Novel Therapeutic Agents for Management of Diabetes Mellitus: A Hope for Drug Designing against Diabetes Mellitus
Abstract
:1. Introduction
2. Current Anti-Diabetes Therapeutic Regimens
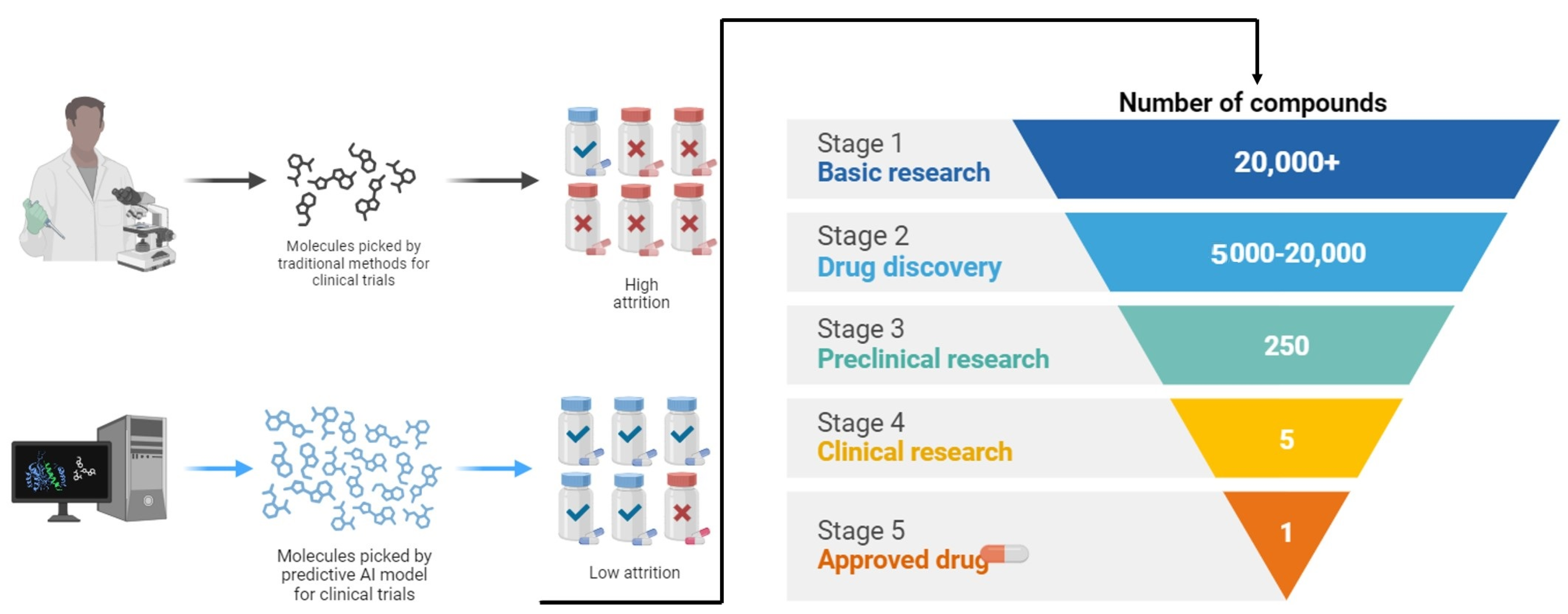
3. Novel Therapeutic Agents
3.1. Stem Cell Therapy: An Emerging Arrow for Targeting Diabetes Mellitus
3.2. Transdermal Drug Delivery System (TDDS)
3.3. Nanotechnology
3.4. Novel Candidate Drugs for Management of DM
3.4.1. Fucoidan
3.4.2. SGLT-2 (Sodium–Glucose Transporter-2) Inhibitors
3.4.3. Statin Therapy
3.4.4. Quercetin Shielding against Diabetes
3.5. Immunological Approach
3.5.1. Cyclosporin A (CsA)
3.5.2. Rituximab
3.5.3. Anti-TNF-α
3.5.4. GAD-65 (Glutamic Acid Decarboxylase 65)
3.5.5. Insulin Secretagogues (TAK-875)
3.6. Ethno-Medicine
3.7. Dietetics
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bangalore, S.; Fakheri, R.; Toklu, B.; Messerli, F.H. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: Systematic review and meta-analysis of randomized trials. BMJ 2016, 352, i438. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc. Res. 2013, 87, 65–74. [Google Scholar] [CrossRef]
- Emdin, C.A.; Rahimi, K.; Neal, B.; Callender, T.; Perkovic, V.; Patel, A. Blood pressure lowering in type 2 diabetes: A systematic review and meta-analysis. JAMA 2015, 313, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, R.; Dutta, K.N. A review on herbs used in the treatment of diabetes mellitus. J. Pharm. Chem. Biol. Sci. 2014, 2, 86–92. [Google Scholar]
- Dos Santos, J.M.; Tewari, S.; Mendes, R.H. The role of oxidative stress in the development of diabetes mellitus and its complications. J. Diabetes Res. 2019, 2019, 4189813. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.-E.; Al-Antary, N.; Yasmeen, A. High-Risk Human Papillomavirus and Colorectal Carcinogenesis. In Human Papillomavirus-Research in a Global Perspective; IntechOpen: London, UK, 2016. [Google Scholar]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Larsen, F.J.; Nyström, T.; Hezel, M.; Borniquel, S.; Weitzberg, E.; Lundberg, J.O. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17716–17720. [Google Scholar] [CrossRef]
- Coniff, R.F.; Shapiro, J.A.; Seaton, T.B.; Bray, G.A. Multicenter, placebo-controlled trial comparing acarbose (BAY g 5421) with placebo, tolbutamide, and tol-butamide-plus-acarbose in non-insulin-dependent diabetes mellitus. Am. J. Med. 1995, 98, 443–451. [Google Scholar] [CrossRef]
- Drummond, R.S.; Lyall, M.; McKnight, J. Statins should be routinely prescribed in all adults with diabetes. Pract. Diabetes Int. 2010, 27, 404–406. [Google Scholar] [CrossRef]
- Emer, J.J.; Claire, W. Rituximab: A review of dermatological applications. J. Clin. Aesthetic Dermatol. 2009, 2, 29–37. [Google Scholar]
- El-Wakf, A.M.; Hassan, H.A.; Mahmoud, A.Z.; Habza, M.N. Fenugreek potent activity against nitrate-induced diabetes in young and adult male rats. Cytotechnology 2014, 67, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.D.; Matsagar, V.A.; Gupta, A.K.; Marburg, S. An abridged review of blast wave parameters. Def. Sci. J. 2012, 62, 300–306. [Google Scholar] [CrossRef]
- Ghosh, P.; Azam, S.; Karim, A.; Hassan, M.; Roy, K.; Jonkman, M. A Comparative Study of Different Machine Learning Tools in Detecting Diabetes. Procedia Comput. Sci. 2021, 192, 467–477. [Google Scholar] [CrossRef]
- Jafri, M.; Aslam, M.; Javed, K.; Singh, S. Effect of Punica granatum Linn. (flowers) on blood glucose level in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2000, 70, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Tillin, T.; Dhutia, H.; Chambers, J.; Malik, I.; Coady, E.; Mayet, J.; Wright, A.R.; Kooner, J.; Shore, A.; Thom, S.; et al. South Asian men have different patterns of coronary artery disease when compared with European men. Int. J. Cardiol. 2008, 129, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, T.; Groop, L.C.; Zimmet, P.Z.; Rowley, M.J.; Knowles, W.; Mackay, I.R. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non—Insulin-dependent onset of disease. Diabetes 1993, 42, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Edstorp, J.; Wei, Y.; Ahlqvist, E.; Alfredsson, L.; Grill, V.; Groop, L.; Rasouli, B.; Sørgjerd, E.P.; Thorsby, P.M.; Tuomi, T.; et al. Smoking, use of smokeless tobacco, HLA genotypes and incidence of latent autoimmune diabetes in adults. Diabetologia 2022, 66, 70–81. [Google Scholar] [CrossRef]
- Alieva, A.; Khalilova, D.; Saidova, S.; Salimova, A. Post-COVID diabetes rmellitus in cross-sectional study: Autoimmune disease with endothelial dysfunction? BMC 2023. [Google Scholar] [CrossRef]
