Congenital Zika Virus Syndrome: Microcephaly and Orofacial Anomalies
Abstract
:1. Introduction
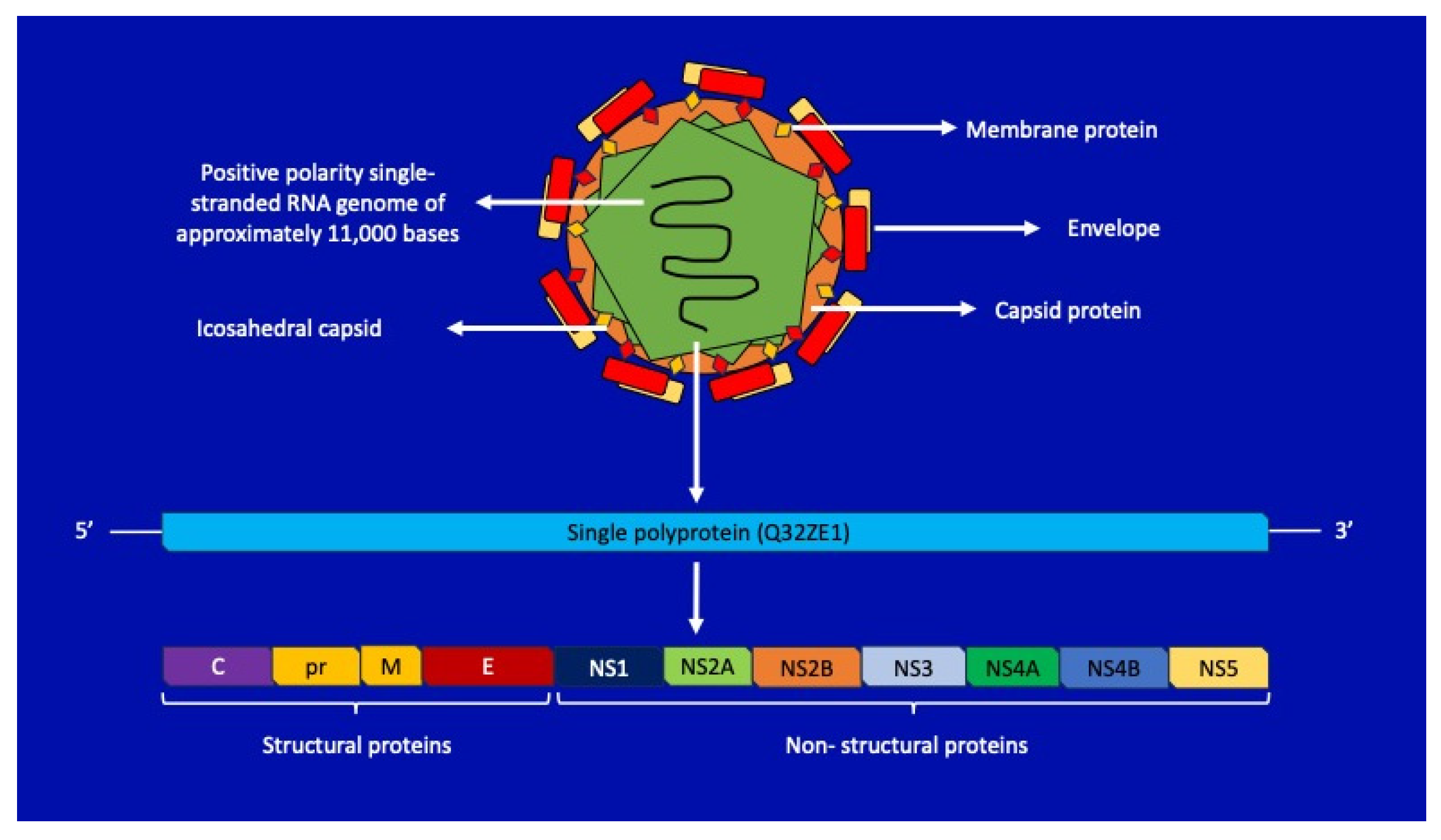
2. Zika Virus: Structure and Pathogenic Insights
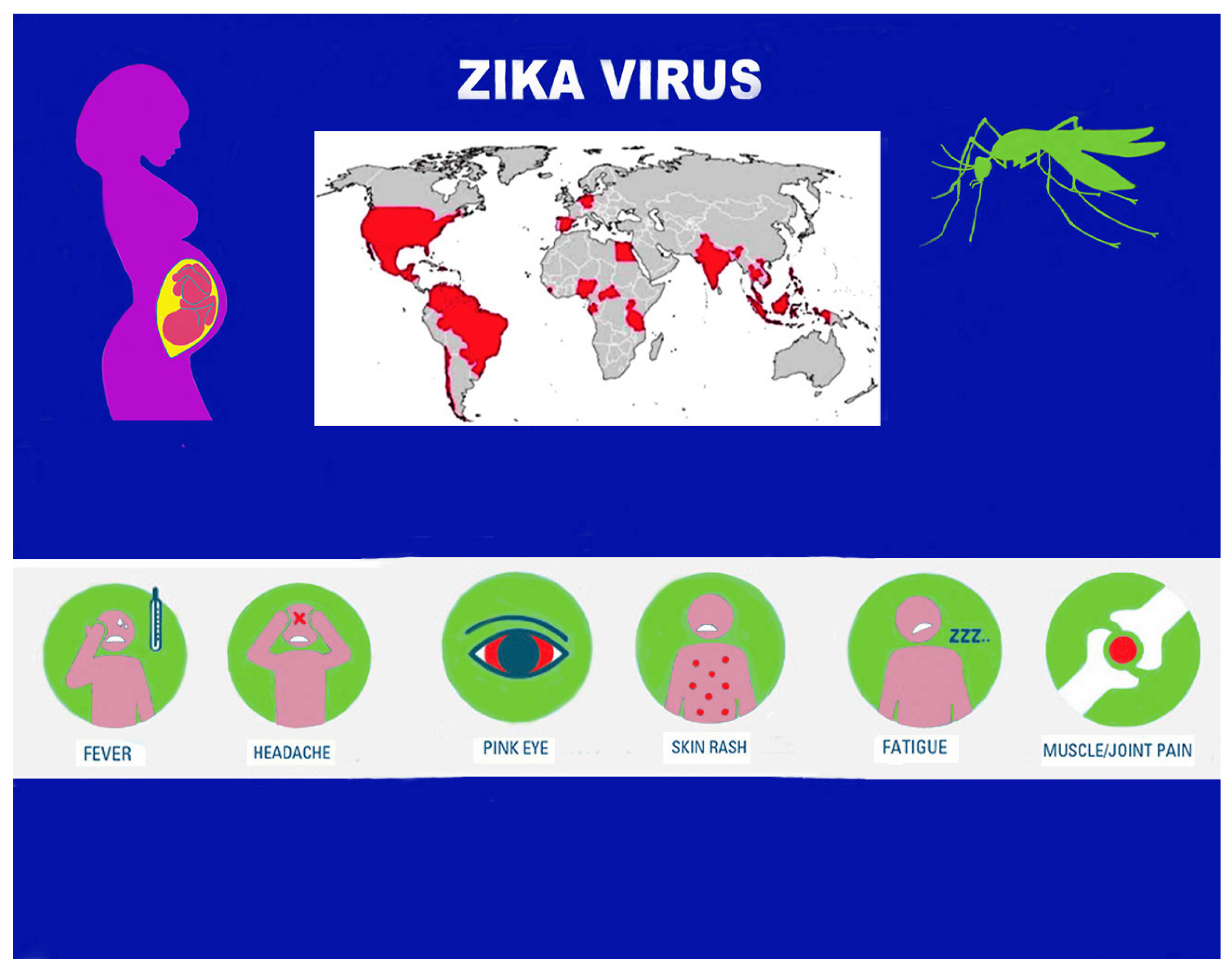
3. Epidemiological Aspects
4. Transmission Mode
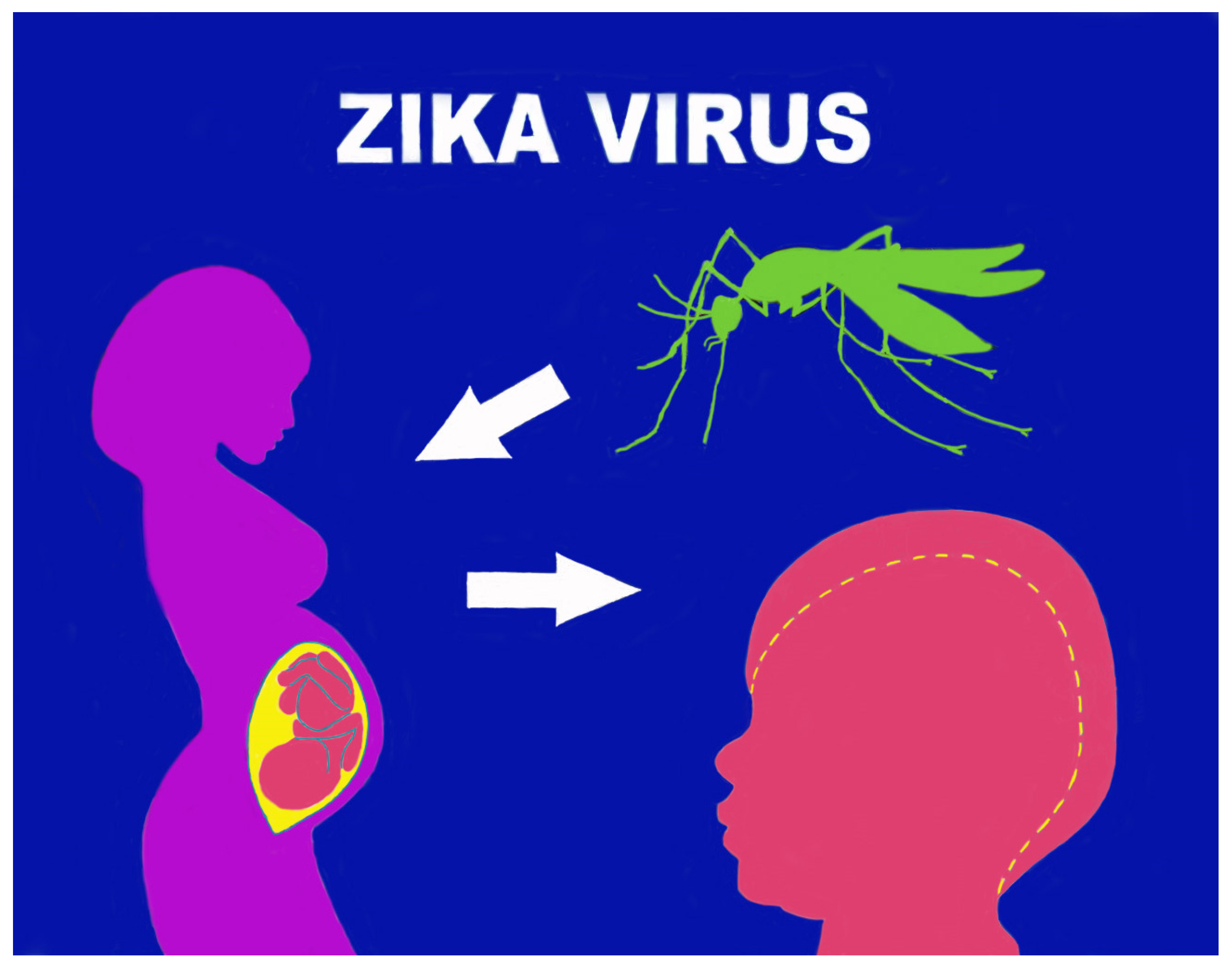
5. Pathogenetic Mechanisms in Pregnancy
6. Microcephaly and Other Brain Anomalies Due to CZS
6.1. Microcephaly
6.2. Orofacial Anomalies
7. Conclusions
Funding
Conflicts of Interest
References
- Musso, D.; Rodriguez-Morales, A.J.; Levi, J.E.; Cao-Lormeau, V.-M.; Gubler, D.J. Unexpected outbreaks of arbovirus infections: Lessons learned from the Pacific and tropical America. Lancet Infect. Dis. 2018, 18, e355–e361. [Google Scholar] [CrossRef]
- Huntington, M.K.; Allison, J.; Nair, D. Emerging Vector-Borne Diseases. Am. Fam. Physician 2016, 94, 551–557. [Google Scholar]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Wimalasiri-Yapa, B.; Yapa, H.E.; Huang, X.; Hafner, L.M.; Kenna, T.J.; Frentiu, F.D. Zika Virus and Arthritis/Arthralgia: A Systematic Review and Meta-Analysis. Viruses 2020, 12, 1137. [Google Scholar] [CrossRef]
- Tognarelli, J.; Ulloa, S.; Villagra, E.; Lagos, J.; Aguayo, C.; Fasce, R.; Parra, B.; Mora, J.; Becerra, N.; Lagos, N.; et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch. Virol. 2016, 161, 665–668. [Google Scholar] [CrossRef]
- Cofre, F. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet. Gynecol. 2016, 33, 96. [Google Scholar] [CrossRef]
- Postler, T.S.; Beer, M.; Blitvich, B.J.; Bukh, J.; de Lamballerie, X.; Drexler, J.F.; Imrie, A.; Kapoor, A.; Karganova, G.G.; Lemey, P.; et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023, 168, 224. [Google Scholar] [CrossRef]
- Sirohi, D.; Kuhn, R.J. Share Zika Virus Structure, Maturation, and Receptors. J. Infect. Dis. 2017, 216 (Suppl. S10), S935–S944. [Google Scholar] [CrossRef]
