Comparing Methodologies for Stomatal Analyses in the Context of Elevated Modern CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Sampling and Preparation
2.2. Microscopy
2.3. C Isotope Measurements
2.4. Gas Exchange Model
3. Results
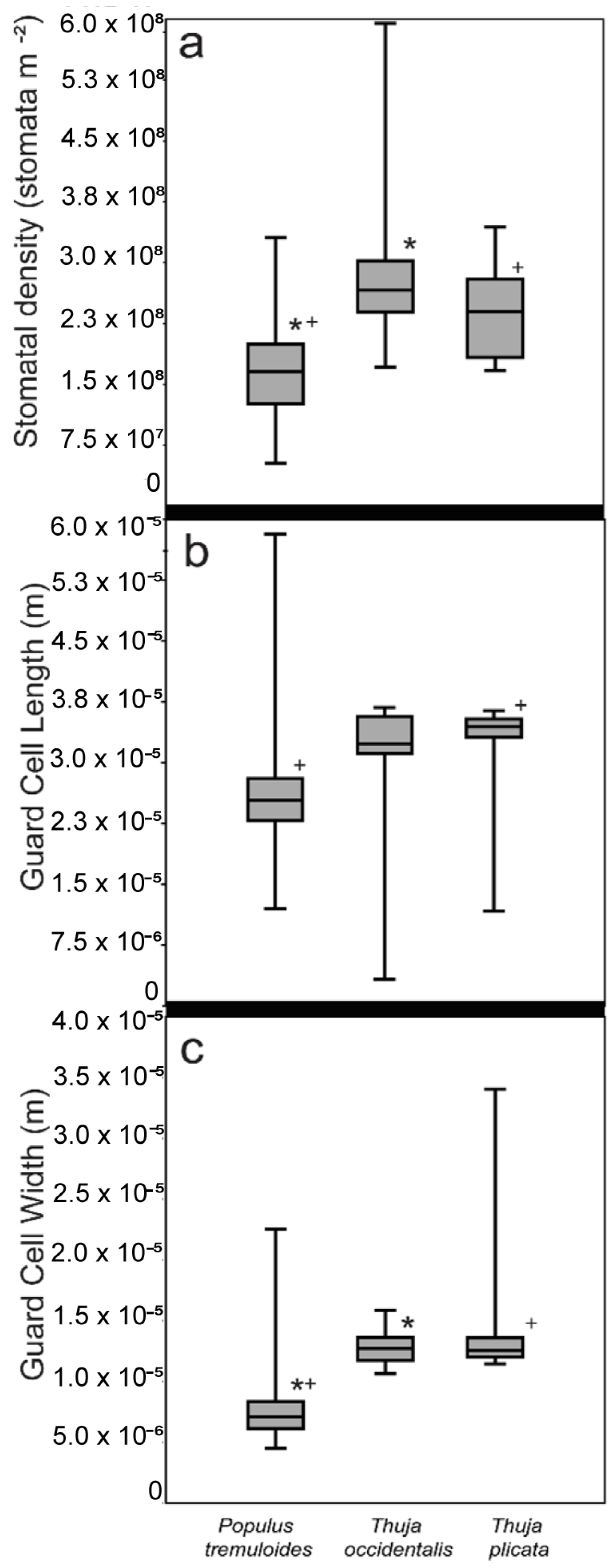
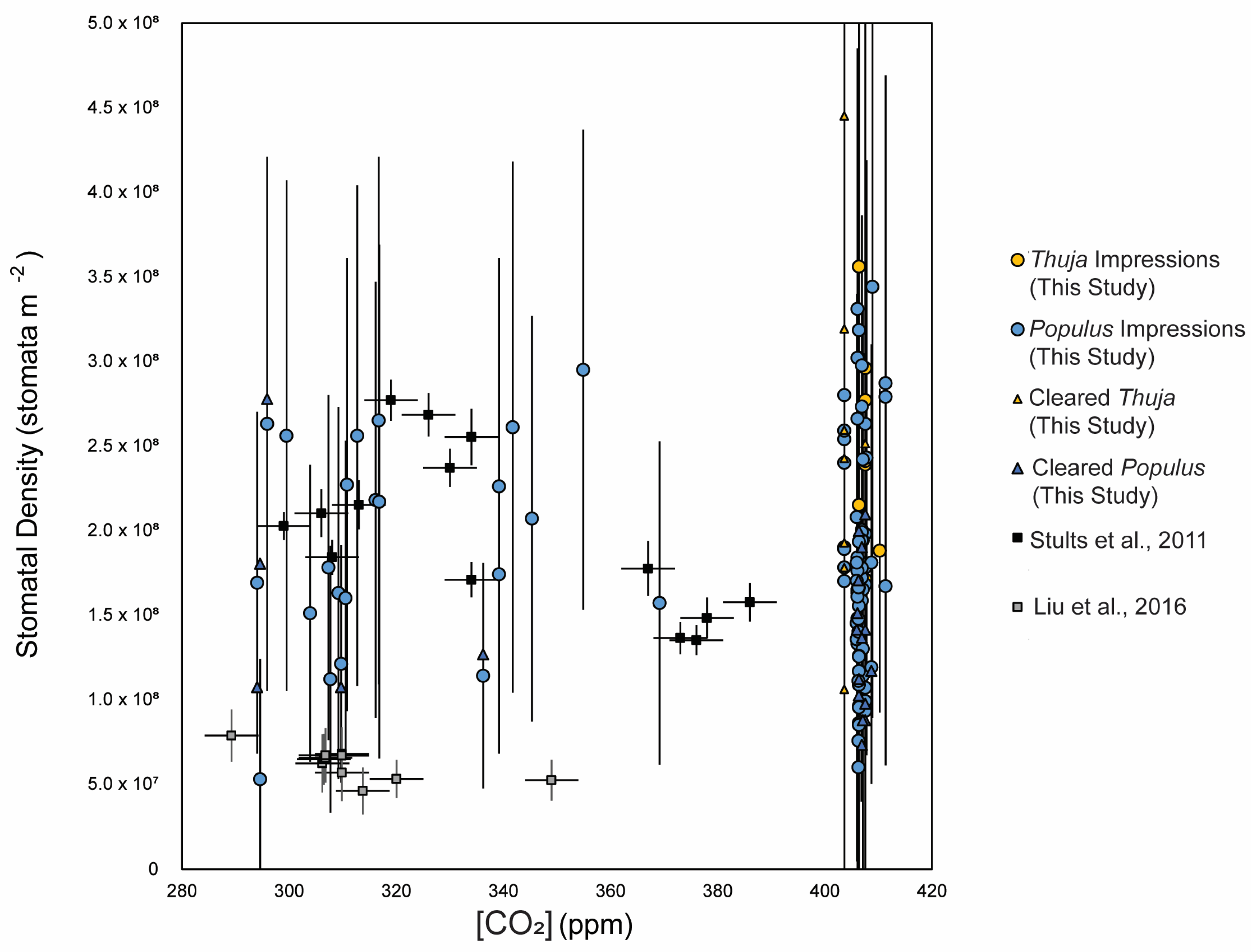
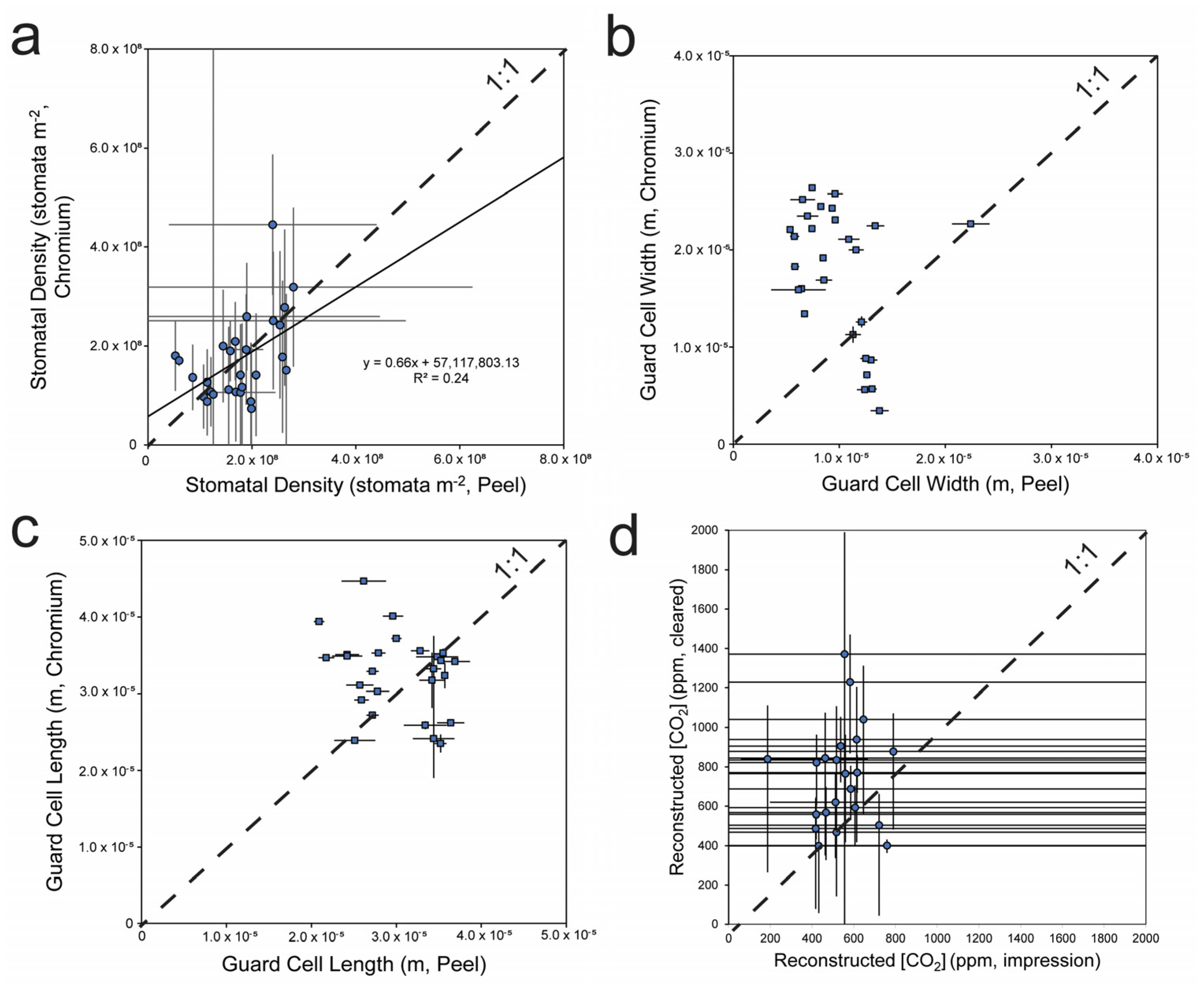
3.1. Method Comparisons
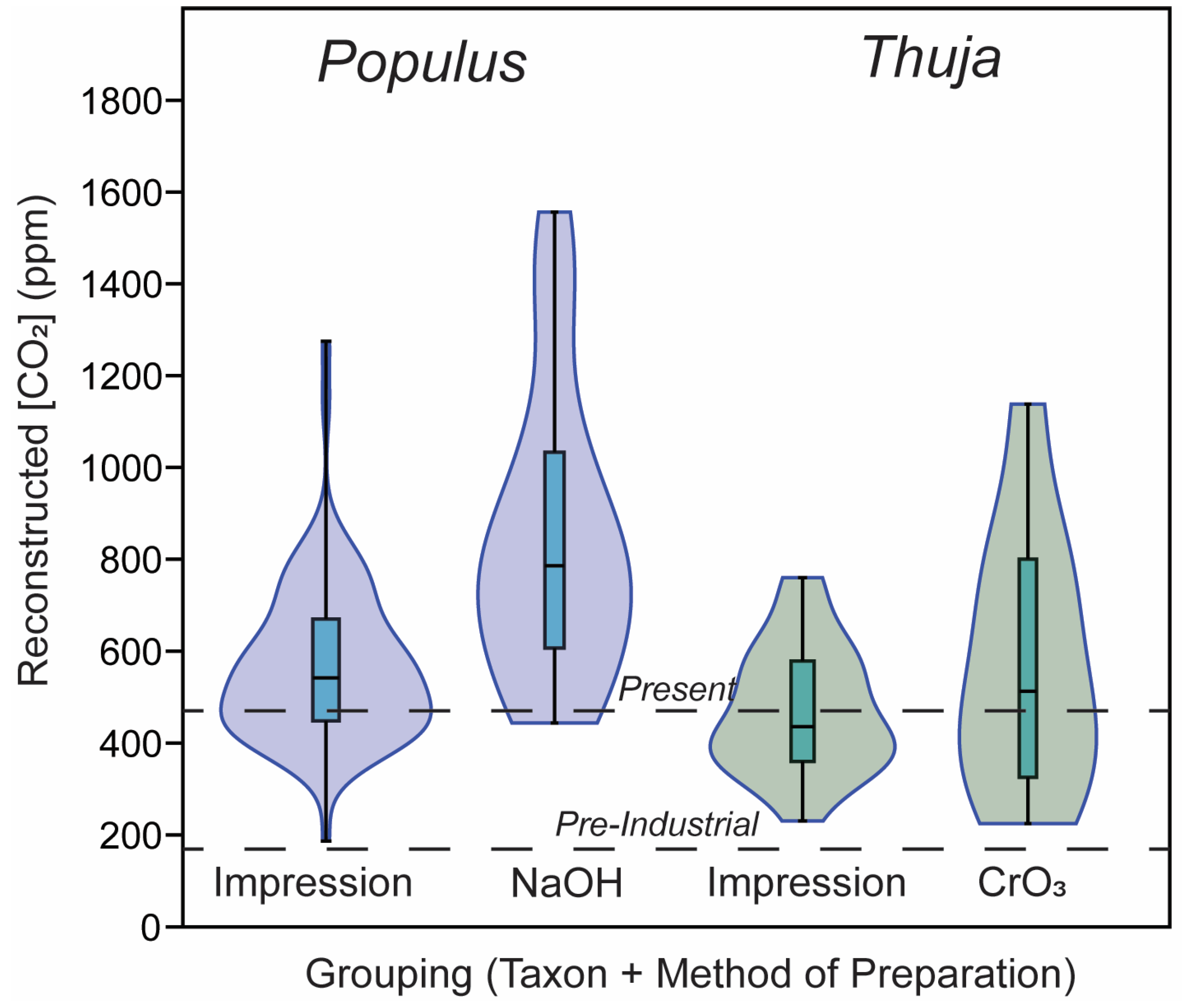
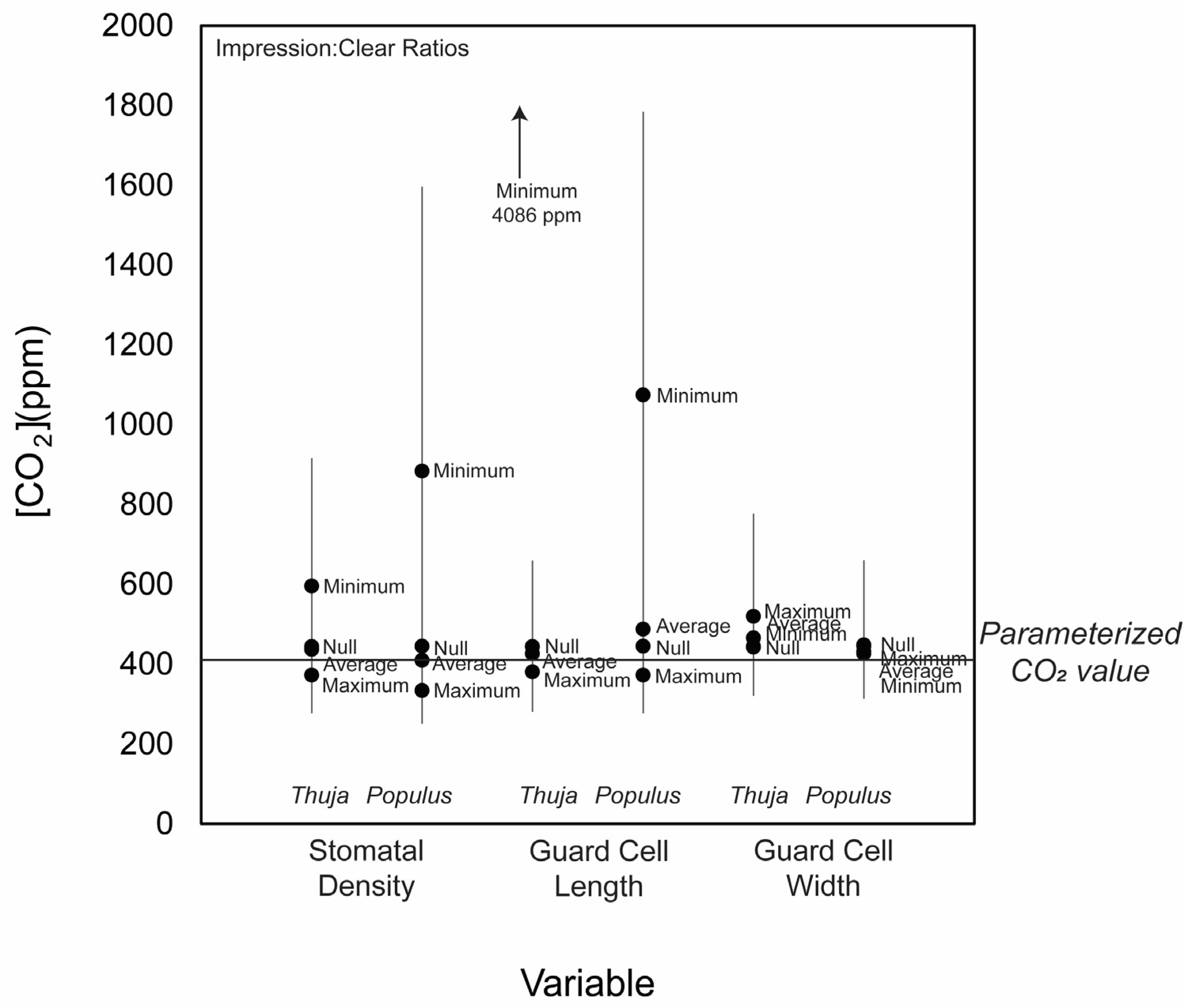
3.2. CO2 Reconstructions Using Both Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beerling, D.J.; McElwain, J.C.; Osborne, C.P. Stomatal responses of the ‘living fossil’ Ginkgo biloba L. to changes in atmospheric CO2 concentrations. J. Exp. Bot. 1998, 49, 1603–1607. [Google Scholar] [CrossRef]
- Beerling, D.J.; Royer, D.L. Reading a CO2 signal from fossil stomata. New Phytol. 2002, 153, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Steinthorsdottir, M.; Jeram, A.J.; McElwain, J.C. Extremely elevated CO2 concentrations at the Triassic/Jurassic boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 308, 418–432. [Google Scholar] [CrossRef]
- Franks, P.J.; Royer, D.L. Comment on “Was atmospheric CO2 capped at 1000 ppm over the past 300 million years?” by McElwain JC et al. [Palaeogeogr. Palaeoclimatol. Palaeoecol. 441 (2016) 653–658]. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 472, 256–259. [Google Scholar] [CrossRef]
- Bange, G.G.J. On the quantitative explanation of stomatal transpiration. Acta Bot. Neerl. 1953, 2, 255–297. [Google Scholar] [CrossRef]
- Jarvis, P.G.; McNaughton, K.G. Stomatal control of transpiration: Scaling up from leaf to region. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 1986; Volume 15, pp. 1–49. [Google Scholar]