- Bally, K.; Ji, B.; Soni, L. COVID-19 Vaccine-Induced Latent Autoimmune Diabetes in Adults. Cureus 2023, 15, e33762. [Google Scholar] [CrossRef]
- Lee, N.; Prabhu, P.; Swaminath, S.; Amini, S.S. Development of Islet Antigen 2 (IA2) Antibodies Post-COVID-19 Infection: A Sign of Autoimmunity or Latent Autoimmune Diabetes Mellitus in Adults (LADA)? Cureus 2023, 15, e40971. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Addendum. 11. Chronic Kidney Disease and Risk Management: Standards of Medical Care in Diabetes—2022: Diabetes Care. Diabetes Care 2022, 45 (Suppl. S1), S175–S184. [Google Scholar] [CrossRef] [PubMed]
- Corson, A. Addressing Therapeutic Inertia in Diabetes Management. Ph.D. Thesis, Oregon Health & Science University, Portland, OR, USA, 2022. [Google Scholar]
- Chernausek, S.D.; Arslanian, S.; Caprio, S.; Copeland, K.C.; El Ghormli, L.; Kelsey, M.M.; Koontz, M.B.; Orsi, C.M.; Wilfley, D. Relationship Between Parental Diabetes and Presentation of Metabolic and Glycemic Function in Youth with Type 2 Diabetes: Baseline Findings from the TODAY Trial. Diabetes Care 2015, 39, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Choe, E.; Lee, Y.H.; Seo, J.W.; Choi, Y.; Yun, Y.; Wang, H.J.; Ahn, C.W.; Cha, B.S.; Lee, H.C. Risk of diabetes in patients treated with HMG-CoA reductase inhibitors. Metabolism 2015, 64, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.A.; Bruce, C.R.; Smith, A.C.; Lopaschuk, G.; Dyck, D.J. Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am. J. Physiol. Metab. 2006, 291, E182–E189. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Enhancing Incretin Action for the Treatment of Type 2 Diabetes. Diabetes Care 2003, 26, 2929–2940. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous silica nanoparticles: Synthesis, pharmaceutical applications, biodistribution, and biosafety as-sessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Gu, Z.; Dang, T.T.; Ma, M.; Tang, B.C.; Cheng, H.; Jiang, S.; Dong, Y.; Zhang, Y.; Anderson, D.G. Glucose-Responsive Microgels Integrated with Enzyme Nanocapsules for Closed-Loop Insulin Delivery. ACS Nano 2013, 7, 6758–6766. [Google Scholar] [CrossRef]
- Jacobsen, L.V.; Flint, A.; Olsen, A.K.; Ingwersen, S.H. Liraglutide in Type 2 Diabetes Mellitus: Clinical Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2015, 55, 657–672. [Google Scholar] [CrossRef]
- Kawahara, T.; Suzuki, G.; Inazu, T.; Mizuno, S.; Kasagi, F.; Okada, Y.; Tanaka, Y. Rationale and design of Diabetes Prevention with active Vitamin D (DPVD): A randomised, double-blind, placebo-controlled study. BMJ Open 2016, 6, e011183. [Google Scholar] [CrossRef]
- Galea, E.; Weinstock, L.D.; Larramona-Arcas, R.; Pybus, A.F.; Giménez-Llort, L.; Escartin, C.; Wood, L.B. Multi-transcriptomic analysis points to early organelle dysfunction in human astrocytes in Alzheimer’s disease. Neurobiol. Dis. 2022, 166, 105655. [Google Scholar] [CrossRef]
- Gagliardini, E.; Conti, S.; Benigni, A.; Remuzzi, G.; Remuzzi, A. Imaging of the Porous Ultrastructure of the Glomerular Epithelial Filtration Slit. J. Am. Soc. Nephrol. 2010, 21, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Fernandes, A.; Stein, F.; Brites, D. Protective Signature of IFNγ-Stimulated Microglia Relies on miR-124-3p Regulation from the Secretome Re-leased by Mutant APP Swedish Neuronal Cells. Front. Pharmacol. 2022, 13, 833066. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. Novel therapeutic approaches in diabetes. Nov. Diabetes 2016, 31, 43–56. [Google Scholar]
- Giovannini, P.; Howes, M.-J.R.; Edwards, S.E. Medicinal plants used in the traditional management of diabetes and its sequelae in Central America: A review. J. Ethnopharmacol. 2016, 184, 58–71. [Google Scholar] [CrossRef]
- Shah, N.N.; Khan, Z.; Ahad, H.; Elderdery, A.Y.; Alomary, M.N.; Atwah, B.; Alhindi, Z.; Alsugoor, M.H.; Elkhalifa, A.M.; Nabi, S.; et al. Mucormycosis an added burden to Covid-19 Patients: An in-depth systematic review. J. Infect. Public Health 2022, 15, 1299–1314. [Google Scholar] [CrossRef]
- Geil, P.; Shane-McWhorter, L. Dietary Supplements in the Management of Diabetes: Potential Risks and Benefits. J. Am. Diet. Assoc. 2008, 108, S59–S65. [Google Scholar] [CrossRef]
- Greer, R.M.; Portelli, S.L.; Hung, B.S.M.; Cleghorn, G.J.; McMahon, S.K.; Batch, J.A.; Conwell, L.S. Serum vitamin D levels are lower in Australian children and adolescents with type 1 diabetes than in children without diabetes. Pediatr. Diabetes 2013, 14, 31–41. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Potential Therapeutic Effects of Nitrate/Nitrite and Type 2 Diabetes Mellitus. Int. J. Endocrinol. Metab. 2013, 11, 63–64. [Google Scholar] [CrossRef]
