Evolution of Consciousness
Abstract
:1. Introduction
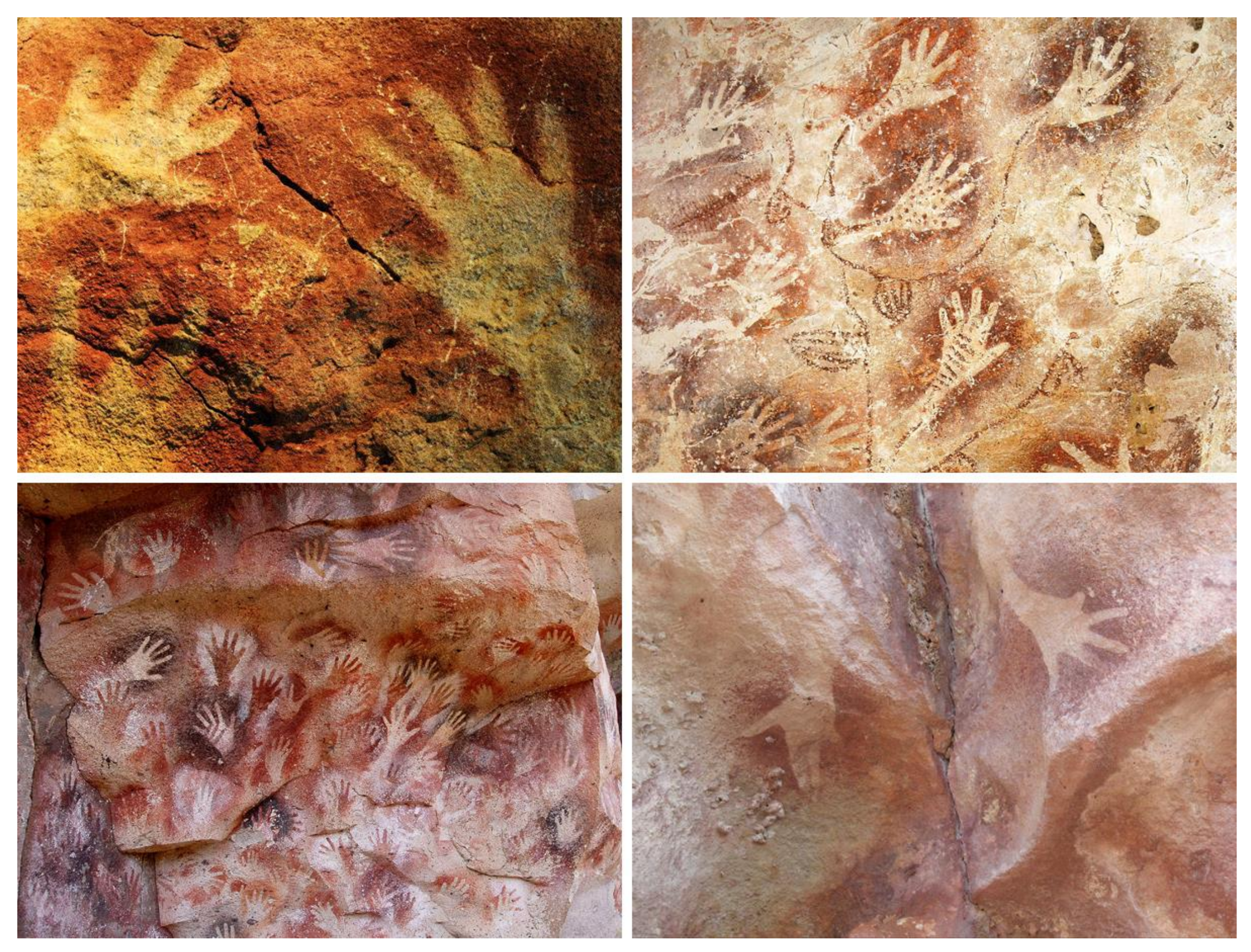
2. Causal Potency of Conscious Experiences Is Manifested in Prehistoric Art
3. Physicalism and Sentience
4. Brain Size and Cognitive Abilities in the Evolutionary Tree of Life
5. Quantum Substrates Inside Neural Tissue of Living Organisms
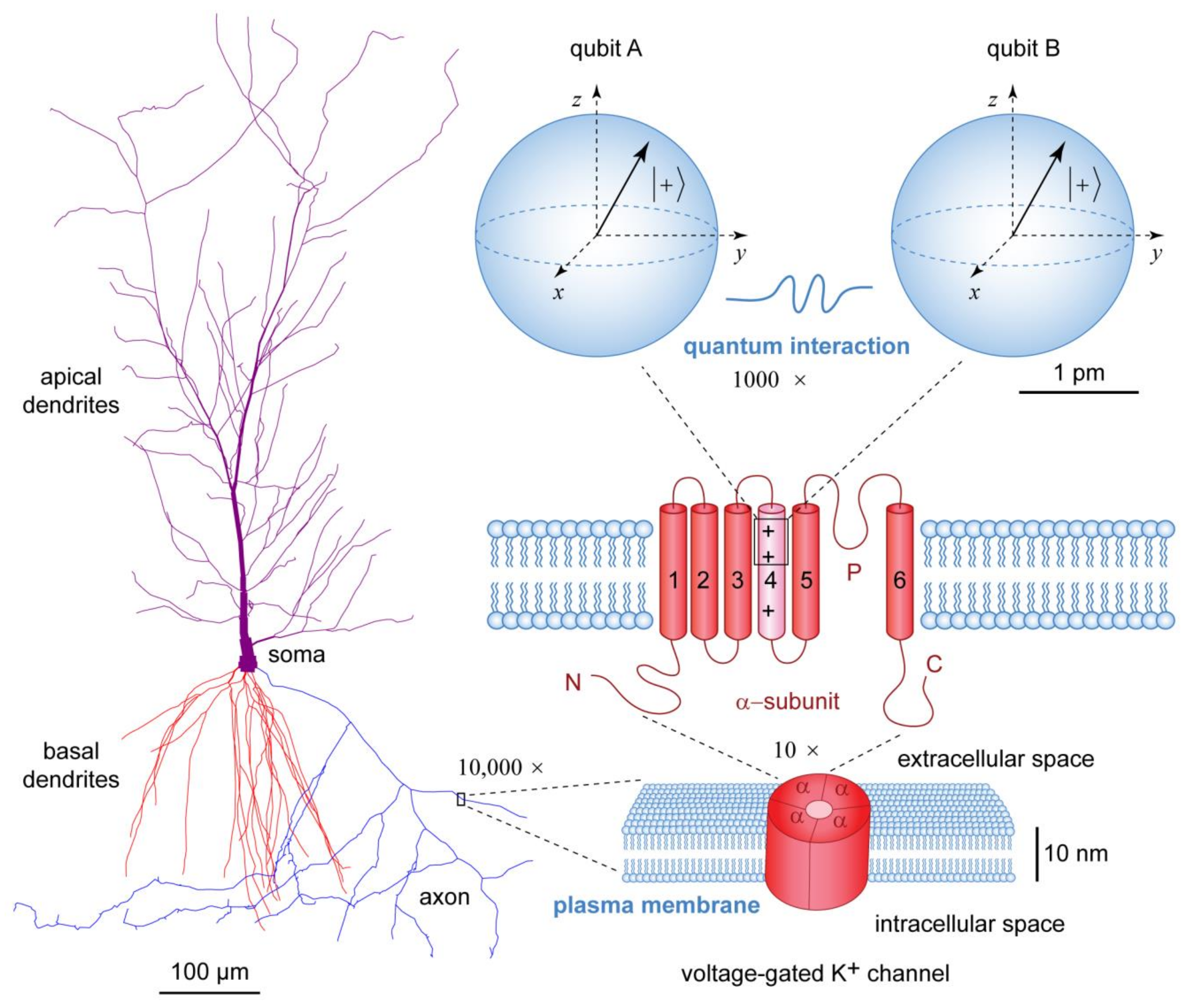
6. Conclusions
7. Glossary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgiev, D.D. Quantum Information and Consciousness: A Gentle Introduction; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Georgiev, D.D. Inner privacy of conscious experiences and quantum information. Biosystems 2020, 187, 104051. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, D.D. Quantum information theoretic approach to the mind-brain problem. Prog. Biophys. Mol. Biol. 2020, 158, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, B. Mirror self-image reactions before age two. Dev. Psychobiol. 1972, 5, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Fuwa, K.; Myowa, M. Chimpanzees recognize their own delayed self-image. R. Soc. Open Sci. 2017, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, S.; Poo, M.-m.; Gong, N. Spontaneous expression of mirror self-recognition in monkeys after learning precise visual-proprioceptive association for mirror images. Proc. Natl. Acad. Sci. USA 2017, 114, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, J.M.; de Waal, F.B.M.; Reiss, D. Self-recognition in an Asian elephant. Proc. Natl. Acad. Sci. USA 2006, 103, 17053–17057. [Google Scholar] [CrossRef] [PubMed]
- Reiss, D.; Marino, L. Mirror self-recognition in the bottlenose dolphin: A case of cognitive convergence. Proc. Natl. Acad. Sci. USA 2001, 98, 5937–5942. [Google Scholar] [CrossRef]
- Kohda, M.; Bshary, R.; Kubo, N.; Awata, S.; Sowersby, W.; Kawasaka, K.; Kobayashi, T.; Sogawa, S. Cleaner fish recognize self in a mirror via self-face recognition like humans. Proc. Natl. Acad. Sci. USA 2023, 120, e2208420120. [Google Scholar] [CrossRef]
- Nengo, I.; Tafforeau, P.; Gilbert, C.C.; Fleagle, J.G.; Miller, E.R.; Feibel, C.; Fox, D.L.; Feinberg, J.; Pugh, K.D.; Berruyer, C.; et al. New infant cranium from the African Miocene sheds light onape evolution. Nature 2017, 548, 169–174. [Google Scholar] [CrossRef]
- Schrago, C.G. On the time scale of new world primate diversification. Am. J. Phys. Anthropol. 2007, 132, 344–354. [Google Scholar] [CrossRef]
- Gheerbrant, E. Paleocene emergence of elephant relatives and the rapid radiation of African ungulates. Proc. Natl. Acad. Sci. USA 2009, 106, 10717–10721. [Google Scholar] [CrossRef] [PubMed]
- Morton, F.B.; Robinson, L.M.; Brando, S.; Weiss, A. Personality structure in bottlenose dolphins (Tursiops truncatus). J. Comp. Psychol. 2021, 135, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.P.; Daeschler, E.B.; Jenkins, F.A.; Shubin, N.H. The cranial endoskeleton of Tiktaalik roseae. Nature 2008, 455, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Cell: A Molecular Approach, 8th ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Copley, S.D. Evolution and the enzyme. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 9–46. [Google Scholar] [CrossRef]
