Exploring the Causal Association between Morning Diurnal Preference and Psychiatric Disorders: A Bidirectional Two-Sample Mendelian Randomization Analysis
Abstract
1. Introduction
2. Materials and Methods
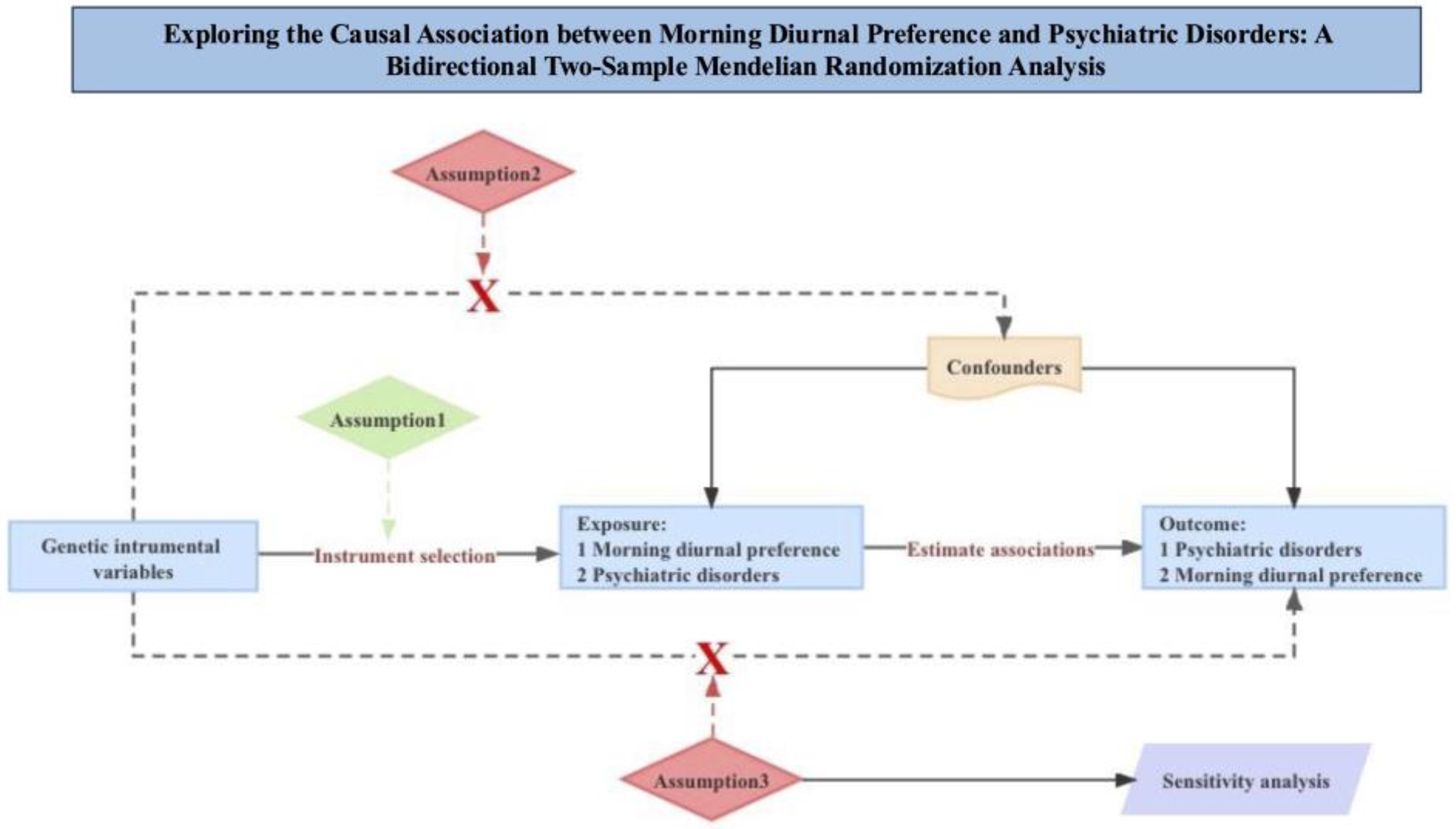
2.1. Study Design
2.2. Data Sources
2.2.1. Genetic Instrumental Variables