- Forman, E.M.; Hoffman, K.L.; Juarascio, A.S.; Butryn, M.L.; Herbert, J.D. Comparison of acceptance-based and standard cognitive-based coping strategies for craving sweets in overweight and obese women. Eat. Behav. 2013, 14, 64–68. [Google Scholar] [CrossRef]
- van Poelje, P.D.; Dang, Q.; Erion, M.D. Fructose-1,6-bisphosphatase as a therapeutic target for type 2 diabetes. Drug Discov. Today Ther. Strat. 2007, 4, 103–109. [Google Scholar] [CrossRef]
- Patra, N.; Kar, D.; Pal, A.; Behera, A. Antibacterial, anticancer, anti-diabetic and catalytic activity of bio-conjugated metal nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035001. [Google Scholar] [CrossRef]
- Pastors, J.G.; Warshaw, H.; Daly, A.; Franz, M.; Kulkarni, K. The Evidence for the Effectiveness of Medical Nutrition Therapy in Diabetes Management. Diabetes Care 2002, 25, 608–613. [Google Scholar] [CrossRef]
- Bantle, A.E.; Thomas, W.; Bantle, J.P. Metabolic effects of alcohol in the form of wine in persons with type 2 diabetes mellitus. Metabolism 2008, 57, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 1–14. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Shin, S.; Shigihara, T.; Hahm, E.; Liu, M.-J.; Han, J.; Yoon, J.-W.; Jun, H.-S. Glucagon-Like Peptide-1 Gene Therapy in Obese Diabetic Mice Results in Long-Term Cure of Diabetes by Improving Insulin Sensitivity and Reducing Hepatic Gluconeogenesis. Diabetes 2007, 56, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.H.; Wang, X.; Martinez, M.P.; Walthall, J.C.; Curry, E.S.; Page, K.; Buchanan, T.A.; Coleman, K.J.; Getahun, D. Association of Maternal Diabetes with Autism in Offspring. JAMA 2015, 313, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Iqbal Hydrie, M.Z.; Basit, A.; Shera, A.S.; Hussain, A. Effect of intervention in subjects with high risk of diabetes mellitus in Pakistan. J. Nutr. Metab. 2012, 2012, 867604. [Google Scholar] [CrossRef]
- Naujok, O.; Lenzen, S. Pluripotent stem cells for cell replacement therapy of diabetes. Dtsch. Med. Wochenschr. (1946) 2012, 137, 1062–1066. [Google Scholar]
- Naidu, K.C.; Pullaiah, T. Antidiabetic Plants in India and Herbal Based Antidiabetic Research; Regency: New Delhi, India, 2003. [Google Scholar]
- Daga, R.A.; Laway, B.A.; Shah, Z.A.; Mir, S.A.; Kotwal, S.K.; Zargar, A.H. High prevalence of vitamin D deficiency among newly diagnosed youth-onset diabetes mellitus in north India. Arq. Bras. Endocrinol. Metabol. 2012, 56, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Li, Q.; Xia, L.; Song, J.; Xu, L.; Zhang, J.; Xie, Y.; Song, H. Au-modified three-dimensional In2O3 inverse opals: Synthesis and improved performance for acetone sensing toward diagnosis of diabetes. Nanoscale 2015, 7, 13051–13060. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Hou, D.; Zhao, X.; Wang, L.; Hu, Y.; Liu, J.; Cheng, H.; Yang, P.; Shan, X.; Yan, Y.; et al. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine 2015, 50, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Abouzaripour, M.; Kashani, I.R.; Pasbakhsh, P.; Atlasy, N. Intravenous Transplantation of Very Small Embryonic Like Stem Cells in Treatment of Diabetes Mellitus. Avicenna J. Med. Biotechnol. 2015, 7, 22–31. [Google Scholar] [PubMed]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2016, 16, 115–130. [Google Scholar] [CrossRef]
- Moses, A.M.; Howanitz, J.; Miller, M. Diuretic Action of Three Sulfonylurea Drugs. Ann. Intern. Med. 1973, 78, 541–544. [Google Scholar] [CrossRef]
- Nadkarni, P.; Chepurny, O.G.; Holz, G.G. Regulation of glucose homeostasis by GLP-1. Prog. Mol. Biol. Transl. Sci. 2014, 121, 23–65. [Google Scholar]
- Thakkar, U.G.; Trivedi, H.L.; Vanikar, A.V.; Dave, S.D. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow–derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy 2015, 17, 940–947. [Google Scholar] [CrossRef]
- Nam, S.; Song, Y. Role of self-efficacy in the relationship between patient-provider relationships and psychological insulin resistance among patients with type 2 diabetes. J. Contemp. Diabetes Res. 2014, 1, 1. [Google Scholar]
- Snarski, E.; Milczarczyk, A.; Hałaburda, K.; Torosian, T.; Paluszewska, M.; Urbanowska, E.; Król, M.; Boguradzki, P.; Jedynasty, K.; Franek, E.; et al. Immunoablation and autologous hematopoietic stem cell transplantation in the treatment of new-onset type 1 diabetes mellitus: Long-term observations. Bone Marrow Transplant. 2015, 51, 398–402. [Google Scholar] [CrossRef]
- Jeon, C.Y.; Haan, M.N.; Cheng, C.; Clayton, E.R.; Mayeda, E.R.; Miller, J.W.; Aiello, A.E. Helicobacter pylori Infection Is Associated with an Increased Rate of Diabetes. Diabetes Care 2012, 35, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z. Pharmacovigilance of Herbal Medicine: Herbavigilance. Adv. Pharmacoepidemiol. Drug Saf. 2016, 5, 1000208. [Google Scholar] [CrossRef]