- Mojzsis, S.J.; Arrhenius, G.; McKeegan, K.D.; Harrison, T.M.; Nutman, A.P.; Friend, C.R.L. Evidence for life on Earth before 3,800 million years ago. Nature 1996, 384, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Boussau, B.; Blanquart, S.; Necsulea, A.; Lartillot, N.; Gouy, M. Parallel adaptations to high temperatures in the Archaean eon. Nature 2008, 456, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Levison, H.F.; Tsiganis, K.; Morbidelli, A. Origin of the cataclysmic Late Heavy Bombardment period of the terrestrial planets. Nature 2005, 435, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef]
- Miller, S.L.; Urey, H.C. Organic compound synthesis on the primitive earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef]
- Roberts, A. The Incredible Human Journey; Bloomsbury Publishing: London, UK, 2009. [Google Scholar]
- Stringer, C. The Origin of Our Species; Penguin: London, UK, 2012. [Google Scholar]
- Wood, B. Human Evolution: A Very Short Introduction, 2nd ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Tobias, P.V. The brain of Homo habilis: A new level of organization in cerebral evolution. J. Hum. Evol. 1987, 16, 741–761. [Google Scholar] [CrossRef]
- Rightmire, G.P. Brain size and encephalization in early to Mid-Pleistocene Homo. Am. J. Phys. Anthropol. 2004, 124, 109–123. [Google Scholar] [CrossRef]
- Langdon, J.H. Human Evolution: Bones, Cultures, and Genes; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Kumar, V.; Campbell, R. A Better Ape: The Evolution of the Moral Mind and How It Made Us Human; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Georgiev, D.D. Causal potency of consciousness in the physical world. Int. J. Mod. Phys. B 2023, 2450256. [Google Scholar] [CrossRef]
- Georgiev, D.D. Quantum propensities in the brain cortex and free will. BioSystems 2021, 208, 104474. [Google Scholar] [CrossRef] [PubMed]
- James, W. Are we automata? Mind 1879, 4, 1–22. [Google Scholar] [CrossRef]
- James, W. The Principles of Psychology; Henry Holt and Company: New York, NY, USA, 1890; Volume 1. [Google Scholar]
- Eccles, J.C.; Popper, K.R. The Self and Its Brain; Springer: Berlin, Germany, 1977. [Google Scholar] [CrossRef]
- Kim, J. Mind in a Physical World: An Essay on the Mind-Body Problem and Mental Causation; MIT Press: Cambridge, MA, USA, 1998. [Google Scholar] [CrossRef]
- Huxley, T.H. On the hypothesis that animals are automata, and its history. Fortn. Rev. 1874, 16, 555–580. [Google Scholar]
- Broad, C.D. The Mind and Its Place in Nature; Routledge: London, UK, 1925. [Google Scholar] [CrossRef]
- Eidelman, N.Y. Looking for My Ancestors; Molodaya Gvardiya: Moscow, Russia, 1967. [Google Scholar]
- Stuart, A.J. Vanished Giants: The Lost World of the Ice Age; University of Chicago Press: Chicago, IL, USA, 2021. [Google Scholar] [CrossRef]
- Sanz de Sautuola, M. Breves Apuntes Sobre Algunos Objetos Prehistóricos de la Provincia de Santander; Imprenta y Litografía de Telesforo Martínez: Santander, Spain, 1880; Available online: http://simurg.bibliotecas.csic.es/view/CSIC000073342/27/ (accessed on 1 December 2023).
