Migraine Comorbidities
Abstract
1. Introduction
2. Common Types and Red Flags
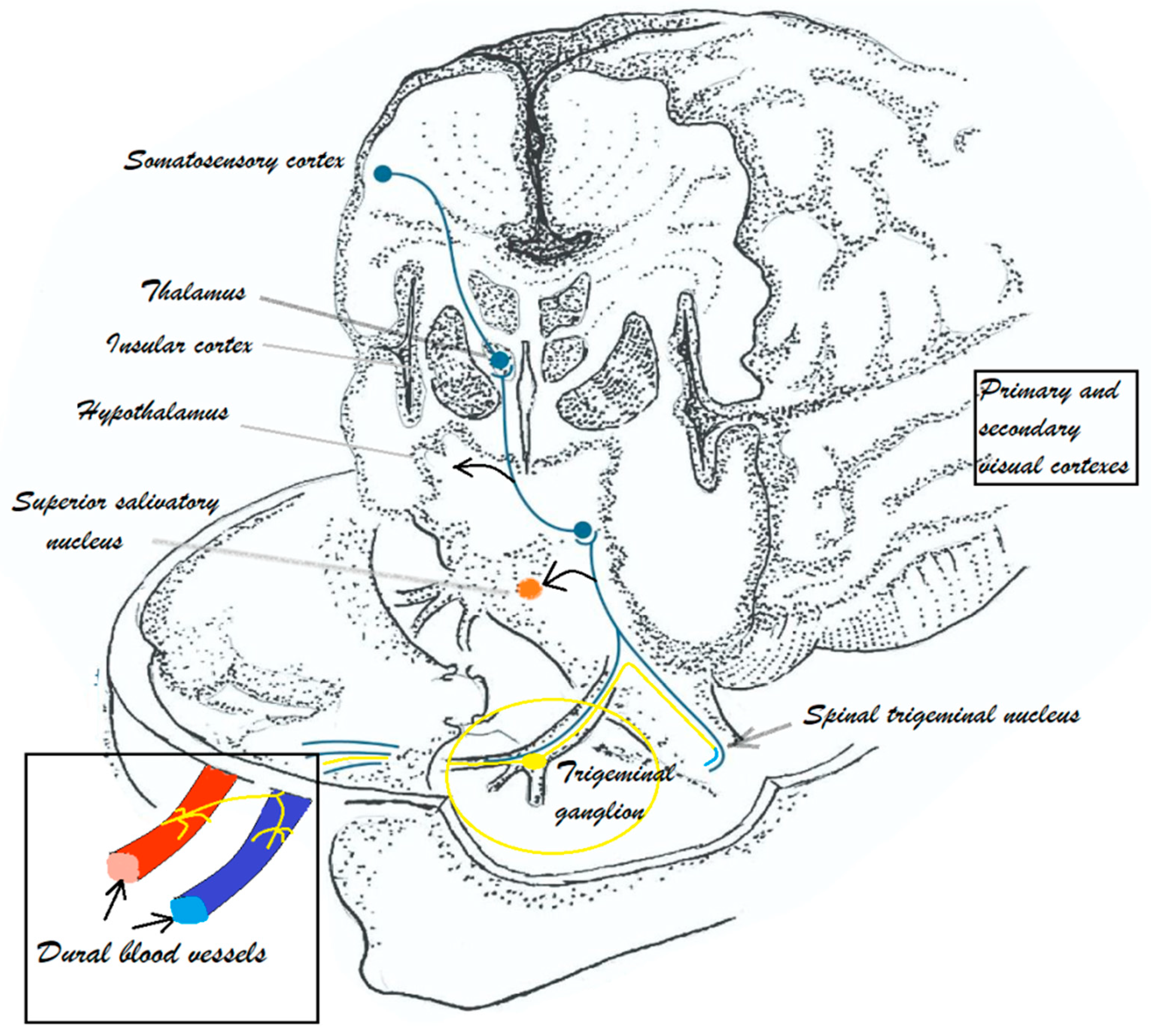
3. Migraine Pathophysiology
4. Epilepsy and Migraine
5. Depression, Anxiety and Migraine
6. Migraine, the Gut–Brain Axis and Irritable Bowel Syndrome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPA | a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ASD | anti-seizure drugs |
| BDNF | brain-derived neurotrophic factor |
| CGRP | calcitonin gene-related peptide |
| CSD | cortical spreading depression |
| FDA | Food and Drug Administration |
| FFAR3 | free fatty acid receptor 3 |
| FHM | familial hemiplegic migraine |
| FMT | fecal microbiota transplantation |
| GABA | gamma-amino-butyric acid |
| GAD | generalized anxiety disorder |
| HPA | hypothalamic-pituitary-adrenal axis |
| 5-HT | 5-hydroxytryptamine |
| IBS | irritable bowel syndrome |
| LPS | lipopolysaccharides |
| MTHFR | methylenetetrahydrofolate reductase |
| NTG | Nitroglycerine |
| NMDA | N-methyl-D-aspartate |
| OCD | obsessive compulsive disorder |
| PACAP | adenylate cyclase activating peptides |
| PD | panic disorder |
| SCFAs | short-chain fatty acids |
| SSN | superior salivatory nucleus |
| SSRIs | selective 5-HT reuptake inhibitors |
References
- Grech, O.; Mollan, S.P.; Wakerley, B.R.; Fulton, D.; Lavery, G.G.; Sinclair, A.J. The Role of Metabolism in Migraine Pathophysiology and Susceptibility. Life 2021, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Kazeminasab, S.; Nejadghaderi, S.A.; Mohammadinasab, R.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front. Neurol. 2022, 12, 800605. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: Epidemiology and systems of care. Lancet 2021, 397, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Lipton, R.B.; Ferrari, M.D. Migraine--current understanding and treatment. N. Engl. J. Med. 2002, 346, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Lipton, R.B.; Celentano, D.D.; Reed, M.L. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 1992, 267, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Stewart, W.F.; Kolodner, K.; Liberman, J.; Lipton, R.B. Epidemiology of migraine in England. Cephalalgia 1999, 19, 305–306. [Google Scholar]
- Scher, A.I.; Stewart, W.F.; Lipton, R.B. Migraine and headache: A meta-analytic approach. In The Epidemiology of Pain; Crombie, I.K., Ed.; IASP Press: Seattle, WA, USA, 1999; pp. 159–170. [Google Scholar]
- Lipton, R.B.; Bigal, M.E.; Kolodner, K.; Stewart, W.F.; Liberman, J.N.; Steiner, T.J. The family impact of migraine: Population-based studies in the USA and UK. Cephalalgia 2003, 23, 429–440. [Google Scholar] [CrossRef]
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd ed.; Sage Publications: New York, NY, USA, 2018; Volume 38, pp. 1–211. [Google Scholar] [CrossRef]
- Pescador Ruschel, M.A.; De Jesus, O. Migraine Headache. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Viana, M.; Tronvik, E.A.; Do, T.P.; Zecca, C.; Hougaard, A. Clinical features of visual migraine aura: A systematic review. J. Headache Pain 2019, 20, 64. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, B.K.; Lee, W.; Hwang, H.; Heo, K.; Chu, M.K. Prevalence and impact of visual aura in migraine and probable migraine: A population study. Sci. Rep. 2022, 12, 426. [Google Scholar] [CrossRef]