- Talreja, S.; Kaur, C.D. Fighting diabetes with herbal technological developments. World J. Pharm. Res. 2014, 3, 2842–2867. [Google Scholar]
- Waseem, R.; Muhee, A.; Malik, H.U.; Akhoon, Z.A.; Munir, K.; Nabi, S.U.; Taifa, S. Isolation and Identification of Major Mastitis Causing Bacteria from Clinical Cases of Bovine Mastitis in Kashmir Valley. Indian J. Anim. Res. 2020, 54, 1428–1432. [Google Scholar] [CrossRef]
- Rosalie, I.O.; Ekype, E. Antidiabetic potentials of common herbal plants and plant products: A glance. Int. J. Herb. Med. 2016, 4, 90–97. [Google Scholar]
- Yao, B.; Fang, H.; Xu, W.; Yan, Y.; Xu, H.; Liu, Y.; Mo, M.; Zhang, H.; Zhao, Y. Dietary fiber intake and risk of type 2 diabetes: A dose–response analysis of prospective studies. Eur. J. Epidemiol. 2014, 29, 79–88. [Google Scholar] [CrossRef] [PubMed]
- DiSanto, R.M.; Subramanian, V.; Gu, Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Bedekar, A.; Shah, K.; Koffas, M. Natural products for type II diabetes treatment. Adv. Appl. Microbiol. 2010, 71, 21–73. [Google Scholar]
- Jarald, E.; Joshi, S.B.; Jain, D. Diabetes and herbal medicines. Iran. J. Pharmacol. Ther. 2008, 7, 97–106. [Google Scholar]
- Patel, D.; Kumar, R.; Laloo, D.; Hemalatha, S. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pac. J. Trop. Dis. 2012, 2, 239–250. [Google Scholar] [CrossRef]
- Pirags, V.; Lebovitz, H.; Fouqueray, P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes. Metab. 2012, 14, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Neumiller, J.J.; Wood, L.; Campbell, R.K. Dipeptidyl Peptidase-4 Inhibitors for the Treatment of Type 2 Diabetes Mellitus. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Zmistowski, B.; Restrepo, C.; Hess, J.; Adibi, D.; Cangoz, S.; Parvizi, J. Unplanned readmission after total joint arthroplasty: Rates, reasons, and risk factors. J. Bone Jt. Surg. 2013, 95, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Yatoo, M.I.; Saxena, A.; Jhambh, R.; Nabi, S.; Melepad, D.P.; Kumar, P.; Dimri, U.; Sharma, M.C. Status of trace mineral deficiency in sheep and goat in Kashmir Valley. Res. J. Vet. Pract. 2013, 1, 43–45. [Google Scholar]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Tyagi, A.C.; Sen, U.; Mishra, P.K. Synergy of microRNA and stem cell: A novel therapeutic approach for diabetes mellitus and cardiovascular diseases. Curr. Diabetes Rev. 2011, 7, 367–376. [Google Scholar] [CrossRef]
- Radican, L.; Fu, A.Z.; Zhang, Q.; Pentakota, S.R.; Seck, T. Underutilisation of statins in patients with type 2 diabetes treated with an antihyperglycaemic regimen. In Diabetologia; Springer: New York, NY, USA, 2010. [Google Scholar]
- Chen, L.-K.; Chen, Y.-M.; Lin, M.-H.; Peng, L.-N.; Hwang, S.-J. Care of elderly patients with diabetes mellitus: A focus on frailty. Ageing Res. Rev. 2010, 9, S18–S22. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, N.D.; Garg, S.; Goyal, L.; Gupta, A.; Khan, S.; Moin, S. The effect of type 2 diabetes mellitus and smoking on periodontal parameters and salivary matrix metallopro-teinase-8 levels. J. Oral Sci. 2016, 58, 1–6. [Google Scholar] [CrossRef]
- Ryan, G.J.; Jobe, L.J.; Martin, R. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin. Ther. 2005, 27, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, A.H.; Darsow, T.; Maggs, D.G. Incretin-based therapies. J. Diabetes 2012, 4, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Marek-Trzonkowska, N.; Myśliwiec, M.; Dobyszuk, A.; Grabowska, M.; Derkowska, I.; Juścińska, J.; Owczuk, R.; Szadkowska, A.; Witkowski, P.; Młynarski, W.; et al. Therapy of type 1 diabetes with CD4+CD25highCD127-regulatory T cells prolongs survival of pancreatic islets—Results of one year follow-up. Clin. Immunol. 2014, 153, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Henriksen, K.J.; Boden, E.K.; Tooley, A.J.; Ye, J.; Subudhi, S.K.; Zheng, X.X.; Strom, T.B.; Bluestone, J.A. Cutting Edge: CD28 Controls Peripheral Homeostasis of CD4+CD25+ Regulatory T Cells. J. Immunol. 2003, 171, 3348–3352. [Google Scholar] [CrossRef] [PubMed]
- Nabi, S.; Dey, S.; Shah, O.; Hussain, T.; Amin, U.; Vala, J.; Jan, A.; Ramdas, A.; Muhee, A.; Hussain, A.; et al. Incidence of renal disorders in canines and its relation with age breed and sex. Pharma Innov. J. 2018, 7, 87–89. [Google Scholar]
- Rahimi, G.; Jafari, N.; Khodabakhsh, M.; Shirzad, Z.; Dogaheh, H.P. Upregulation of microRNA Processing Enzymes Drosha and Dicer in Gestational Diabetes Mellitus. Gynecol. Endocrinol. 2014, 31, 156–159. [Google Scholar] [CrossRef]