- Jones, H.G. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Woodward, F.I. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 1987, 327, 617–618. [Google Scholar] [CrossRef]
- Lin, J.; Jach, M.E.; Ceulemans, R. Stomatal density and needle anatomy of Scots pine (Pinus sylvestris) are affected by elevated CO2. New Phytol. 2001, 150, 665–674. [Google Scholar] [CrossRef]
- Teng, N.; Jin, B.; Wang, Q.; Hao, H.; Ceulemans, R.; Kuang, T.; Lin, J. No detectable maternal effects of elevated CO2 on Arabidopsis over 15 generations. PLoS ONE 2009, 4, e6035. [Google Scholar]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 response of stomata and its dependence on Environmental Factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Yu, J.; Liu, F. Effect of the transgenerational exposure to elevated CO2 on the drought response of winter wheat: Stomatal control and water use efficiency. Environ. Exp. Bot. 2017, 136, 78–84. [Google Scholar] [CrossRef]
- Franks, P.J.; Royer, D.L.; Beerling, D.J.; Van de Water, P.K.; Cantrill, D.J.; Barbour, M.M.; Berry, J.A. New constraints on atmospheric CO2 concentration for the Phanerozoic. Geophys. Res. Lett. 2014, 41, 4685–4694. [Google Scholar] [CrossRef]
- Grein, M.; Konrad, W.; Wilde, V.; Utescher, T.; Roth-Nebelsick, A. Reconstruction of atmospheric CO2 during the early middle Eocene by application of a gas exchange model to fossil plants from the Messel Formation, Germany. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 309, 383–391. [Google Scholar] [CrossRef]
- Woodward, F.I.; Bazzaz, F.A. The responses of stomatal density to CO2 partial pressure. J. Exp. Bot. 1988, 39, 1771–1781. [Google Scholar] [CrossRef]