- Mitsikostas, D.D.; Ashina, M.; Craven, A.; Diener, H.C.; Goadsby, P.J.; Ferrari, M.D.; Lampl, C.; Paemeleire, K.; Pascual, J.; Siva, A.; et al. European Headache Federation consensus on technical investigation for primary headache disorders. J. Headache Pain 2015, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Sheftell, F.D.; Tepper, S.J.; Bigal, M.E. Migraine: Barriers for care. Neurol. Sci. 2005, 26, s140–s142. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Hansen, J.M.; Do, T.P.; MeloCarrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Arulmozhi, D.K.; Veeranjaneyulu, A.; Bodhankar, S.L. Migraine: Current concepts and emerging therapies. Vasc. Pharmacol. 2005, 43, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.R.; Wolf, H.G. Mechanism of migraine headache and action of ergotamine tartrate. Arch. Neurol. Psychiatry 1938, 39, 737–763. [Google Scholar] [CrossRef]
- Shevel, E. The Extracranial Vascular Theory of Migraine—A Great Story Confirmed by the Facts. Headache J. Head Face Pain 2011, 51, 409–417. [Google Scholar] [CrossRef]
- Olesen, J.; Larsen, B.; Lauritzen, M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann. Neurol. 1981, 9, 344–352. [Google Scholar] [CrossRef]
- May, A.; Goadsby, P.J. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J. Cereb. Blood Flow. Metab. 1999, 19, 115–127. [Google Scholar] [CrossRef]
- Moskowitz, M.A. The neurobiology of vascular head pain. Ann. Neurol. 1984, 16, 157–168. [Google Scholar] [CrossRef]
- Dodick, D.; Silberstein, S. Central sensitization theory of migraine: Clinical implications. Headache 2006, 46, S182–S191. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Hansen, J.M.; Á Dunga, B.O.; Olesen, J. Human models of migraine—Short-term pain for long-term gain. Nat. Rev. Neurol. 2017, 13, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y. Migraine Pathophysiology Revisited: Proposal of a New Molecular Theory of Migraine Pathophysiology and Headache Diagnostic Criteria. Int. J. Mol. Sci. 2022, 23, 13002. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies-successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L. Role of CGRP in Migraine. Handb. Exp. Pharmacol. 2019, 255, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Caprara, A.L.F. Gepants for Acute and Preventive Migraine Treatment: A Narrative Review. Brain Sci. 2022, 12, 1612. [Google Scholar] [CrossRef]
- Altamura, C.; Brunelli, N.; Marcosano, M.; Fofi, L.; Vernieri, F. Gepants—A long way to cure: A narrative review. Neurol. Sci. 2022, 43, 5697–5708. [Google Scholar] [CrossRef]
- Sevivas, H.; Fresco, P. Treatment of resistant chronic migraine with anti-CGRP monoclonal antibodies: A systematic review. Eur. J. Med. Res. 2022, 27, 86. [Google Scholar] [CrossRef]
- Rogawski, M.A. Migraine and Epilepsy—Shared Mechanisms within the Family of Episodic Disorders. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Lipton, R.B.; Ottman, R.; Ehrenberg, B.L.; Hauser, W.A. Comorbidity of migraine: The connection between migraine and epilepsy. Neurology 1994, 44 (Suppl. S7), S28–S32. [Google Scholar] [PubMed]
- Altamura, C.; Corbelli, I.; de Tommaso, M.; Di Lorenzo, C.; Di Lorenzo, G.; Di Renzo, A.; Filippi, M.; Jannini, T.B.; Messina, R.; Parisi, P.; et al. Pathophysiological Bases of Comorbidity in Migraine. Front. Hum. Neurosci. 2021, 15, 640574. [Google Scholar] [CrossRef] [PubMed]
