.G. HER2-low breast cancers: Current insights and future directions. Semin. Diagn. Pathol. 2022, 39, 305–312. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Eiger, D.; Agostinetto, E.; Saude-Conde, R.; de Azambuja, E. The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers 2021, 13, 1015. [Google Scholar] [CrossRef]
- Fergenbaum, J.H.; Garcia-Closas, M.; Hewitt, S.M.; Lissowska, J.; Sakoda, L.C.; Sherman, M.E. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol. Biomark. Prev. 2004, 13, 667–672. [Google Scholar] [CrossRef]
- Combs, S.E.; Han, G.; Mani, N.; Beruti, S.; Nerenberg, M.; Rimm, D.L. Loss of antigenicity with tissue age in breast cancer. Lab. Investig. 2016, 96, 264–269. [Google Scholar] [CrossRef]
- Ehinger, A.; Bendahl, P.O.; Ryden, L.; Ferno, M.; Alkner, S. Stability of oestrogen and progesterone receptor antigenicity in formalin-fixed paraffin-embedded breast cancer tissue over time. APMIS 2018, 126, 746–754. [Google Scholar] [CrossRef]
- Kim, K.; Ylaya, K.; Perry, C.; Lee, M.Y.; Kim, J.W.; Chung, J.Y.; Hewitt, S.M. Quality Assessment of Proteins and RNA Following Storage in Archival Formalin-Fixed Paraffin-Embedded Human Breast Cancer Tissue Microarray Sections. Biopreserv. Biobank 2023, 21, 493–503. [Google Scholar] [CrossRef]
- Werner, M.; Von Wasielewski, R.; Komminoth, P. Antigen retrieval, signal amplification and intensification in immunohistochemistry. Histochem. Cell Biol. 1996, 105, 253–260. [Google Scholar] [CrossRef]
- Toda, Y.; Kono, K.; Abiru, H.; Kokuryo, K.; Endo, M.; Yaegashi, H.; Fukumoto, M. Application of tyramide signal amplification system to immunohistochemistry: A potent method to localize antigens that are not detectable by ordinary method. Pathol. Int. 1999, 49, 479–483. [Google Scholar] [CrossRef]
- Shi, S.R.; Shi, Y.; Taylor, C.R. Antigen retrieval immunohistochemistry: Review and future prospects in research and diagnosis over two decades. J. Histochem. Cytochem. 2011, 59, 13–32. [Google Scholar] [CrossRef]
- Shi, S.R.; Cote, R.J.; Taylor, C.R. Antigen retrieval immunohistochemistry: Past, present, and future. J. Histochem. Cytochem. 1997, 45, 327–343. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N. On the chemistry of formaldehyde fixation and its effects on immunohistochemical reactions. Histochemistry 1985, 82, 201–204. [Google Scholar] [CrossRef]
- Thavarajah, R.; Mudimbaimannar, V.K.; Elizabeth, J.; Rao, U.K.; Ranganathan, K. Chemical and physical basics of routine formaldehyde fixation. J. Oral. Maxillofac. Pathol. 2012, 16, 400–405. [Google Scholar] [CrossRef]
- Olsen, D.A.; Ostergaard, B.; Bokmand, S.; Wamberg, P.A.; Jakobsen, E.H.; Brandslund, I. HER-2 protein concentrations in breast cancer cells increase before immunohistochemical and fluorescence in situ hybridization analysis turn positive. Clin. Chem. Lab Med. 2007, 45, 177–182. [Google Scholar] [CrossRef]
- Ross, J.S.; Fletcher, J.A.; Linette, G.P.; Stec, J.; Clark, E.; Ayers, M.; Symmans, W.F.; Pusztai, L.; Bloom, K.J. The Her-2/neu gene and protein in breast cancer 2003: Biomarker and target of therapy. Oncologist 2003, 8, 307–325. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhou, L.; Wu, Q.; Jia, L.; Diao, X.; Kang, Q.; Huang, X.; Liu, Y.; Hu, T.; Long, M. Loss of Human Epidermal Receptor 2 Expression in Formalin-Fixed Paraffin-Embedded Breast Cancer Samples and the Rescuing Effect of Enhanced Antigen Retrieval and Signal Amplification. Life 2024, 14, 31. https://doi.org/10.3390/life14010031
Ma X, Zhou L, Wu Q, Jia L, Diao X, Kang Q, Huang X, Liu Y, Hu T, Long M. Loss of Human Epidermal Receptor 2 Expression in Formalin-Fixed Paraffin-Embedded Breast Cancer Samples and the Rescuing Effect of Enhanced Antigen Retrieval and Signal Amplification. Life. 2024; 14(1):31. https://doi.org/10.3390/life14010031
Chicago/Turabian StyleMa, Xiuli, Lixin Zhou, Qi Wu, Ling Jia, Xinting Diao, Qiang Kang, Xiaozheng Huang, Yiqiang Liu, Taobo Hu, and Mengping Long. 2024. "Loss of Human Epidermal Receptor 2 Expression in Formalin-Fixed Paraffin-Embedded Breast Cancer Samples and the Rescuing Effect of Enhanced Antigen Retrieval and Signal Amplification" Life 14, no. 1: 31. https://doi.org/10.3390/life14010031