- Van de Water, P.K.; Leavitt, S.W.; Betancourt, J.L. Trends in stomatal density and 13C/12C ratios of Pinus flexilis needles during last glacial-interglacial cycle. Science 1994, 264, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Liancourt, P.; Spence, L.A.; Song, D.S.; Lkhagva, A.; Sharkhuu, A.; Boldgiv, B.; Helliker, B.R.; Petraitis, P.S.; Casper, B.B. Plant response to climate change varies with topography, interactions with neighbors, and ecotype. Ecology 2013, 94, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo) ecology and (paleo) climate. Proc. Natl. Acad. Sci. USA 2010, 107, 19691–19695. [Google Scholar] [CrossRef]
- Sheldon, N.D.; Smith, S.Y.; Stein, R.; Ng, M. Carbon isotope ecology of gymnosperms and implications for paleoclimatic and paleoecological studies. Glob. Planet. Chang. 2020, 184, 103060. [Google Scholar] [CrossRef]
- Stein, R.A.; Sheldon, N.D.; Smith, S. Rapid response to anthropogenic climate change by Thuja occidentalis: Implications for past climate reconstructions and future climate predictions. PeerJ 2019, 7, e7378. [Google Scholar] [CrossRef]
- Stein, R.A.; Sheldon, N.D.; Smith, S.Y. C3 plant carbon isotope discrimination does not respond to CO2 concentration on decadal to centennial timescales. New Phytol. 2021, 229, 2576–2585. [Google Scholar] [CrossRef]
- Field, C.B. Plant physiology of the “missing” carbon sink. Plant Physiol. 2001, 125, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Scurlock, J.M.O.; Hall, D.O. The global carbon sink: A grassland perspective. Glob. Chang. Biol. 1998, 4, 229–233. [Google Scholar] [CrossRef]