- Haddow, A.D.; Woodall, J.P. Distinguishing between Zika and Spondweni viruses. Bull. World Health Organ. 2016, 94, 711. [Google Scholar] [CrossRef]
- White, M.K.; Wollebo, H.S.; David Beckham, J.; Tyler, K.L.; Khalili, K. Zika virus: An emergent neuropathological agent. Ann. Neurol. 2016, 80, 479–489. [Google Scholar] [CrossRef]
- van den Elsen, K.; Quek, J.P.; Luo, D. Molecular Insights into the Flavivirus Replication Complex. Viruses 2021, 13, 956. [Google Scholar] [CrossRef]
- Silva, L.R.; Souza, A.M. Zika virus: What do we know about the viral structure, mechanisms of transmission, and neurological outcomes? Rev. Soc. Bras. Med. Trop. 2016, 49, 267–273. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, L. Systematic Analysis of Structure Similarity between Zika Virus and Other Flaviviruses. ACS Infect. Dis. 2019, 5, 1070–1080. [Google Scholar] [CrossRef]
- Steen, H.C.; Gamero, A.M. The role of signal transducer and activator of transcription-2 in the interferon response. J. Interferon. Cytokine Res. 2012, 32, 103–110. [Google Scholar] [CrossRef]
- Gabaglia, C.R. Zika virus and diagnostics. Curr. Opin. Pediatr. 2017, 29, 107–113. [Google Scholar] [CrossRef]
- Song, B.-H.; Yun, S.-I.; Woolley, M.; Lee, Y.-M. Lee YM. Zika virus: History, epidemiology, transmission, and clinical presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Younger, D.S. Epidemiology of Zika Virus. Neurol. Clin. 2016, 34, 1049–1056. [Google Scholar] [CrossRef]
- Hills, S.L.; Fischer, M.; Petersen, L.R. Epidemiology of Zika Virus Infection. J. Infect. Dis. 2017, 216 (Suppl. S10), S868–S874. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Ma, Y.; Wu, J.; Mao, C.; Yuan, L.; Lu, J. Prevalence of Zika virus in blood donations: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 590. [Google Scholar] [CrossRef]
- Runge-Ranzinger, S.; Morrison, A.C.; Manrique-Saide, P.; Horstick, O. Zika transmission patterns: A meta-review. Trop. Med. Int. Health 2019, 24, 523–529. [Google Scholar] [CrossRef]