- Rasoul, M.A.; Al-Mahdi, M.; Al-Kandari, H.; Dhaunsi, G.S.; Haider, M.Z. Low serum vitamin-D status is associated with high prevalence and early onset of type-1 diabetes mellitus in Kuwaiti children. BMC Pediatr. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Sørensen, I.M.; Joner, G.; Jenum, P.A.; Eskild, A.; Torjesen, P.A.; Stene, L.C. Maternal Serum Levels of 25-Hydroxy-Vitamin D During Pregnancy and Risk of Type 1 Diabetes in the Offspring. Diabetes 2011, 61, 175–178. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Zhang, W.; Chen, J.J.; Zhang, Z.-L.; Han, S.-F.; Qin, L.-Q. Vitamin D Intake and Risk of Type 1 Diabetes: A Meta-Analysis of Observational Studies. Nutrients 2013, 5, 3551–3562. [Google Scholar] [CrossRef]
- Stene, L.C.; Magnus, P.; Lie, R.T.; Søvik, O.; Joner, G. No Association between Preeclampsia or Cesarean Section and Incidence of Type 1 Diabetes among Children: A Large, Population-Based Cohort Study. Pediatr. Res. 2003, 54, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Raab, J.; Giannopoulou, E.Z.; Schneider, S.; Warncke, K.; Krasmann, M.; Winkler, C.; Ziegler, A.-G. Prevalence of vitamin D deficiency in pre-type 1 diabetes and its association with disease progression. Diabetologia 2014, 57, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, M.; Mykkänen, J.; Koskinen, M.; Simell, V.; Veijola, R.; Hyöty, H.; Ilonen, J.; Knip, M.; Simell, O.; Toppari, J. Serum 25-Hydroxyvitamin D Concentrations in Children Progressing to Autoimmunity and Clinical Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.; Brady, H.; Yin, X.; Seifert, J.; Barriga, K.; Hoffman, M.; Bugawan, T.; Barón, A.E.; Sokol, R.J.; Eisenbarth, G.; et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: The Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 2011, 54, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.; Shukla, K.; Tyagi, M.K.; Garg, P.; Gambhir, J.K.; Shukla, R. Antidiabetic and antihyperlipidemic effects of the stem of Musa sapientum Linn. in streptozotocin-induced diabetic rats. J. Diabetes 2012, 4, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Rak, K.; Bronkowska, M. Immunomodulatory Effect of Vitamin D and Its Potential Role in the Prevention and Treatment of Type 1 Diabetes Mellitus—A Narrative Review. Molecules 2018, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S. Association between Patient Interpretation of Diabetic Peripheral Neuropathy and Foot Self-Care Behaviors. Int. J. Nurs. Didact. 2018, 8, 43–59. [Google Scholar] [CrossRef]
- Federico, G.; Genoni, A.; Puggioni, A.; Saba, A.; Gallo, D.; Randazzo, E.; Salvatoni, A.; Toniolo, A. Vitamin D status, enterovirus infection, and type 1 diabetes in Italian children/adolescents. Pediatr. Diabetes 2018, 19, 923–929. [Google Scholar] [CrossRef]
- Grant, S.F. The TCF7L2 Locus: A Genetic Window into the Pathogenesis of Type 1 and Type 2 Diabetes. Diabetes Care 2019, 42, 1624–1629. [Google Scholar] [CrossRef]
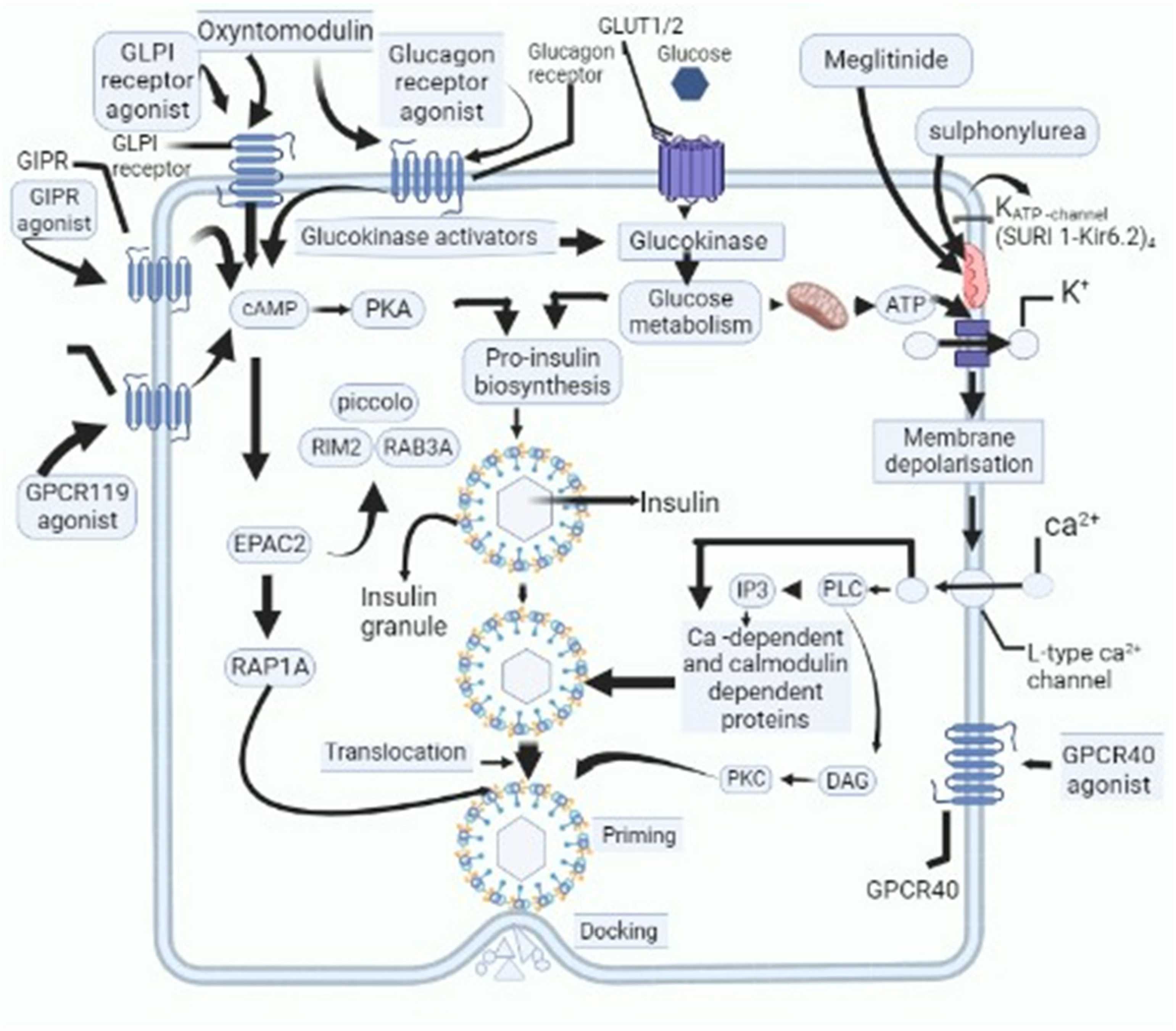

| Alpha Glucosidase Inhibitors | These Are Budding Therapeutics Aimed at Blocking α-Glucosidase, Henceforth, Postponing Breakdown of Carbohydrates Which Consequently Diminishes Its Intestinal Assimilation [5] | ||||
|---|---|---|---|---|---|
| Drugs | Commercial Name | Mechanism of Action | Advantages | Side Effects | References |
| (a) Acarbose | Precose | There is amendable blockage of glucosidases esp. glucoamylase, sucrose, maltose, and α-amylase of brush border epithelium | Decreased postprandial hyperglycemia in T2DM | Borborygmic, abdominal fullness, diarrhea, intestinal flatulence | [32] |
| (b) Migtitol | Glycet | Works by reducing the disintegration and assimilation of sugar in small intestine | Considerable hypoglycemia by achievement of normoglycemia in patients via enhancement of glucose tolerance | Lesser GIT after-effects | [5,27,33] |
| (c) Voglibose | Newly added significant sucrose blocker | Slows down the glucose assimilation, accordingly decreasing the possibility of macrovascular complications | [5] | ||
| (d) Insulin sensitizers | Aid the activity of insulin in liver, adipose tissue, and muscles in addition to decreasing the peripheral insulin resistance in skeletal muscles and adipocytes | Hypoglycemia, weight gain, diarrhea, and greater chance of cardiovascular diseases | [34] | ||
| Sulfonylureas | Sulfonylureas Are a Class of Oral Antidiabetic Drugs Commonly Used in the Treatment of Type 2 Diabetes. They Work by Stimulating Insulin Release from the Beta Cells of the Pancreas | ||||
|---|---|---|---|---|---|
| Drugs | Commercial Name | Mechanism of Action | Advantages | Side Effects | References |
| |||||
|