- Hudson, H.; Hetreed, O.; López-Linares, J.L.; Banderas, A.; Sibony, C.; Everett, R. Finding Altamira; Eagle Films: Beirut, Lebanon, 2016. [Google Scholar]
- Cartailhac, É. Les cavernes ornées de dessins. La grotte d’Altamira, Espagne. Mea Culpa d’un sceptique. L’Anthropologie 1902, 13, 348–354. [Google Scholar]
- Pike, A.W.G.; Hoffmann, D.L.; García-Diez, M.; Pettitt, P.B.; Alcolea, J.; De Balbín, R.; González-Sainz, C.; de las Heras, C.; Lasheras, J.A.; Montes, R.; et al. U-series dating of paleolithic art in 11 caves in Spain. Science 2012, 336, 1409–1413. [Google Scholar] [CrossRef]
- Valladas, H.; Cachier, H.; Maurice, P.; de Quirost, F.B.; Clottes, J.; Valdés, V.C.; Uzquiano, P.; Arnold, M. Direct radiocarbon dates for prehistoric paintings at the Altamira, El Castillo and Niaux caves. Nature 1992, 357, 68–70. [Google Scholar] [CrossRef]
- Bataille, G. Prehistoric Painting: Lascaux or the Birth of Art; Skira: Milan, Italy, 1955. [Google Scholar]
- Ducasse, S.; Langlais, M. Twenty years on, a new date with Lascaux. Reassessing the chronology of the cave’s Paleolithic occupations through new 14C AMS dating. Paléo Rev. D’archéologie Préhistorique 2019, 30, 130–147. [Google Scholar] [CrossRef]
- Chauvet, J.-M.; Deschamps, E.B.; Hillaire, C. Dawn of Art: The Chauvet Cave; Harry N. Abrams: New York, NY, USA, 1996. [Google Scholar]
- Chauvet, J.-M.; Deschamps, E.B. Chauvet Cave: The Discovery of the World’s Oldest Paintings; Thames & Hudson: London, UK, 2001. [Google Scholar]
- Cuzange, M.-T.; Delqué-Količ, E.; Goslar, T.; Grootes, P.M.; Higham, T.; Kaltnecker, E.; Nadeau, M.-J.; Oberlin, C.; Paterne, M.; van der Plicht, J.; et al. Radiocarbon intercomparison program for Chauvet cave. Radiocarbon 2016, 49, 339–347. [Google Scholar] [CrossRef]
- Herzog, W. Cave of Forgotten Dreams; Creative Differences Productions, Inc.: Los Angeles, CA, USA, 2010. [Google Scholar]
- Aubert, M.; Setiawan, P.; Oktaviana, A.A.; Brumm, A.; Sulistyarto, P.H.; Saptomo, E.W.; Istiawan, B.; Ma’rifat, T.A.; Wahyuono, V.N.; Atmoko, F.T.; et al. Palaeolithic cave art in Borneo. Nature 2018, 564, 254–257. [Google Scholar] [CrossRef]
- de las Heras, C.; Lasheras, J.A.; Arrizabalaga, A.; de la Rasilla, M. Pensando el Gravetiense: Nuevos Datos Para la Región Cantábrica, en su Contexto Peninsular y Pirenaico; Ministerio de Educación y Cultura: Madrid, Spain, 2012. [CrossRef]
- Fage, L.-H. The rock art of Borneo: Presentation and new observations on some exceptional hand stencils. Palethnol. Archéologie Et Sci. Hum. 2013, 5, 186–188. [Google Scholar] [CrossRef]
- Fage, L.-H.; Chazine, J.-M. Borneo: Memory of the Caves; Le Kalimanthrope: Caylus, France, 2010. [Google Scholar]
- Aguerre, A.M. A propósito de un nuevo fechado radiocarbónico para la “Cueva de las Manos”. Alto Río Pinturas—Provincia de Santa Cruz. Relac. Soc. Argent. Antropol. 1977, 11, 129–142. [Google Scholar]
- Taçon, P.S.C. It’s Time Rock Art Was Better Protected. Apollo Magazine 17 August 2016. Available online: https://www.apollo-magazine.com/its-time-rock-art-was-better-protected/ (accessed on 1 December 2023).