- Phillips, O.L.; Lewis, S.L. Evaluating the tropical forest carbon sink. Glob. Chang. Biol. 2014, 20, 2039–2041. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A.; Idso, S.B. Increasing atmospheric CO2: Effects on crop yield, water use and climate. Agric. Water Manag. 1983, 7, 55–72. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Hillel, D. Climate Change and the Global Harvest: Potential Impacts of the Greenhouse Effect on Agriculture; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Tubiello, F.N.; Donatelli, M.; Rosenzweig, C.; Stockle, C.O. Effects of climate change and elevated CO2 on cropping systems: Model predictions at two Italian locations. Eur. J. Agron. 2000, 13, 179–189. [Google Scholar] [CrossRef]
- Machado, R.; Loram-Lourenço, L.; Farnese, F.S.; Alves, R.D.F.B.; de Sousa, L.F.; Silva, F.G.; Filho, S.C.V.; Torres-Ruiz, J.M.; Cochard, H.; Menezes-Silva, P.E. Where do leaf water leaks come from? Trade-offs underlying the variability in minimum conductance across tropical savanna species with contrasting growth strategies. New Phytol. 2021, 229, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Jordan, G.J.; Carpenter, R.J.; Koutoulis, A.; Price, A.; Brodribb, T.J. Environmental adaptation in stomatal size independent of the effects of genome size. New Phytol. 2015, 205, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Leitch, I.J.; Patel, S.; Pendharkar, A.; Knight, C.A. Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol. 2008, 179, 975–986. [Google Scholar] [CrossRef]
- Zenil-Ferguson, R.; Ponciano, J.M.; Burleigh, J.G. Evaluating the role of genome downsizing and size thresholds from genome size distributions in angiosperms. Am. J. Bot. 2016, 103, 1175–1186. [Google Scholar] [CrossRef]
- Bhadra, S.; Leitch, I.J.; Onstein, R.E. From genome size to trait evolution during angiosperm radiation. Trends Genet. 2023, 39, 728–735. [Google Scholar] [CrossRef]