- Gregory, C.J.; Oduyebo, T.; Brault, A.C.; Brooks, J.T.; Chung, K.-W.; Hills, S.; Kuehnert, M.J.; Mead, P.; Meaney-Delman, D.; Rabe, I.; et al. Modes of Transmission of Zika Virus. J. Infect. Dis. 2017, 216 (Suppl. S10), S875–S883. [Google Scholar] [CrossRef]
- Nogueira, M.; Júnior, N.N.; Estofolete, C.; Terzian, A.B.; Guimarães, G.; Zini, N.; da Silva, R.A.; Silva, G.D.; Franco, L.J.; Rahal, P.; et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in Sao Jose do Rio Preto, Brazil. Clin. Microbiol. Infect. 2018, 24, 646–652. [Google Scholar] [CrossRef]
- Gutiérrez-Bugallo, G.; Piedra, L.A.; Rodriguez, M.; Bisset, J.A.; Lourenço-De-Oliveira, R.; Weaver, S.C.; Vasilakis, N.; Vega-Rúa, A. Vector-borne transmission and evolution of Zika virus. Nat. Ecol. Evol. 2019, 3, 561–569. [Google Scholar] [CrossRef]
- Brasil, P.; Vasconcelos, Z.; Kerin, T.; Gabaglia, C.R.; Ribeiro, I.P.; Bonaldo, M.C.; Damasceno, L.; Pone, M.V.; Pone, S.; Zin, A.; et al. Zika virus vertical transmission in children with confirmed antenatal exposure. Nat. Commun. 2020, 11, 3510. [Google Scholar] [CrossRef]
- Martins, M.M.; Alves da Cunha, A.J.L.; Robaina, J.R.; Raymundo, C.E.; Barbosa, A.P.; Medronho, R.A. Fetal, neonatal, and infant outcomes associated with maternal Zika virus infection during pregnancy: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246643. [Google Scholar] [CrossRef]
- Marbán-Castro, E.; Goncé, A.; Fumadó, V.; Romero-Acevedo, L.; Bardají, A. Zika virus infection in pregnant women and their children: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 265, 162–168. [Google Scholar] [CrossRef]
- Mysorekar, I.U.; Diamond, M.S. Modeling Zika Virus Infection in Pregnancy. N. Engl. J. Med. 2016, 375, 481–484. [Google Scholar] [CrossRef]