- Malafouris, L. Making hands and tools: Steps to a process archaeology of mind. World Archaeol. 2021, 53, 38–55. [Google Scholar] [CrossRef]
- Etxepare, R.; Irurtzun, A. Gravettian hand stencils as sign language formatives. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200205. [Google Scholar] [CrossRef] [PubMed]
- George, A. Messages from the Stone Age. New Sci. 2023, 257, 38–42. [Google Scholar] [CrossRef]
- Aubert, M.; Lebe, R.; Oktaviana, A.A.; Tang, M.; Burhan, B.; Hamrullah; Jusdi, A.; Abdullah; Hakim, B.; Zhao, J.-X.; et al. Earliest hunting scene in prehistoric art. Nature 2019, 576, 442–445. [Google Scholar] [CrossRef]
- Woodhouse, H.C. Prehistoric hunting methods as depicted in the rock paintings of Southern Africa. South Afr. J. Sci. 1966, 62, 169–171. [Google Scholar]
- Gross, M. Cave art reveals human nature. Curr. Biol. 2020, 30, R95–R98. [Google Scholar] [CrossRef]
- Harman, G.H. The inference to the best explanation. Philos. Rev. 1965, 74, 88–95. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Darwin, C. From So Simple a Beginning: The Four Great Books of Charles Darwin (The Voyage of the Beagle, On the Origin of Species, The Descent of Man, The Expression of the Emotions in Man and Animals); Wilson, E.O., Ed.; W. W. Norton & Company: New York, NY, USA, 2006. [Google Scholar]
- Georgiev, D.D. Quantum no-go theorems and consciousness. Axiomathes 2013, 23, 683–695. [Google Scholar] [CrossRef]
- Duhem, P.M.M. The Aim and Structure of Physical Theory; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar] [CrossRef]
- Hume, D. An Enquiry Concerning Human Understanding and Selections from A Treatise of Human Nature; Open Court: La Salle, IL, USA, 1907. [Google Scholar]
- Popper, K.R. The Logic of Scientific Discovery, 2nd ed.; Routledge: London, UK, 2002. [Google Scholar] [CrossRef]
- Popper, K.R. Postscript to the Logic of Scientific Discovery. Vol. 1: Realism and the Aim of Science; Rowman and Littlefield: Totowa, NJ, USA, 1983. [Google Scholar]
- Popper, K.R. Postscript to the Logic of Scientific Discovery. Vol. 2: The Open Universe: An Argument for Indeterminism; Rowman and Littlefield: Totowa, NJ, USA, 1982. [Google Scholar]
- Popper, K.R. Postscript to the Logic of Scientific Discovery. Vol. 3: Quantum Theory and the Schism in Physics; Rowman and Littlefield: Totowa, NJ, USA, 1982. [Google Scholar]
- Popper, K.R. Conjectures and Refutations: The Growth of Scientific Knowledge, 4th ed.; Routledge & Kegan Paul: London, UK, 1981. [Google Scholar]
- Lakatos, I. Proofs and Refutations: The Logic of Mathematical Discovery; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Planck, M. The Origin and Development of the Quantum Theory; Oxford University Press: Oxford, UK, 1922. [Google Scholar]
- Planck, M. Ueber das Gesetz der Energieverteilung im Normalspectrum. Ann. Der Phys. 1901, 309, 553–563. [Google Scholar] [CrossRef]
- Planck, M. Entropie und Temperatur strahlender Wärme. Ann. Der Phys. 1900, 306, 719–737. [Google Scholar] [CrossRef]
- Planck, M. The Theory of Heat Radiation; P. Blakiston’s Son & Co.: Philadelphia, PA, USA, 1914. [Google Scholar]
- Einstein, A. On a heuristic point of view about the creation and conversion of light. In The Collected Papers of Albert Einstein. Volume 2: The Swiss Years: Writings, 1900–1909 (English Translation Supplement); Princeton University Press: Princeton, NJ, USA, 1905; pp. 86–103. [Google Scholar]
- Einstein, A. Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt. Ann. Der Phys. 1905, 17, 132–148. [Google Scholar] [CrossRef]
- de Broglie, L. Recherches sur la théorie des quanta. Ann. Phys. 1925, 10, 22–128. [Google Scholar] [CrossRef]
- de Broglie, L. Waves and quanta. Nature 1923, 112, 540. [Google Scholar] [CrossRef]
- Schrödinger, E. Collected Papers on Wave Mechanics; Blackie & Son: London, UK, 1928. [Google Scholar]
- Schrödinger, E. An undulatory theory of the mechanics of atoms and molecules. Phys. Rev. 1926, 28, 1049–1070. [Google Scholar] [CrossRef]
- Dirac, P.A.M. The Principles of Quantum Mechanics, 4th ed.; Oxford University Press: Oxford, UK, 1967. [Google Scholar]
- von Neumann, J. Mathematical Foundations of Quantum Mechanics; Princeton University Press: Princeton, NJ, USA, 1955. [Google Scholar] [CrossRef]
- von Neumann, J. Mathematische Grundlagen der Quantenmechanik; Springer: Berlin, Germany, 1932. [Google Scholar] [CrossRef]
- Born, M. Zur Quantenmechanik der Stoßvorgänge. Z. Für Phys. 1926, 37, 863–867. [Google Scholar] [CrossRef]
- Born, M. Statistical interpretation of quantum mechanics. Science 1955, 122, 675–679. [Google Scholar] [CrossRef]
- Werth, A. Avoiding the pitfall of progress and associated perils of evolutionary education. Evol. Educ. Outreach 2012, 5, 249–265. [Google Scholar] [CrossRef]
- Werth, A. The Problems of Evolution as a “March of Progress”. SAPIENS–Anthropology Magazine 16 August 2022. Available online: https://www.sapiens.org/biology/evolution-march-of-progress/ (accessed on 1 December 2023).
- Gould, S.J. Wonderful Life: The Burgess Shale and the Nature of History; W. W. Norton & Company: New York, NY, USA, 1990. [Google Scholar]
- Jones, E.M. Apollo Lunar Surface Journal; National Aeronautics and Space Administration: Washington, DC, USA, 2018.