| These cause stimulation of the secretion of insulin from pancreas | Considerable hypoglycemia is achieved | GIT interferences and hemolytic anemia | [22] |
| |||||
|
| All of these promote the secretion of insulin from β cells of pancreas | Considerable hypoglycemia is achieved | GIT interferences and hemolytic anemia | [5,21,35] |
| Biguanides | They Primarily Work by Reducing Hepatic Glucose Production and Improving Peripheral Insulin Sensitivity | |||
|---|---|---|---|---|
| Drugs | Mechanism of Action | Advantages | Side Effects | References |
| Metformin (extracted from plant Galega officinalis) | Works by improving the serum glucose levels via hindering the liver glucose production along with boosting the uptake of glucose by muscle fibers | Declining triglycerides and low-density lipids and has lesser frequency of hypoglycemia | Lactic acidosis, vit B12 deficiency, congestive heart failure | [9] |
| Thiazolidinediones (TZD) | These Drugs Work by Targeting the Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ), a Nuclear Receptor Involved in Glucose and Lipid Metabolism | ||||
|---|---|---|---|---|---|
| Drugs | Commercial Name | Mechanism of Action | Advantages | Side Effects | References |
|
| Enhances insulin sensitivity of all target tissues and acts as ligands for PPAR (peroxisomes proliferator-activated gamma) complex located inside the nucleus | Effect on plasma low-density lipids/cholesterol |
| [5,38,39] |
| Peptide Analogues | These Analogues Are Synthetic Compounds Designed to Mimic the Actions of Endogenous Peptides Involved in Glucose Homeostasis | |||
|---|---|---|---|---|
| Drugs | Mechanism of Action | Advantages | Side Effects | References |
| Incretin mimetics | Liberated in riposte to the ingestion of food, eliciting the glucose-induced insulin response | Appetence decreasing positive impact of these agents on cardiovascular, inflammation, and the central nervous system | Does not hinder the glucagon emission | [43,44] |
| Glucagon-like peptide analogs and agonists (GLP-1) | Retarding the gastric clearing, boosting the insulin secretion alongside hampering the glucagon secretion from pancreas | Prevents gastric acid secretion in addition to promoting insulin secretion | Not reported | [35,45,46] |
| Glucose-dependent insulinotropic polypeptide analogs (GIP) | These analogs are synthetic compounds designed to mimic the actions of endogenous peptides involved in glucose homeostasis | Reducing the postprandial glucose levels and glycosylated hemoglobin | Lesser danger of hypoglycemia with the use of this agent | [20,47] |
| DPP-4 inhibitors | GIP produced out of the k cells of the upper small intestine works by affecting the metabolism of lipids | Fasting plasma glucose levels reduced along with the changes in glycosylated hemoglobin | Effects like vomiting and anorexia | [8,12] |
| Liraglutide | Action via enzymatic incretin disintegration Agonist for receptor (GLP-1) | Appetence decreasing positive impact of these agents on cardiovascular, inflammation, and the central nervous system | Compared to the short-acting forms, long-acting ones have less chance of causing hypoglycemia | [9,48,49,50] |
| Novel Anti-Diabetic Agents | The Latest Developments in Pharmacotherapy, Focusing on Innovative Approaches That Address the Complex Challenges of Diabetes | |||
|---|---|---|---|---|
| Drugs | Mechanism of Action | Advantages | Side Effects | References |
| Significant decline in the levels of glucose in T1DM and T2DM | Decreases the blood glucose level by reducing the glucagon secretion | Used as solo therapy or sometimes in combination with orally active anti-diabetic agents. | [23,52,53,54] |
| Secreted and stored in combination with insulin and hindering the glucagon secretion in addition to slowing down the unloading of gastric contents | If there is some beta cell function remaining, then at that time, replacement curative aided with basal insulin can prove to be useful | Increased arterial pressure, inducing kidney dysfunction, onset of hypertension, boosting the occurrence of diabetes and hypothyroidism | [23,52,53,54] |
| Molecules produced from L-arginine via the enzymes nitric oxide synthase (NOS) namely inducible, neuronal, endothelial, and mitochondrial have been observed to abate the levels of triglycerides in serum | Regulation of TG levels in blood | Promotion of fat deposition in liver | [23,52,53,54] |
| Fasting plasma insulin and HbA1c levels are declined by administration of vitamin C. Moreover, there is seen to be refinement of insulin action. Likewise, with the administration of β carotene, reduction of oxidative low-density lipids has been noted | New effective therapy for the cure of T2DM patients is antioxidant therapy, which might reflect a significant role in diminishing the chances of diabetic hyperglycemia and thereafter its associated drawbacks | [23,52,53,54] | |