- Lomax, B.H.; Woodward, F.I.; Leitch, I.J.; Knight, C.A.; Lake, J.A. Genome size as a predictor of guard cell length in Arabidopsis thaliana is independent of environmental conditions. New Phytol. 2009, 181, 311–314. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Nejad, A.R.; van Meeteren, U. Stomatal response characteristics of Tradescantia virginiana grown at high relative air humidity. Physiol. Plant. 2005, 125, 324–332. [Google Scholar] [CrossRef]
- Pearce, D.W.; Millard, S.; Bray, D.F.; Rood, S.B. Stomatal characteristics of riparian poplar species in a semi-arid environment. Tree Physiol. 2006, 26, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.A.B.; Marenco, R.A. Density, size and distribution of stomata in 35 rainforest tree species in Central Amazonia. Acta Amaz. 2011, 41, 205–212. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Materassi, A.; Raschi, A.; Scutt, C.P.; Centritto, M. The functional significance of the stomatal size to density relationship: Interaction with atmospheric [CO2] and role in plant physiological behaviour. Sci. Total Environ. 2023, 863, 160908. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N. Leaf clearing protocol to observe stomata and other cells on leaf surface. Bio-protocol 2017, 7, e2538. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, B. Using clear nail polish to make Arabidopsis epidermal impressions for measuring the change of stomatal aperture size in immune response. In Plant Pattern Recognition Receptors; Humana Press: New York, NY, USA, 2017; pp. 243–248. [Google Scholar]
- Brown, R.; Rickless, P. A new method for the study of cell division and cell extension with some preliminary observations on the effect of temperature and of nutrients. Proc. R. Soc. Lond. B 1949, 136, 110–115. [Google Scholar] [PubMed]