- Sances, G.; Guaschino, E.; Perucca, P.; Allena, M.; Ghiotto, N.; Manni, R. Migralepsy: A call for a revision of the definition. Epilepsia 2009, 50, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, M.; Cestèle, S. Pathophysiological mechanisms of migraine and epilepsy: Similarities and differences. Neurosci. Lett. 2018, 667, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Manso-Calderón, R. Links between headaches and epilepsy: Current knowledge and terminology. Neurología 2014, 29, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Zarcone, D.; Corbetta, S. Shared mechanisms of epilepsy, migraine and affective disorders. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017, 38 (Suppl. S1), 73–76. [Google Scholar] [CrossRef] [PubMed]
- Barker-Haliski, M.; White, H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef]
- Hoffmann, J.; Charles, A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurother. J. Am. Soc. Exp. Neurother. 2018, 15, 361–370. [Google Scholar] [CrossRef]
- Castro, M.J.; Stam, A.H.; Lemos, C. First mutation in the voltage-gated NaV1.1 subunit gene SCN1A with cooccurring familial hemiplegic migraine and epilepsy. Cephalalgia 2009, 29, 308–313. [Google Scholar] [CrossRef]
- Liao, J.; Tian, X.; Wang, H.; Xiao, Z. Epilepsy and migraine-Are they comorbidity? Genes. Dis. 2018, 5, 112–118. [Google Scholar] [CrossRef]
- Riant, F.; De Fusco, M.; Aridon, P. ATP1A2 mutations in 11 families with familial hemiplegic migraine. Hum. Mutat. 2005, 26, 281. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Goadsby, P.J. Topiramate inhibits trigeminovascular activation: An intravital microscopy study. Br. J. Pharmacol. 2005, 146, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Demarquay, G.; Rheims, S. Relationships between migraine and epilepsy: Pathophysiological mechanisms and clinical implications. Rev. Neurol. 2021, 177, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Romero-Reyes, M. Targeting the central projection of the dural trigeminovascular system for migraine prophylaxis. J. Cereb. Blood Flow Metab. 2019, 39, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Cutrer, F.; Limmroth, V.; Moskowitz, M. Possible Mechanisms of Valproate in Migraine Prophylaxis. Cephalalgia 1997, 17, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.H.; Kuan, Y.C.; Tam, K.W.; Chung, C.C.; Hong, C.T.; Huang, Y.H. Efficacy of levetiracetam for migraine prophylaxis: A systematic review and meta-analysis. J. Formos. Med. Assoc. Taiwan. Yi Zhi 2021, 120 Pt 3, 755–764. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Gong, T. High-field MRS study of GABA+ in patients with migraine: Response to levetiracetam treatment. Neuroreport 2018, 29, 1007–1010. [Google Scholar] [CrossRef]
- Gotra, P.; Bhardwaj, N.; Ludhiadch, A.; Singh, G.; Munshi, A. Epilepsy and Migraine Shared Genetic and Molecular Mechanisms: Focus on Therapeutic Strategies. Mol. Neurobiol. 2021, 58, 3874–3883. [Google Scholar] [CrossRef]
- HELP (Headache in Epileptic Patients) Study Group. Multi-Center Study on Migraine and Seizure-Related Headache in Patients with Epilepsy. Yonsei Med. J. 2010, 51, 219–224. [Google Scholar] [CrossRef]