| Mode of action via inhibiting the FBPase enzyme (rate-limiting enzyme in gluconeogenesis pathway) | Liver hyperplasia, liver hypertrophy, and liver carcinogenesis | [23,52,53,54] | |
| Recently, Swift-liberated bromocriptine evolved in favor of T2DM amelioration but the mode of action is not clear yet | The literature has proved that after 24 weeks of therapy, the average glycated hemoglobin levels declined by 0.0% to 0.2% | [23,52,53,54] | |
| Quite effective in the sense that it stimulates glucose uptake in muscles, liver gluconeogenesis is depressed along with boost in the sugar-dependent insulin release | Hampering oxidative phosphorylation | [23,52,53,54] | |
| Mostly participating in controlling the energy homeostasis supplementary to reduction of triglycerides | [23,52,53,54] | ||
| Phytomedicine with Family | Portion | Active Chemical Constituents | Mechanism of Action | References |
|---|---|---|---|---|
| Allium cepa (Onion) Alliaceae | Corm | S-methyl cysteine sulphoxide and allyl propyl disulfide | Arouse the action of enzymes hexokinase and reductase in addition to the production of insulin | [66] |
| Carica papaya Caricaceae | Seed and extract of leaves | ------ | Alleviate wounds in alloxan-induced diabetic rats in addition to reducing the serum glucose level | [37] |
| Catharantus roseus (Vinca roses) Apocynaceae | Leaves and twigs | ------- | Boosting the biosynthesis of insulin from the pancreatic islets | [91] |
| Acacia Arabica Fabaceae | Bark and seed | Polyphenols and tannins | Commencement of insulin secretion from pancreatic β cells | [92] |
| Allium sativum (Garlic) Alliaceae | Corm | Allicin and allyl propyl disulfide | Modifies the action of enzymes glucose-6-phosphate, HMG CoA reductase, and hexokinase, in addition to managing glucose levels in serum and tissues | [51] |
| Aloe barbadensis (Aloevera/Ghikanwar) Liliaceae | Leaf | Barbaloin and alloin | Revitalizing the process of hepatogluconeogenesis/glycogenolysis alongside the liberation of insulin from the pancreas. In addition, the glutathione levels in diabetic rats were elevated by a factor of 4 | [4,63] |
| Beta vulgaris (Beet root) | Root | Betacyanins and phenolics | Non-enzymatic glycosylation of serum glucose and skin proteins declined | [92] |
| Azadirachta indica (Neem) Meliaceae | Seed and leaf | Nimbin and azadirachtin | β cells of the pancreas are revived/revitalized Also, it has been noted to amend blood circulation via dilating blood vessels (Mishra et al., 2011) | [93,94] |
| Brassica nigra (Mustard) Brassicaceae | Whole plant | Sinignin, isorhamnetin, diglucoside, and isothiocynate | The conduct of glycogen synthetase is boosted unlike the action of glycogen phosphorylase and gluconeogenic enzymes, which is reduced thereby depressing glycogenolysis and gluconeogenesis | [51] |
| Cassia auriculata (Senna) Leguminaceae | Flower | Sennoside A and Sennosede B | The activity of hepatic hexokinase and phosphofructokinase enzymes is amplified while the activity of glucose-6-phosphate and fructose-1,6-biphosphatase enzymes is suppressed. Further, there is an increase in the no. of islets and beta cells in pancreas | [51] |
| Andrographis Paniculata (Kalmegh) Acanthaceae | Whole plant | Andrographolide, kalmeghin, and diterpenoid lactone | Glucose assimilation from the intestinal wall is countered | [51,62] |
| Gymnema sylvestre (Gudmar) Asclepiadaceae | Leaf | Gymnema saponins and gymnemic acid | Boosting the number of β cells along with insulin secretion | [63] |
| Ficus benglenesis (Banyan) Moraceae | Bark and leaf | Tannin, taraxasterol, quercetin-3-galactoside, and rutin | Blood insulin levels in type 2 diabetes mellitus were triggered via the action of hypoglycemic components separated | [63] |
| Capsicum frutescens (Mirch) Solanaceae | Entire plant or Fruit | Capsaicin, protein | There is the devaluation of insulin binding on insulin receptors along with boosting insulin voiding | [51] |
| Coriandrum sativum (Coriander fruits) Umbelliferae | Seed | Blood glucose level declined including the activity of beta cells escalated thereafter augmenting insulin release | [62] | |
| Cuminum cyminum (Jira) Umbelliferaceae | Seed | Geraniol, coriandrol, pinene, coriendrlyacetate | Depletion in glycosylated hemoglobin, blood urea nitrogen, and blood glucose, and at the same time serum insulin content is enhanced | [62] |