- Rosenberg, A.Z.; Yu, W.; Hill, D.A.; Reyes, C.A.; Schwartz, D.A. Placental Pathology of Zika Virus: Viral infection of the placenta Induces Villous Stromal Macrophage (Hofbauer Cell) proliferation and hyperplasia. Arch. Pathol. Lab. Med. 2017, 141, 43–48. [Google Scholar] [CrossRef]
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; Ma, H.; O’Neal, J.T.; Rajakumar, A.; Wrammert, J.; Rimawi, B.H.; Pulendran, B.; et al. Zika virus infects human placental macrophages. Cell Host Microbe 2016, 20, 83–90. [Google Scholar] [CrossRef]
- Merfeld, E.; Ben-Avi, L.; Kennon, M.; Cerveny, K.L. Potential mechanisms of Zika-linked microcephaly. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e273. [Google Scholar] [CrossRef]
- Robbins, J.R.; Bakardjiev, A.I. Pathogens and the placental fortress. Curr. Opin. Microbiol. 2012, 15, 36–43. [Google Scholar] [CrossRef]
- Sarkola, M.E.; Grenman, S.E.; Rintala, M.A.; Syrjanen, K.J.; Syrjanen, S.M. Human papillomavirus in the placenta and umbilical cord blood. Acta Obstet. Gynecol. Scand. 2008, 87, 1181–1188. [Google Scholar] [CrossRef]
- Mazziotta, C.; Pellielo, G.; Tognon, M.; Martini, F.; Rotondo, J.C. Significantly Low Levels of IgG Antibodies Against Oncogenic Merkel Cell Polyomavirus in Sera From Females Affected by Spontaneous Abortion. Front. Microbiol. 2021, 12, 789991. [Google Scholar] [CrossRef]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Freitas, D.A.; Souza-Santos, R.; Carvalho, L.M.A.; Barros, W.B.; Neves, L.M.; Brasil, P.; Wakimoto, M.D. Congenital Zika syndrome: A systematic review. PLoS ONE 2020, 15, e0242367. [Google Scholar] [CrossRef]
- Vhp, L.; Aragão, M.M.; Pinho, R.S.; Hazin, A.N.; Paciorkowski, A.R.; Penalva De Oliveira, A.C.; Masruha, M.R. Congenital Zika Virus Infection: A Review with Emphasis on the Spectrum of Brain Abnormalities. Curr. Neurol. Neurosci. Rep. 2020, 20, 49. [Google Scholar] [CrossRef]