- Georgiev, D.D.; Kolev, S.K.; Cohen, E.; Glazebrook, J.F. Computational capacity of pyramidal neurons in the cerebral cortex. Brain Res. 2020, 1748, 147069. [Google Scholar] [CrossRef]
- Marino, L.; Connor, R.C.; Fordyce, R.E.; Herman, L.M.; Hof, P.R.; Lefebvre, L.; Lusseau, D.; McCowan, B.; Nimchinsky, E.A.; Pack, A.A.; et al. Cetaceans have complex brains for complex cognition. PLoS Biol. 2007, 5, e139. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S. The human brain in numbers: A linearly scaled-up primate brain. Front. Hum. Neurosci. 2009, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.; Dicke, U. Evolution of the brain and intelligence. Trends Cogn. Sci. 2005, 9, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.S.; Gloe, T.; Hegedus, J.; Bitterman, K.; Billings, B.K.; Chengetanai, S.; Bentil, S.; Wang, V.X.; Ng, J.C.; Tang, C.Y.; et al. Brain gyrification in wild and domestic canids: Has domestication changed the gyrification index in domestic dogs? J. Comp. Neurol. 2020, 528, 3209–3228. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S.; Avelino-de-Souza, K.; Neves, K.; Porfírio, J.; Messeder, D.; Mattos Feijó, L.; Maldonado, J.; Manger, P.R. The elephant brain in numbers. Front. Neuroanat. 2014, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, S.H.; Carlin, K.P.; Van Alstyne, K.R.; Hanson, A.C.; Tarpley, R.J. Comparison of dolphins’ body and brain measurements with four other groups of cetaceans reveals great diversity. Brain Behav. Evol. 2017, 88, 235–257. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, S.H.; Brownson, R.H. Relative brain sizes and cortical surface areas in odontocetes. Acta Zool. Fenn. 1984, 172, 149–152. [Google Scholar]
- Hofman, M.A. Size and shape of the cerebral cortex in mammals: I. The cortical surface. Brain Behav. Evol. 2008, 27, 28–40. [Google Scholar] [CrossRef]
- Grimm, D. Is a dolphin a person? Science 2010, 327, 1070–1071. [Google Scholar] [CrossRef]
- Dudzinski, K.M.; Frohoff, T. Dolphin Mysteries: Unlocking the Secrets of Communication; Yale University Press: New Haven, CT, USA, 2008. [Google Scholar] [CrossRef]
- Ryabov, V.A. The study of acoustic signals and the supposed spoken language of the dolphins. St. Petersburg Polytech. Univ. J. Phys. Math. 2016, 2, 231–239. [Google Scholar] [CrossRef]
- Caldwell, M.C.; Caldwell, D.K. Individualized whistle contours in bottle-nosed dolphins (Tursiops truncatus). Nature 1965, 207, 434–435. [Google Scholar] [CrossRef]
- Harder, J.H.; Hill, H.M.; Dudzinski, K.M.; Sanabria, K.T.; Guarino, S.; Kuczaj, S.A., II. The development of echolocation in bottlenose dolphins. Int. J. Comp. Psychol. 2016, 29, 17. [Google Scholar] [CrossRef]
- Starkhammar, J.; Moore, P.W.; Talmadge, L.; Houser, D.S. Frequency-dependent variation in the two-dimensional beam pattern of an echolocating dolphin. Biol. Lett. 2011, 7, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Ladegaard, M.; Mulsow, J.; Houser, D.S.; Jensen, F.H.; Johnson, M.; Madsen, P.T.; Finneran, J.J. Dolphin echolocation behaviour during active long-range target approaches. J. Exp. Biol. 2019, 222, jeb189217. [Google Scholar] [CrossRef]
- Jensen, F.H.; Rocco, A.; Mansur, R.M.; Smith, B.D.; Janik, V.M.; Madsen, P.T. Clicking in shallow rivers: Short-range echolocation of Irrawaddy and Ganges river dolphins in a shallow, acoustically complex habitat. PLoS ONE 2013, 8, e59284. [Google Scholar] [CrossRef]
- Martin, M.J.; Elwen, S.H.; Kassanjee, R.; Gridley, T. To buzz or burst-pulse? The functional role of Heaviside’s dolphin, Cephalorhynchus heavisidii, rapidly pulsed signals. Anim. Behav. 2019, 150, 273–284. [Google Scholar] [CrossRef]
- Blomqvist, C.; Amundin, M. High-frequency burst-pulse sounds in agonistic/aggresive interactions in bottlenose dolphins, Tursiops truncatus. In Echolocation in Bats and Dolphins; Thomas, J.A., Moss, C.F., Vater, M., Eds.; University of Chicago Press: Chicago, IL, USA, 2004; pp. 425–431. [Google Scholar]
- Overstrom, N.A. Association between burst-pulse sounds and aggressive behavior in captive Atlantic bottlenosed dolphins (Tursiops truncatus). Zoo Biol. 1983, 2, 93–103. [Google Scholar] [CrossRef]
- Perazio, C.E.; Kuczaj II, S.A. Vocalizations produced by bottlenose dolphins (Tursiops truncatus) during mouth actions in aggressive and non-aggressive contexts. Int. J. Comp. Psychol. 2017, 30, 7. [Google Scholar] [CrossRef]
- Carzon, P.; Delfour, F.; Dudzinski, K.M.; Oremus, M.; Clua, É. Cross-genus adoptions in delphinids: One example with taxonomic discussion. Ethology 2019, 125, 669–676. [Google Scholar] [CrossRef]
- Avramidis, S.; Avramidou, E. Animal rescuers: A review. Int. J. Aquat. Res. Educ. 2008, 2, 346–354. [Google Scholar] [CrossRef]
- Jones, S. Dolphins Save Swimmers from Shark. Guardian 24 November 2004. Available online: https://www.theguardian.com/science/2004/nov/24/internationalnews (accessed on 1 December 2023).
- Celizic, M. Dolphins Save Surfer from Becoming Shark’s Bait. Today 8 November 2007. Available online: https://www.today.com/news/dolphins-save-surfer-becoming-sharks-bait-2d80555123 (accessed on 1 December 2023).
- Barreto, D.B. Saved from a Shark; National Geographic Wild: Washington, DC, USA, 2023. [Google Scholar]
- Bas-Wohlert, C. Faroe Islands Mass Dolphin Slaughter Casts Shadow over Tradition. Phys.org 20 September 2021. Available online: https://phys.org/news/2021-09-faroe-islands-mass-dolphin-slaughter.html (accessed on 1 December 2023).
- Haq, S.N.; Ravindran, J.; Halasz, S.; Goodwin, A.; Braithwaite, S. Faroe Islands Sets Quota of 500 Dolphins to be Killed in Controversial Annual Whale hunt. CNN 11 July 2022. Available online: https://edition.cnn.com/2022/07/11/europe/faroe-islands-whale-hunt-dolphin-limit-Intl-scli-scn/index.html (accessed on 1 December 2023).
- Ravindran, J.; Halasz, S.; Goodwin, A.; Braithwaite, S. 1400 Dolphins Were Killed in the Faroe Islands in One Day, Shocking Even Some Pro-Whalers. CNN 15 September 2021. Available online: https://edition.cnn.com/2021/09/15/europe/faroe-dolphin-killing-record-scli-intl-scn/index.html (accessed on 1 December 2023).