- Louter, M.A.; Pijpers, J.A.; Wardenaar, K.J.; van Zwet, E.W.; van Hemert, A.M.; Zitman, F.G.; Ferrari, M.D.; Penninx, B.W.; Terwindt, G.M. Symptom dimensions of affective disorders in migraine patients. J. Psychosom. Res. 2015, 79, 458–463. [Google Scholar] [CrossRef]
- Jahangir, S.; Adjepong, D.; Al-Shami, H.A.; Malik, B.H. Is There an Association Between Migraine and Major Depressive Disorder? A Narrative Review. Cureus 2020, 12, e8551. [Google Scholar] [CrossRef] [PubMed]
- Baskin, S.M.; Lipchik, G.L.; Smitherman, T.A. Mood and anxiety disorders in chronic headache. Headache 2006, 46 (Suppl. S3), S76–S87. [Google Scholar] [CrossRef] [PubMed]
- Vuralli, D.; Wattiez, A.S.; Russo, A.F.; Bolay, H. Behavioral and cognitive animal models in headache research. J. Headache Pain 2019, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, P.; Flint, A. Diagnosis and Management of Anxiety Disorders. Contin. Lifelong Learn. Neurol. 2018, 24, 893–919. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, V.B.; Bogdanova, O.V.; Koulchitsky, S.V.; Chauvel, V.; Multon, S.; Makarchuk, M.Y.; Brennan, K.C.; Renshaw, P.F.; Schoenen, J. Behavior in the open field predicts the number of KCl-induced cortical spreading depressions in rats. Behav. Brain Res. 2013, 236, 90–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, R.; Asif, S.; Bali, A.; Dang, A.K.; Gonzalez, D.A. The Development and Impact of Anxiety With Migraines: A Narrative Review. Cureus 2022, 14, e26419. [Google Scholar] [CrossRef]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Winter, G.; Hart, R.A.; Charlesworth, R.P.G.; Sharpley, C.F. Gut microbiome and depression: What we know and what we need to know. Rev. Neurosci. 2018, 29, 629–643. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Liu, W.H.; Chuang, H.L.; Huang, Y.T.; Wu, C.C.; Chou, G.T.; Wang, S.; Tsai, Y.C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef]
- Horii, Y.; Nakakita, Y.; Fujisaki, Y.; Yamamoto, S.; Itoh, N.; Miyazaki, K.; Kaneda, H.; Oishi, K.; Shigyo, T.; Nagai, K. Effects of intraduodenal injection of Lactobacillus brevis SBC8803 on autonomic neurotransmission and appetite in rodents. Neurosci. Lett. 2013, 539, 32–37. [Google Scholar] [CrossRef]
- Lantéri-Minet, M.; Radat, F.; Chautard, M.H.; Lucas, C. Anxiety and depression associated with migraine: Influence on migraine subjects’ disability and quality of life, and acute migraine management. Pain 2005, 118, 319–326. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, A.; Jiang, Z.; Tsai, H.; Liu, W. The exploration of mechanisms of comorbidity between migraine and depression. J. Cell. Mol. Med. 2019, 23, 4505–4513. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Saxena, P.R. On serotonin and migraine: A clinical and pharmacological review. Cephalalgia Int. J. Headache 1993, 13, 151–165. [Google Scholar] [CrossRef]
- Peterlin, B.L.; Katsnelson, M.J.; Calhoun, A.H. The associations between migraine, unipolar psychiatric comorbidities, and stress-related disorders and the role of estrogen. Curr. Pain Headache Rep. 2009, 13, 404–412. [Google Scholar] [CrossRef]
- Warnock, J.K.; Cohen, L.J.; Blumenthal, H.; Hammond, J.E. Hormone-Related Migraine Headaches and Mood Disorders: Treatment with Estrogen Stabilization. Pharmacotherapy 2017, 37, 120–128. [Google Scholar] [CrossRef]