- Saad, T.; PennaeCosta, A.A.; de Góes, F.V.; de Freitas, M.; de Almeida, J.V.; de Santa Ignêz, L.J.; Amancio, A.P.; Alvim, R.J.; Antunes Kramberger, L.A. Neurological manifestations of congenital Zika virus infection. Child’s Nerv. Syst. 2018, 34, 73–78. [Google Scholar] [CrossRef]
- Venceslau, E.M.; Guida, J.P.; Amaral, E.; Modena, J.L.P.; Costa, M.L. Characterization of placental infection by Zika virus in humans: A review of the literature. Rev. Bras. Ginecol. Obstet. Rev. Fed. Bras. Soc. Ginecol. Obstet. 2020, 42, 577–585. [Google Scholar] [CrossRef]
- Pomar, L.; Musso, D.; Malinger, G.; Vouga, M.; Panchaud, A.; Baud, D. Zika virus during pregnancy: From maternal exposure to congenital Zika virus syndrome. Prenat. Diagn. 2019, 39, 420–430. [Google Scholar] [CrossRef]
- von der Hagen, M.; Pivarcsi, M.; Liebe, J.; von Bernuth, H.; Didonato, N.; Hennermann, J.B.; Bührer, C.; Wieczorek, D.; Kaindl, A.M. Diagnostic approach to microcephaly in childhood: A two-center study and review of the literature. Dev. Med. Child Neurol. 2014, 56, 732–741. [Google Scholar] [CrossRef]
- Pool, K.-L.; Adachi, K.; Karnezis, S.; Salamon, N.; Romero, T.; Nielsen-Saines, K.; Pone, S.; Aibe, M.; da Silva, T.G.; Ribeiro, C.T.M.; et al. Association between neonatal neuroimaging and clinical outcomes in Zika-Exposed infants from Rio de Janeiro, Brazil. JAMA Netw. Open 2019, 2, e198124. [Google Scholar] [CrossRef]
- Lemke, G. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- Ji, R.; Meng, L.; Jiang, X.; Cvm, N.K.; Ding, J.; Li, Q.; Lu, Q. TAM receptors support neural stem cell survival, proliferation and neuronal differentiation. PLoS ONE 2014, 9, e115140. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Sandoval-Espinosa, C.; Bershteyn, M.; Kriegstein, A.R. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 2016, 18, 591–596. [Google Scholar] [CrossRef]