- Sellheim, N. Livelihood, Cruelty, Extinction? The Recent Killings of Atlantic White-Sided Dolphins in the Faroe Islands. Polar Research and Policy Initiative 30 November 2021. Available online: https://polarconnection.org/dolphin-hunting-faroe-islands/ (accessed on 1 December 2023).
- Rollin, B.E. Animal research: A moral science. EMBO Rep. 2007, 8, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, A.; Sorenson, J. Human consequences of animal exploitation: Needs for redefining social welfare. J. Sociol. Soc. Welf. 2013, 40, 7–32. [Google Scholar] [CrossRef]
- Francione, G.L. Animals as Persons: Essays on the Abolition of Animal Exploitation; Columbia University Press: New York, NY, USA, 2009. [Google Scholar]
- Fraisl, D.; Hager, G.; Bedessem, B.; Gold, M.; Hsing, P.-Y.; Danielsen, F.; Hitchcock, C.B.; Hulbert, J.M.; Piera, J.; Spiers, H.; et al. Citizen science in environmental and ecological sciences. Nat. Rev. Methods Primers 2022, 2, 64. [Google Scholar] [CrossRef]
- Fahlquist, J.N. Moral responsibility for environmental problems—Individual or institutional? J. Agric. Environ. Ethics 2009, 22, 109–124. [Google Scholar] [CrossRef]
- Piccolo, J.J.; Taylor, B.; Washington, H.; Kopnina, H.; Gray, J.; Alberro, H.; Orlikowska, E. “Nature’s contributions to people” and peoples’ moral obligations to nature. Biol. Conserv. 2022, 270, 109572. [Google Scholar] [CrossRef]
- Busch, P.; Lahti, P.J.; Mittelstaedt, P. The Quantum Theory of Measurement; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar] [CrossRef]
- Busch, P. Is the quantum state (an) observable? In Potentiality, Entanglement and Passion-at-a-Distance: Quantum Mechanical Studies for Abner Shimony, Volume Two; Cohen, R.S., Horne, M., Stachel, J., Eds.; Boston Studies in the Philosophy of Science; Kluwer: Dordrecht, The Netherlands, 1997; pp. 61–70. [Google Scholar] [CrossRef]
- Busch, P.; Gudder, S.P. Effects as functions on projective Hilbert space. Lett. Math. Phys. 1999, 47, 329–337. [Google Scholar] [CrossRef]
- Heisenberg, W. Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik. Z. Für Phys. 1927, 43, 172–198. [Google Scholar] [CrossRef]
- Maddox, J. The reality of the quantum jump. Nature 1986, 323, 577. [Google Scholar] [CrossRef]
- Nagourney, W.; Sandberg, J.; Dehmelt, H. Shelved optical electron amplifier: Observation of quantum jumps. Phys. Rev. Lett. 1986, 56, 2797–2799. [Google Scholar] [CrossRef]
- Sauter, T.; Neuhauser, W.; Blatt, R.; Toschek, P.E. Observation of quantum jumps. Phys. Rev. Lett. 1986, 57, 1696–1698. [Google Scholar] [CrossRef]
- Bergquist, J.C.; Hulet, R.G.; Itano, W.M.; Wineland, D.J. Observation of quantum jumps in a single atom. Phys. Rev. Lett. 1986, 57, 1699–1702. [Google Scholar] [CrossRef]
- Hills, T.T. Neurocognitive free will. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190510. [Google Scholar] [CrossRef] [PubMed]
- Garson, J.W. Chaos and free will. Philos. Psychol. 1995, 8, 365–374. [Google Scholar] [CrossRef]
- Schuster, H.G.; Just, W. Deterministic Chaos: An Introduction, 4th ed.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar] [CrossRef]
- Smith, L. Chaos: A Very Short Introduction; Oxford University Press: Oxford, UK, 2007. [Google Scholar] [CrossRef]
- Hawking, S.W.; Mlodinow, L. The Grand Design; Bantam Press: London, UK, 2010. [Google Scholar]
- Conway, J.H.; Kochen, S.B. The free will theorem. Found. Phys. 2006, 36, 1441–1473. [Google Scholar] [CrossRef]
- Conway, J.H.; Kochen, S. The strong free will theorem. In Deep Beauty: Understanding the Quantum World through Mathematical Innovation; Halvorson, H., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 443–454. [Google Scholar] [CrossRef]
- Susskind, L.; Friedman, A. Quantum Mechanics: The Theoretical Minimum. What You Need to Know to Start Doing Physics; Basic Books: New York, NY, USA, 2014. [Google Scholar]
- Durrett, R. Stochastic Calculus: A Practical Introduction; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Klebaner, F.C. Introduction to Stochastic Calculus with Applications; Imperial College Press: London, UK, 2005. [Google Scholar]
- Ishikawa, Y. Stochastic Calculus of Variations: For Jump Processes, 2nd ed.; De Gruyter: Berlin, Germany, 2016. [Google Scholar]
- Dyson, F. Disturbing The Universe; Basic Books: New York, NY, USA, 1979. [Google Scholar]
- Georgiev, D.D. Quantum information in neural systems. Symmetry 2021, 13, 773. [Google Scholar] [CrossRef]
- Li, X.-Z.; Walker, B.; Michaelides, A. Quantum nature of the hydrogen bond. Proc. Natl. Acad. Sci. USA 2011, 108, 6369–6373. [Google Scholar] [CrossRef]
- Zolotaryuk, A.V. One-dimensional lattice dynamics of hydrogen bonded systems. Theor. Math. Phys. 1986, 68, 916–923. [Google Scholar] [CrossRef]
- Melkikh, A.V. Thinking as a quantum phenomenon. Biosystems 2019, 176, 32–40. [Google Scholar] [CrossRef]
- Melkikh, A.V. Thinking, holograms, and the quantum brain. Biosystems 2023, 229, 104926. [Google Scholar] [CrossRef]
- Fields, C.; Glazebrook, J.F. Information flow in context-dependent hierarchical Bayesian inference. J. Exp. Theor. Artif. Intell. 2022, 34, 111–142. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology, 14th ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Fields, C.; Friston, K.J.; Glazebrook, J.F.; Levin, M. A free energy principle for generic quantum systems. Prog. Biophys. Mol. Biol. 2022, 173, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.; Friston, K.J.; Glazebrook, J.F.; Levin, M.; Marcianò, A. The free energy principle drives neuromorphic development. Neuromorphic Comput. Eng. 2022, 2, 042002. [Google Scholar] [CrossRef]