- Dean, C.; Leech, R.M. Genome expression during normal leaf development. 1. Cellular and chloroplast numbers and DNA, RNA and protein levels in tissues of different ages within a seven-day old wheat leaf. Plant Physiol. 1982, 69, 904–910. [Google Scholar] [CrossRef]
- Ghouse, A.K.M.; Farooqui, F.; Sabir, D. Use of sodium hydroxide in the preparation of epidermal peels from certain coniferous leaves rich in resins and tannins. Stain Technol. 1974, 49, 125–128. [Google Scholar] [CrossRef]
- Smith, R.Y.; Greenwood, D.R.; Basinger, J.F. Estimating paleoatmospheric pCO2 during the early Eocene climatic optimum from stomatal frequency of ginkgo, Okanagan highlands, British Columbia, Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 293, 120–131. [Google Scholar] [CrossRef]
- Stockey, R.A.; Taylor, T.N. Cuticular features and epidermal patterns in the genus Araucaria de Jussieu. Bot. Gaz. 1978, 139, 490–498. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with Image J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43 (Suppl. S1), S25–S30. [Google Scholar] [CrossRef]
- Poole, I. Stomatal density and index: The practice. In Fossil Plants and Spores: Modern Techniques; The Geological Society: London, UK, 1999; pp. 257–260. [Google Scholar]
- White, J.W.C.; Vaughn, B.H.; Michel, S.E. Stable Isotopic Composition of Atmospheric Carbon Dioxide (13C and 18O) from the NOAA ESRL Carbon Cycle Cooperative Global Air Sampling Network, 1990–2014; Version 2015-10-26; University of Colorado, Institute of Arctic and Alpine Research (INSTAAR): Boulder, CO, USA, 2015. [Google Scholar]
- PRISM Climate Group. Oregon State University. 2004. Available online: http://prism.oregonstate.edu (accessed on 23 March 2021).