- Bhagat, R.; Prajapati, B.; Narwal, S.; Agnihotri, N.; Adlakha, Y.K.; Sen, J.; Mani, S.; Seth, P. Zika virus E protein alters the properties of human fetal neural stem cells by modulating microRNA circuitry. Cell Death Differ. 2018, 25, 1837–1854. [Google Scholar] [CrossRef]
- Bhagat, R.; Rajpara, P.; Kaur, G.; Gupta, K.; Seth, P. Zika virus E protein dysregulate mir-204/WNT2 signalling in human fetal neural stem cells. Brain Res. Bull. 2021, 176, 93–102. [Google Scholar] [CrossRef]
- Lottini, G.; Baggiani, M.; Chesi, G.; D’orsi, B.; Quaranta, P.; Lai, M.; Pancrazi, L.; Onorati, M.; Pistello, M.; Freer, G.; et al. Zika virus induces FOXG1 nuclear displacement and downregulation in human neural progenitors. Stem Cell Rep. 2022, 17, 1683–1698. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Mattos, A.; Meneghim, M.d.C.; Vedovello, S.A.S.; Borges, T.M.D.; Santamaria, M. Oral and maxillofacial outcomes in children with microcephaly associated with the congenital Zika syndrome. Eur. J. Orthod. 2021, 43, 346–352. [Google Scholar] [CrossRef]
- Gomes, P.N.; Amaral, B.A.D.; Azevedo, I.D.; Maia, H.C.d.M.; Arrais, N.M.R.; de Lima, K.C. Association of congenital Zika syndrome with dental alterations in children with microcephaly. PLoS ONE 2022, 17, e0276931. [Google Scholar] [CrossRef]
- D’Agostino, E.S.; Chagas, J.R.L.P.; Cangussu, M.C.T.; Vianna, M.I.P. Chronology and sequence of deciduous teeth eruption in children with microcephaly associated to the Zika virus. Spec. Care Dent. 2020, 40, 3–9. [Google Scholar] [CrossRef]
- Carvalho, A.; Brites, C.; Mochida, G.; Ventura, P.; Fernandes, A.; Lage, M.L.; Taguchi, T.; Brandi, I.; Silva, A.; Franceschi, G.; et al. Clinical and neurodevelopmental features in children with cerebral palsy and probable congenital Zika. Brain Dev. 2019, 41, 587–594. [Google Scholar] [CrossRef]
- Carvalho, I.F.; Alencar, P.N.B.; de Andrade, M.D.C.; Silva, P.G.d.B.; Carvalho, E.D.F.; Araújo, L.S.; Cavalcante, M.P.M.; Sousa, F.B. Clinical and x-ray oral evaluation in patients with congenital Zika Virus. J. Appl. Oral Sci. 2019, 27, e20180276. [Google Scholar] [CrossRef]