- Kuchling, F.; Friston, K.; Georgiev, G.; Levin, M. Morphogenesis as Bayesian inference: A variational approach to pattern formation and control in complex biological systems. Phys. Life Rev. 2020, 33, 88–108. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, D.D.; Glazebrook, J.F. Thermal stability of solitons in protein α-helices. Chaos Solitons Fractals 2022, 155, 111644. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Glazebrook, J.F. Launching of Davydov solitons in protein α-helix spines. Phys. E Low-Dimens. Syst. Nanostructures 2020, 124, 114332. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Glazebrook, J.F. Quantum transport and utilization of free energy in protein α-helices. Adv. Quantum Chem. 2020, 82, 253–300. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Glazebrook, J.F. Quantum tunneling of Davydov solitons through massive barriers. Chaos Solitons Fractals 2019, 123, 275–293. [Google Scholar] [CrossRef]
- Chapman, J.B.; Johnson, E.A.; Kootsey, J.M. Electrical and biochemical properties of an enzyme model of the sodium pump. J. Membr. Biol. 1983, 74, 139–153. [Google Scholar] [CrossRef]
- Chapp, A.D.; Schum, S.; Behnke, J.E.; Hahka, T.; Huber, M.J.; Jiang, E.; Larson, R.A.; Shan, Z.; Chen, Q.-H. Measurement of cations, anions, and acetate in serum, urine, cerebrospinal fluid, and tissue by ion chromatography. Physiol. Rep. 2018, 6, e13666. [Google Scholar] [CrossRef]
- Larsen, B.R.; Stoica, A.; MacAulay, N. Managing brain extracellular K+ during neuronal activity: The physiological role of the Na+/K+-ATPase subunit isoforms. Front. Physiol. 2016, 7, 141. [Google Scholar] [CrossRef]
- Somjen, G.G.; Giacchino, J.L. Potassium and calcium concentrations in interstitial fluid of hippocampal formation during paroxysmal responses. J. Neurophysiol. 1985, 53, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Somjen, G.G. Na+ and K+ concentrations, extra- and intracellular voltages, and the effect of TTX in hypoxic rat hippocampal slices. J. Neurophysiol. 2000, 83, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Leibfried, D.; Blatt, R.; Monroe, C.; Wineland, D. Quantum dynamics of single trapped ions. Rev. Mod. Phys. 2003, 75, 281–324. [Google Scholar] [CrossRef]
- Ge, W.; Sawyer, B.C.; Britton, J.W.; Jacobs, K.; Bollinger, J.J.; Foss-Feig, M. Trapped ion quantum information processing with squeezed phonons. Phys. Rev. Lett. 2019, 122, 030501. [Google Scholar] [CrossRef] [PubMed]
- Häffner, H.; Roos, C.F.; Blatt, R. Quantum computing with trapped ions. Phys. Rep. 2008, 469, 155–203. [Google Scholar] [CrossRef]
- Davydov, A.S. Biology and Quantum Mechanics; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Sakmann, B.; Neher, E. Single-Channel Recording, 2nd ed.; Springer: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Johnston, D.; Wu, S.M.-S. Foundations of Cellular Neurophysiology; MIT Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Hodgkin, A.L. The ionic basis of nervous conduction. Science 1964, 145, 1148–1154. [Google Scholar] [CrossRef]
- Maffeo, C.; Bhattacharya, S.; Yoo, J.; Wells, D.; Aksimentiev, A. Modeling and simulation of ion channels. Chem. Rev. 2012, 112, 6250–6284. [Google Scholar] [CrossRef]
- Sigg, D. Modeling ion channels: Past, present, and future. J. Gen. Physiol. 2014, 144, 7–26. [Google Scholar] [CrossRef]
- Clerx, M.; Beattie, K.A.; Gavaghan, D.J.; Mirams, G.R. Four ways to fit an ion channel model. Biophys. J. 2019, 117, 2420–2437. [Google Scholar] [CrossRef]
- Kariev, A.M.; Njau, P.; Green, M.E. The open gate of the Kv1.2 channel: Quantum calculations show the key role of hydration. Biophys. J. 2014, 106, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Kariev, A.M.; Green, M.E. Quantum calculation of proton and other charge transfer steps in voltage sensing in the Kv1.2 channel. J. Phys. Chem. B 2019, 123, 7984–7998. [Google Scholar] [CrossRef] [PubMed]
- Kariev, A.M.; Green, M.E. Quantum calculations on ion channels: Why are they more useful than classical calculations, and for which processes are they essential? Symmetry 2021, 13, 655. [Google Scholar] [CrossRef]
- Kariev, A.M.; Znamenskiy, V.S.; Green, M.E. Quantum mechanical calculations of charge effects on gating the KcsA channel. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.; Glazebrook, J.F.; Levin, M. Minimal physicalism as a scale-free substrate for cognition and consciousness. Neurosci. Conscious. 2021, 7, niab013. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.; Glazebrook, J.F.; Levin, M. Neurons as hierarchies of quantum reference frames. Biosystems 2022, 219, 104714. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Molnár, Z.; Clowry, G.J.; Šestan, N.; Alzu’bi, A.; Bakken, T.; Hevner, R.F.; Hüppi, P.S.; Kostović, I.; Rakic, P.; Anton, E.S.; et al. New insights into the development of the human cerebral cortex. J. Anat. 2019, 235, 432–451. [Google Scholar] [CrossRef]
- Rakic, P. Evolution of the neocortex: A perspective from developmental biology. Nat. Rev. Neurosci. 2009, 10, 724–735. [Google Scholar] [CrossRef]
- Kopić, J.; Junaković, A.; Salamon, I.; Rasin, M.-R.; Kostović, I.; Krsnik, Ž. Early regional patterning in the human prefrontal cortex revealed by laminar dynamics of deep projection neuron markers. Cells 2023, 12, 231. [Google Scholar] [CrossRef]
- Eckermann, M.; van der Meer, F.; Cloetens, P.; Ruhwedel, T.; Möbius, W.; Stadelmann, C.; Salditt, T. Three-dimensional virtual histology of the cerebral cortex based on phase-contrast X-ray tomography. Biomed. Opt. Express 2021, 12, 7582–7598. [Google Scholar] [CrossRef] [PubMed]