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Keeling, C.D.; Piper, S.C.; Bacastow, R.B.; Wahlen, M.; Whorf, T.P.; Heimann, M.; Meijer, H.A. Atmospheric CO2 and 13CO2 exchange with the terrestrial biosphere and oceans from 1978 to 2000: Observations and carbon cycle implications. In A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems; Springer: New York, NY, USA, 2005; pp. 83–113. [Google Scholar]
- Etheridge, D.M.; Steele, L.P.; Langenfelds, R.L.; Francey, R.J.; Barnola, J.M.; Morgan, V.I. Historical CO2 records from the Law Dome DE08, DE08-2, and DSS ice cores. In Trends: A Compendium of Data on Global Change; US Department of Energy: Washington, DC, USA, 1998; pp. 351–364. [Google Scholar]
- Stults, D.Z.; Wagner-Cremer, F.; Axsmith, B.J. Atmospheric paleo-CO2 estimates based on Taxodium distichum (Cupressaceae) fossils from the Miocene and Pliocene of Eastern North America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 309, 327–332. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, Q.; Han ME, N.G.; Jin, J.H. Estimates of late middle Eocene pCO2 based on stomatal density of modern and fossil Nageia leaves. Clim. Past 2016, 12, 241–253. [Google Scholar] [CrossRef]
- Rae, J.W.; Zhang, Y.G.; Liu, X.; Foster, G.L.; Stoll, H.M.; Whiteford, R.D. Atmospheric CO2 over the past 66 million years from marine archives. Annu. Rev. Earth Planet. Sci. 2021, 49, 609–641. [Google Scholar] [CrossRef]
- Lamont-Doherty Earth Observatory of Columbia University. 2023. Available online: www.paleo-co2.org (accessed on 10 November 2023).
- Upchurch, G.R. Dispersed angiosperm cuticles. In Proceedings of the Meeting of the Paleobotanical Section of the Botanical Society of America, Toronto, ON, Canada, 6 August 1989; pp. 65–92. [Google Scholar]
- Alvin, K.L.; Boulter, M.C. A controlled method of comparative study for taxodiaceous leaf cuticles. Bot. J. Linn. Soc. 1974, 69, 277–286. [Google Scholar] [CrossRef]
- Jellings, A.J.; Leech, R.M. The importance of quantitative anatomy in the interpretation of whole leaf biochemistry in species of Triticum, Hordeum and Avena. New Phytol. 1982, 92, 39–48. [Google Scholar] [CrossRef]
- Stein, R.A.; Sheldon, N.D.; Smith, S.Y. Supplemental data associated with this work. Available online: https://data.mendeley.com/datasets/gs6rn9tjxn/1 (accessed on 13 November 2023).