- Cavalcanti, A.F.C.; Aguiar, Y.P.C.; Melo, A.S.d.O.; Leal, J.I.B.d.F.; Cavalcanti, A.L.; Cavalcanti, S.D.L.B. Teething symptoms in children with congenital Zika syndrome: A 2-year follow-up. Int. J. Paediatr. Dent. 2019, 29, 74–78. [Google Scholar] [CrossRef]
- Gusmão, T.P.d.L.; de Faria, A.B.S.; Filho, J.C.L.; Carvalho, A.d.A.T.; Gueiros, L.A.M.; Leão, J.C. Dental changes in children with congenital Zika syndrome. Oral Dis. 2020, 26, 457–464. [Google Scholar] [CrossRef]
- de Oliveira, A.M.M.; de Melo, E.G.M.; Mendes, M.L.T.; Oliveira, S.J.G.d.S.; Tavares, C.S.S.; Vaez, A.C.; de Vasconcelos, S.J.A.; Santos, H.P.; Santos, V.S.; Martins-Filho, P.R.S. Oral and maxillofacial conditions, dietary aspects, and nutritional status of children with congenital Zika syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 71–77. [Google Scholar] [CrossRef]
- da Silva, M.C.P.M.; Arnaud, M.d.A.; Lyra, M.C.A.; Filho, A.V.d.A.; Rocha, M.W.; Ramos, R.C.F.; Van Der Linden, V.; Caldas, A.d.F.; Heimer, M.V.; Rosenblatt, A. Dental development in children born to Zikv-infected mothers: A case-based study. Arch. Oral Biol. 2020, 110, 104598. [Google Scholar] [CrossRef]
- Sobrinho, A.R.d.S.; Ramos, L.F.S.; Maciel, Y.L.; Maurício, H.d.A.; Cartaxo, R.d.O.; Ferreira, S.J.; Sette-De-Souza, P.H. Orofacial features in children with microcephaly associated with Zika virus: A scoping review. Oral Dis. 2022, 28, 1022–1028. [Google Scholar] [CrossRef]
- Medina, D.T.; dos Santos, A.P.P.; Rodrigues, F.M.D.F.; de Oliveira, B.H. Oral manifestations of congenital Zika virus infection in children with microcephaly: 18-month follow-up case series. Spec. Care Dent. 2022, 42, 343–351. [Google Scholar] [CrossRef]
- Siqueira, R.M.P.; Santos, M.; Cabral, G.M.P. Alterations in the primary teeth of children with microcephaly in Northeast Brazil: A comparative study. Int. J. Paediatr. Dent. 2018, 28, 523–532. [Google Scholar] [CrossRef] [PubMed]