- Markram, H.; Muller, E.; Ramaswamy, S.; Reimann, M.W.; Abdellah, M.; Sanchez, C.A.; Ailamaki, A.; Alonso-Nanclares, L.; Antille, N.; Arsever, S.; et al. Reconstruction and simulation of neocortical microcircuitry. Cell 2015, 163, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castañeda, R.; Zingg, B.; Matho, K.S.; Chen, X.; Wang, Q.; Foster, N.N.; Li, A.; Narasimhan, A.; Hirokawa, K.E.; Huo, B.; et al. Cellular anatomy of the mouse primary motor cortex. Nature 2021, 598, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E.M.; Dong, H.-W.; Ecker, J.R.; Hawrylycz, M.J.; Huang, Z.J.; Lein, E.S.; Ngai, J.; Osten, P.; Ren, B.; Tolias, A.S.; et al. A multimodal cell census and atlas of the mammalian primary motor cortex. Nature 2021, 598, 86–102. [Google Scholar] [CrossRef]
- Chiaradia, I.; Lancaster, M.A. Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat. Neurosci. 2020, 23, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Eichmüller, O.L.; Knoblich, J.A. Human cerebral organoids—A new tool for clinical neurology research. Nat. Rev. Neurol. 2022, 18, 661–680. [Google Scholar] [CrossRef]
- Samarasinghe, R.A.; Miranda, O.A.; Buth, J.E.; Mitchell, S.; Ferando, I.; Watanabe, M.; Allison, T.F.; Kurdian, A.; Fotion, N.N.; Gandal, M.J.; et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat. Neurosci. 2021, 24, 1488–1500. [Google Scholar] [CrossRef]
- Trujillo, C.A.; Gao, R.; Negraes, P.D.; Gu, J.; Buchanan, J.; Preissl, S.; Wang, A.; Wu, W.; Haddad, G.G.; Chaim, I.A.; et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 2019, 25, 558–569.e557. [Google Scholar] [CrossRef]
- Jeziorski, J.; Brandt, R.; Evans, J.H.; Campana, W.; Kalichman, M.; Thompson, E.; Goldstein, L.; Koch, C.; Muotri, A.R. Brain organoids, consciousness, ethics and moral status. Semin. Cell Dev. Biol. 2023, 144, 97–102. [Google Scholar] [CrossRef]
- Niikawa, T.; Hayashi, Y.; Shepherd, J.; Sawai, T. Human brain organoids and consciousness. Neuroethics 2022, 15, 5. [Google Scholar] [CrossRef]
- Nagel, T. What is it like to be a bat? Philos. Rev. 1974, 83, 435–450. [Google Scholar] [CrossRef]
- Nagel, T. Mind and Cosmos: Why the Materialist Neo-Darwinian Conception of Nature Is Almost Certainly False; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Nagel, T. What is the mind-body problem? In Experimental and Theoretical Studies of Consciousness; Bock, G.R., Marsh, J., Eds.; Ciba Foundation Symposium; John Wiley & Sons: Chichester, UK, 1993; pp. 1–13. [Google Scholar] [CrossRef]
- Epstei, R.; Lanza, R.P.; Skinner, B.F. “Self-awareness” in the pigeon. Science 1981, 212, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Skinner, B.F. Science and Human Behavior; Simon and Schuster: New York, NY, USA, 1965. [Google Scholar]
- Skinner, B.F. The Behavior of Organisms; D. Appleton-Century Co.: New York, NY, USA, 1938. [Google Scholar]
- Rayleigh, J.W.S. Remarks upon the law of complete radiation. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1900, 49, 539–540. [Google Scholar] [CrossRef]
- Jeans, J.H. On the partition of energy between matter and æther. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1905, 10, 91–98. [Google Scholar] [CrossRef]
- Rayleigh, J.W.S. The law of partition of kinetic energy. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1900, 49, 98–118. [Google Scholar] [CrossRef]
- Rayleigh, J.W.S. The dynamical theory of gases and of radiation. Nature 1905, 72, 54–55. [Google Scholar] [CrossRef]
- Rayleigh, J.W.S. The constant of radiation as calculated from molecular data. Nature 1905, 72, 243–244. [Google Scholar] [CrossRef]
- Jeans, J.H. The dynamical theory of gases and of radiation. Nature 1905, 72, 101–102. [Google Scholar] [CrossRef]
- Jeans, J.H. A comparison between two theories of radiation. Nature 1905, 72, 293–294. [Google Scholar] [CrossRef]
- Bohr, N. On the constitution of atoms and molecules. Part I. Binding of electrons by positive nuclei. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1913, 26, 1–25. [Google Scholar] [CrossRef]
- Bohr, N. On the constitution of atoms and molecules. Part II. Systems containing only a single nucleus. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1913, 26, 476–502. [Google Scholar] [CrossRef]
- Bohr, N. On the constitution of atoms and molecules. Part III. Systems containing several nuclei. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1913, 26, 857–875. [Google Scholar] [CrossRef]
- Bohr, N. The spectra of helium and hydrogen. Nature 1913, 92, 231–232. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Gudder, S.P. Sensitivity of entanglement measures in bipartite pure quantum states. Mod. Phys. Lett. B 2022, 36, 2250101. [Google Scholar] [CrossRef]
- Wootters, W.K. Quantum entanglement as a quantifiable resource. Philos. Trans. Math. Phys. Eng. Sci. 1998, 356, 1717–1731. [Google Scholar] [CrossRef]
- Chitambar, E.; Gour, G. Quantum resource theories. Rev. Mod. Phys. 2019, 91, 025001. [Google Scholar] [CrossRef]
- Hayashi, M.; Ishizaka, S.; Kawachi, A.; Kimura, G.; Ogawa, T. Introduction to Quantum Information Science; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Hayashi, M. Quantum Information Theory: Mathematical Foundation; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Nielsen, M.A.; Chuang, I.L. Quantum Computation and Quantum Information, 10th Anniversary ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, D.D. Evolution of Consciousness. Life 2024, 14, 48. https://doi.org/10.3390/life14010048
Georgiev DD. Evolution of Consciousness. Life. 2024; 14(1):48. https://doi.org/10.3390/life14010048
Chicago/Turabian StyleGeorgiev, Danko D. 2024. "Evolution of Consciousness" Life 14, no. 1: 48. https://doi.org/10.3390/life14010048
APA StyleGeorgiev, D. D. (2024). Evolution of Consciousness. Life, 14(1), 48. https://doi.org/10.3390/life14010048