| Eucalyptus globulas (Nilgiri, Dinkum oil) | Leaf | Hydrocumin, phellandrene, and cuminaldehyde | Elevation of peripheral glucose uptake | [61,92,95] |
| Curcuma longa Le. (Turmeric) Zingiberaceae | Tuber | Citronella, camphene, pinene, cineole | Mentioned medicine showed promising outcomes in the management of diabetes | [13,62] |
| Eugenia jambolana (jamun) Myrtaceae | Dried seed and pulp | Essential oil, dimethoxy curcumin, curcumin, and Btermennone | Intensifying the emission of insulin in addition to hindrance of the liver and kidney enzyme insulinase | [96] |
| Trigonella foenum Graecum (Methi) Leguminosae | Seed | Oleanolic acid, ellagic acid, alpha glucosidase, Malvidin 3-laminaribiosidea and ferulic acid | Valuable discharge of insulin alongside the inducement of insulin coalescence | [51,97] |
| Tinospora crispa Menispermaceae | Stem | Nicotinic acid, coumarin, saponin-peptide esters, trigonelline, and flavonoids | Stimulation of insulin secretion on account of its anti-diabetic effect and further, there is the regulation of calcium concentration of beta cells | [51,62] |
| Ocimum sanctum L. (Tulsi) Lamiaceae | Leaf | Fraxinus coumarin alkaloids, asco acid, eugenol, and glucoside | Serum glucose level is diminished | [62] |
| Lawsonia inermis (Henna) Lythraceae | Seed and flower | Xanthones and tannin, alkaloids and fatty oil | Concentration of cholesterol, glucose, and triglycerides is depressed | [98] |
| Momordica charantia (Karela) Cucurbitaceae | Leaf | Charantin, momordic I, momordic II, and cucurbitacin B | There may be rejuvenation of moderately damaged cells along with improvement of beta cell production in the pancreas. In addition, said product contains lectin, which mimics the action of insulin | [92] |
| Mangifera indica Anacardiaceae | Leaf | Mangiferin | Intestinal absorption of glucose is decreased | [42] |
| Musa sapientum (Banana) Musaceae | Flower | Glycoside, flavonoids, and steroid | Works by simulating insulin-like action | [99] |
| Tinospora Cardifolia (Guduchi) Menispermaceae | Root, stem, and leaves | Diterpenoid lactones, alkaloid, glycosides, and steroids | Appreciable depression of blood sugar | [100] |
| Psibium guajava Myrtaceae | Fruit | Strictinin, vitamin C, quercetin, and glycon | The blood sugar level is lowered via the glycon existing in fruit | [60] |
| Murraya koenigii (Curry Leaves) Rubaceae | Leaf | Carbazole alkaloids | Declined gluconeogenesis and glycogenolysis | [101] |
| Cajanus cajan (Arhar) Fabaceae | Seed | Cajanin, cajanones, 2-2 methyl cajanone, and isoflavones | Appreciable decrease in levels of blood glucose | [76] |
| Coccinia indica (baby watermelon) Cucurbitaceae | Whole plant | Asparagine and glutamic acid | Due to repressed glucose synthesis, there is a reduction in blood glucose level | [62] |
| Panex ginseng (Ginseng) Araliaceae | Extract of root | Ginsenosides and protopanaxadiol | Decline the assimilation of glucose along with blocking the action of enzyme alpha-glycosidase | [92] |
| Annona squamosa (Sharifa) Annonaceae | Leaf extract | Moupinamide and liriodenin | Glucose tolerance is enhanced | [61] |
| Punica grantum (Pomegranate) Puniaceae | Seed extract | Punicalin and punicalagin | Blood sugar reduction | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkhalifa, A.M.E.; Nazar, M.; Ali, S.I.; Khursheed, I.; Taifa, S.; Ahmad Mir, M.; Shah, I.H.; Malik, M.; Ramzan, Z.; Ahad, S.; et al. Novel Therapeutic Agents for Management of Diabetes Mellitus: A Hope for Drug Designing against Diabetes Mellitus. Life 2024, 14, 99. https://doi.org/10.3390/life14010099
Elkhalifa AME, Nazar M, Ali SI, Khursheed I, Taifa S, Ahmad Mir M, Shah IH, Malik M, Ramzan Z, Ahad S, et al. Novel Therapeutic Agents for Management of Diabetes Mellitus: A Hope for Drug Designing against Diabetes Mellitus. Life. 2024; 14(1):99. https://doi.org/10.3390/life14010099
Chicago/Turabian StyleElkhalifa, Ahmed M. E., Mehak Nazar, Sofi Imtiyaz Ali, Ibraq Khursheed, Syed Taifa, Muzafar Ahmad Mir, Iqra Hussain Shah, Masood Malik, Zahid Ramzan, Shubeena Ahad, and et al. 2024. "Novel Therapeutic Agents for Management of Diabetes Mellitus: A Hope for Drug Designing against Diabetes Mellitus" Life 14, no. 1: 99. https://doi.org/10.3390/life14010099
APA StyleElkhalifa, A. M. E., Nazar, M., Ali, S. I., Khursheed, I., Taifa, S., Ahmad Mir, M., Shah, I. H., Malik, M., Ramzan, Z., Ahad, S., Bashir, N., Elamin, E., Bazie, E. A., Ahmed, E. M., Alruwaili, M. M., Baltoyour, A. W., Alarfaj, A. S., Ali Al Bataj, I., Arabe, A. M. A., & Nabi, S. U. (2024). Novel Therapeutic Agents for Management of Diabetes Mellitus: A Hope for Drug Designing against Diabetes Mellitus. Life, 14(1), 99. https://doi.org/10.3390/life14010099