- da Costa, C.C.G.; Dias, V.O.; Martelli, D.R.B.; Marques, N.C.T.; Coletta, R.D.; Júnior, H.M. First cases of oligodontia as a manifestation of the Zika virus congenital syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, e261–e266. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, R.; Baan, F.; Kramer, G.; Kuijpers-Jagtman, A.; Bergé, S.; Maal, T.; Ongkosuwito, E. Symmetry of palatal shape during the first year of life in healthy infants. Clin. Oral Investig. 2021, 25, 1069–1076. [Google Scholar] [CrossRef]
- Snider, T.N.; Mishina, Y. Cranial neural crest cell contribution to craniofacial formation, pathology, and future directions in tissue engineering. Birth Defects Res. Part C Embryo Today Rev. 2014, 2, 324–332. [Google Scholar] [CrossRef]
- Rios, D.; Rios, M.; Nóbrega, A.C.; de Oliveira, L.B.; Vaz, D.; Sales, H.; de Almeida, B.L.; Lopes, L.S.; de Siqueira, I.C.; Lucena, R. Alterations in deglutition in children with congenital Zika virus syndrome. Codas 2023, 35, e20210270. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotto, G.; Massa, S.; Spirito, F.; Fazio, V. Congenital Zika Virus Syndrome: Microcephaly and Orofacial Anomalies. Life 2024, 14, 55. https://doi.org/10.3390/life14010055
Scotto G, Massa S, Spirito F, Fazio V. Congenital Zika Virus Syndrome: Microcephaly and Orofacial Anomalies. Life. 2024; 14(1):55. https://doi.org/10.3390/life14010055
Chicago/Turabian StyleScotto, Gaetano, Salvatore Massa, Francesca Spirito, and Vincenzina Fazio. 2024. "Congenital Zika Virus Syndrome: Microcephaly and Orofacial Anomalies" Life 14, no. 1: 55. https://doi.org/10.3390/life14010055
APA StyleScotto, G., Massa, S., Spirito, F., & Fazio, V. (2024). Congenital Zika Virus Syndrome: Microcephaly and Orofacial Anomalies. Life, 14(1), 55. https://doi.org/10.3390/life14010055