- Shors, T.J.; Leuner, B. Estrogen-mediated effects on depression and memory formation in females. J. Affect. Disord. 2003, 74, 85–96. [Google Scholar] [CrossRef]
- de Vries Lentsch, S.; van der Arend, B.W.H.; de Boer, I.; van Zwet, E.W.; MaassenVanDenBrink, A.; Terwindt, G.M. Depression and treatment with anti-calcitonin gene related peptide (CGRP) (ligand or receptor) antibodies for migraine. Eur. J. Neurol. 2023, 00, 1–9. [Google Scholar] [CrossRef]
- Hashikawa-Hobara, N.; Ogawa, T.; Sakamoto, Y.; Matsuo, Y.; Ogawa, M.; Zamami, Y.; Hashikawa, N. Calcitonin gene-related peptide pre-administration acts as a novel antidepressant in stressed mice. Sci. Rep. 2015, 5, 12559. [Google Scholar] [CrossRef]
- Mathé, A.A.; Agren, H.; Lindström, L.; Theodorsson, E. Increased concentration of calcitonin gene-related peptide in cerebrospinal fluid of depressed patients. A possible trait marker of major depressive disorder. Neurosci. Lett. 1994, 182, 138–142. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Larrosa, D.; Ramón, C.; Vega, J.; Martínez-Camblor, P.; Pascual, J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013, 81, 1191–1196. [Google Scholar] [CrossRef]
- Yang, Y.; Ligthart, L.; Terwindt, G.M.; Boomsma, D.I.; Rodriguez-Acevedo, A.J.; Nyholt, D.R. Genetic epidemiology of migraine and depression. Cephalalgia Int. J. Headache 2016, 36, 679–691. [Google Scholar] [CrossRef]
- Gardner, A.; Boles, R.G. Beyond the serotonin hypothesis: Mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 730–743. [Google Scholar] [CrossRef]
- Stuart, S.; Griffiths, L.R. A possible role for mitochondrial dysfunction in migraine. Mol. Genet. Genom. MGG 2012, 287, 837–844. [Google Scholar] [CrossRef]
- Celano, C.M.; Freudenreich, O.; Fernandez-Robles, C.; Stern, T.A.; Caro, M.A.; Huffman, J.C. Depressogenic effects of medications: A review. Dialogues Clin. Neurosci. 2011, 13, 103–125. [Google Scholar] [CrossRef]
- Silvestro, M.; Tessitore, A.; Scotto di Clemente, F.; Battista, G.; Tedeschi, G.; Russo, A. Refractory migraine profile in CGRP-monoclonal antibodies scenario. Acta Neurol. Scand. 2021, 144, 325–333. [Google Scholar] [CrossRef]
- Raffaelli, B.; Fitzek, M.; Overeem, L.H.; Storch, E.; Terhart, M.; Reuter, U. Clinical evaluation of super-responders vs. non-responders to CGRP(-receptor) monoclonal antibodies: A real-world experience. J. Headache Pain 2023, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Cho, S.; Kim, B.K. Predictors of response to galcanezumab in patients with chronic migraine: A real-world prospective observational study. Neurol. Sci. 2023, 44, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Kappéter, Á.; Sipos, D.; Varga, A.; Vigvári, S.; Halda-Kiss, B.; Péterfi, Z. Migraine as a Disease Associated with Dysbiosis and Possible Therapy with Fecal Microbiota Transplantation. Microorganisms 2023, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Liu, S.; Tao, F. Gut microbiota and migraine. Neurobiol. Pain 2022, 11, 10090. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Filippone, A.; Ardizzone, A.; Casili, G.; Paterniti, I.; Esposito, E.; Campolo, M. SCFA treatment alleviates pathological signs of migraine and related intestinal alterations in a mouse model of NTG-induced migraine. Cells 2021, 10, 2756. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Tang, W.; Zhang, Y.; Zhang, M.; Liu, J.; Li, Y.; Kong, S.; Zhao, D.; Yu, S. The gut microbiome modulates nitroglycerin-induced migraine-related hyperalgesia in mice. Cephalalgia 2022, 42, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Ustianowska, K.; Ustianowski, Ł.; Machaj, F.; Gorący, A.; Rosik, J.; Szostak, B.; Szostak, J.; Pawlik, A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int. J. Mol. Sci. 2022, 23, 13267. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, S.; Shu, H.; Yanagisawa, L.; Tao, F. Gut Microbiota Dysbiosis Enhances Migraine-Like Pain Via TNFα Upregulation. Mol. Neurobiol. 2020, 57, 461–468. [Google Scholar] [CrossRef]
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577. [Google Scholar] [CrossRef]
- Cook, T.M.; Gavini, C.K.; Jesse, J.; Aubert, G.; Gornick, E.; Bonomo, R.; Gautron, L.; Layden, B.T.; Mansuy-Aubert, V. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Mol. Metab. 2021, 54, 101350. [Google Scholar] [CrossRef]
- Pujo, J.; De Palma, G.; Lu, J.; Galipeau, H.J.; Surette, M.G.; Collins, S.M.; Bercik, P. Gut microbiota modulates visceral sensitivity through calcitonin gene-related peptide (CGRP) production. Gut Microbes 2023, 15, 2188874. [Google Scholar] [CrossRef]
- Hindiyeh, N.A.; Zhang, N.; Farrar, M.; Banerjee, P.; Lombard, L.; Aurora, S.K. The Role of Diet and Nutrition in Migraine Triggers and Treatment: A Systematic Literature Review. Headache 2020, 60, 1300–1316. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Chojnacki, J.; Sobczuk, P.; Chojnacki, C.; Blasiak, J. Nutrition and Calcitonin Gene Related Peptide (CGRP) in Migraine. Nutrients 2023, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.Y.; Lu, C.L. Irritable bowel syndrome and migraine: Bystanders or partners? J. Neurogastroenterol. Motil. 2013, 19, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Bin Abdulrahman, K.A.; Alenazi, N.S.; Albishri, S.B.; Alshehri, F.F. Association of Migraine and Irritable Bowel Syndrome in Saudi Arabia: A Nationwide Survey. BioMed Res. Int. 2022, 2022, 8690562. [Google Scholar] [CrossRef]
- Pytliak, M.; Vargová, V.; Mechírová, V.; Felšöci, M. Serotonin receptors-from molecular biology to clinical applications. Physiol. Res. 2011, 60, 15–25. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, G.; Xu, Y.; He, B.; Wang, Y.; Ma, R.; Chang, Y.; He, D.; Xu, C.; Xiao, Z. Effects of Diet Based on IgG Elimination Combined with Probiotics on Migraine Plus Irritable Bowel Syndrome. Pain Res. Manag. 2019, 2019, 7890461. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuciureanu, D.I.; Bistriceanu, C.E.; Vulpoi, G.-A.; Cuciureanu, T.; Antochi, F.; Roceanu, A.-M. Migraine Comorbidities. Life 2024, 14, 74. https://doi.org/10.3390/life14010074
Cuciureanu DI, Bistriceanu CE, Vulpoi G-A, Cuciureanu T, Antochi F, Roceanu A-M. Migraine Comorbidities. Life. 2024; 14(1):74. https://doi.org/10.3390/life14010074
Chicago/Turabian StyleCuciureanu, Dan Iulian, Cătălina Elena Bistriceanu, Georgiana-Anca Vulpoi, Tudor Cuciureanu, Florina Antochi, and Adina-Maria Roceanu. 2024. "Migraine Comorbidities" Life 14, no. 1: 74. https://doi.org/10.3390/life14010074
APA StyleCuciureanu, D. I., Bistriceanu, C. E., Vulpoi, G.-A., Cuciureanu, T., Antochi, F., & Roceanu, A.-M. (2024). Migraine Comorbidities. Life, 14(1), 74. https://doi.org/10.3390/life14010074