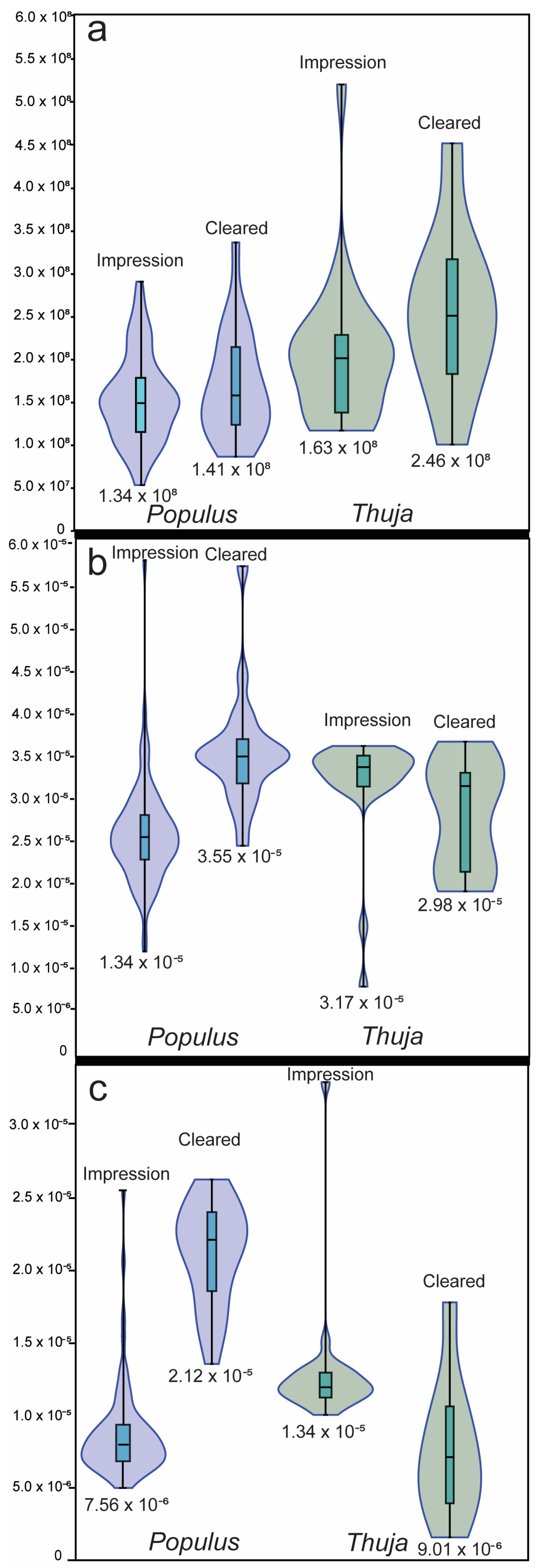

| n | Average Stomata Density (Stomata m−2) | Standard Deviation Stomata Density (Stomata m−2) | Average Guard Cell Length (m) | Guard Cell Length Standard Deviation (m) | Average Guard Cell Width (m) | Guard Cell Width Standard Deviation (m) | |
|---|---|---|---|---|---|---|---|
| Populus tremuloides impressions | 82 | 1.71 × 108 | 5.95 × 107 | 2.58 × 10−5 | 6.07 × 10−6 | 7.56 × 10−6 | 2.78 × 10−6 |
| Thuja impressions | 24 | 2.60 × 108 | 8.92 × 107 | 3.17 × 10−5 | 7.77 × 10−6 | 1.34 × 10−5 | 4.50 × 10−6 |
| All impressions | 106 | 1.91 × 108 | 7.68 × 107 | 2.71 × 10−5 | 6.91 × 10−6 | 8.92 × 10−6 | 4.05 × 10−6 |
| CrO3-cleared Thuja | 10 | 2.46 × 108 | 9.67 × 107 | 2.98 × 10−5 | 4.35 × 10−6 | 9.01 × 10−6 | 4.36 × 10−6 |
| NaOH-cleared Populus | 20 | 1.41 × 108 | 5.10 × 107 | 3.55 × 10−5 | 7.04 × 10−6 | 2.12 × 10−5 | 3.61 × 10−6 |
| All cleared leaves | 30 | 1.76 × 108 | 8.46 × 107 | 3.36 × 10−5 | 6.76 × 10−6 | 1.72 × 10−5 | 6.98 × 10−6 |
| Stomatal Density | Populus tremuloides | Thuja occidentalis |
|---|---|---|
| Populus tremuloides | ||
| Thuja occidentalis | <0.01 | |
| Thuja plicata | <0.01 | 0.13 |
| Guard Cell Length | Populus tremuloides | Thuja occidentalis |
| Populus tremuloides | ||
| Thuja occidentalis | 0.13 | |
| Thuja plicata | <0.01 | 0.56 |
| Guard Cell Width | Populus tremuloides | Thuja occidentalis |
| Populus tremuloides | ||
| Thuja occidentalis | <0.01 | |
| Thuja plicata | <0.01 | 0.41 |
| Value | Stomatal Density (Stomata m−2) | Guard Cell Length (m) | Guard Cell Width (m) |
|---|---|---|---|
| Mean for impressions | 1.74 × 10 8 | 2.97 × 10−5 | 9.95 × 10−6 |
| Mean for cleared leaves | 1.70 × 108 | 3.37 × 10−5 | 1.72 × 10−5 |
| p-value | 0.83 | 0.05 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, R.A.; Sheldon, N.D.; Smith, S.Y. Comparing Methodologies for Stomatal Analyses in the Context of Elevated Modern CO2. Life 2024, 14, 78. https://doi.org/10.3390/life14010078
Stein RA, Sheldon ND, Smith SY. Comparing Methodologies for Stomatal Analyses in the Context of Elevated Modern CO2. Life. 2024; 14(1):78. https://doi.org/10.3390/life14010078
Chicago/Turabian StyleStein, Rebekah A., Nathan D. Sheldon, and Selena Y. Smith. 2024. "Comparing Methodologies for Stomatal Analyses in the Context of Elevated Modern CO2" Life 14, no. 1: 78. https://doi.org/10.3390/life14010078