2.2.2. MR of Morning Diurnal Preference and Psychiatric Disorders
2.2.3. Bidirectional Mendelian Randomization Analyses
2.3. Statistical Analysis
2.4. Power Calculations
3. Results
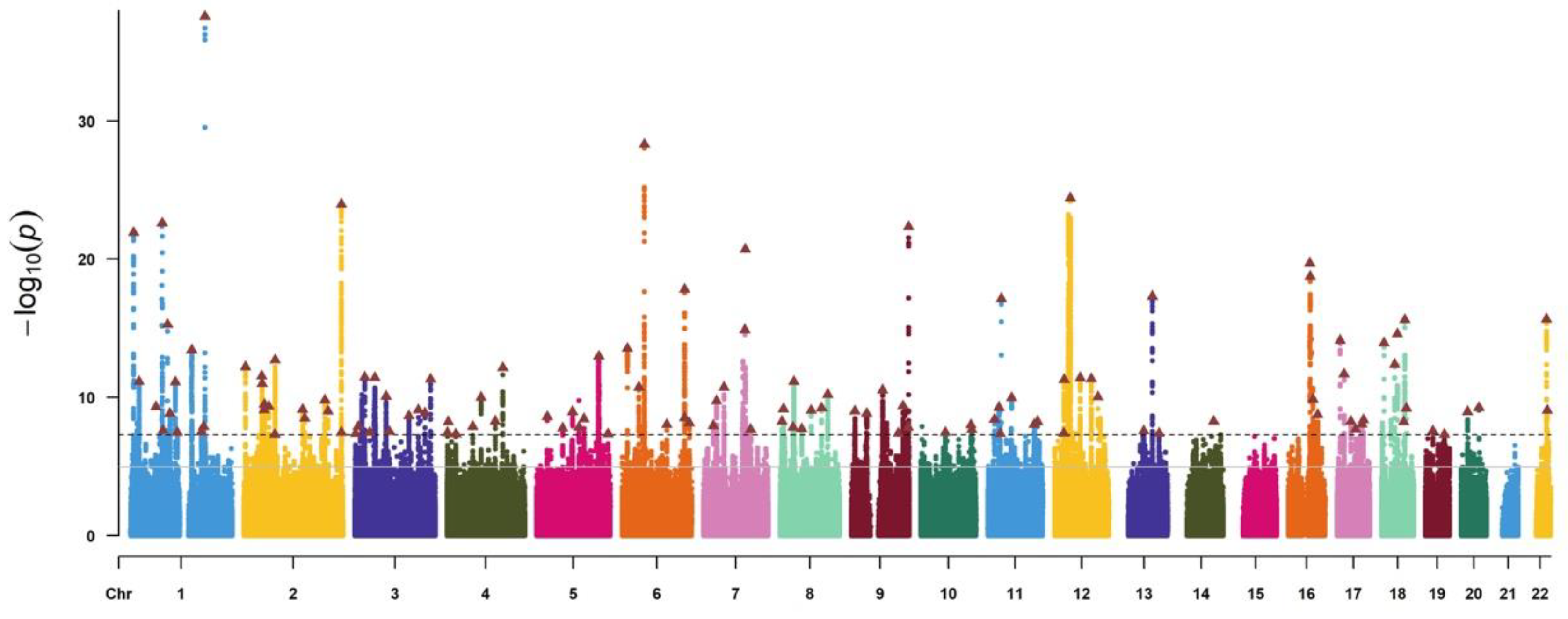
3.1. Genetic Instruments of Exposure and Outcome
3.2. Effects of Morning Diurnal Preference on Psychiatric Disorders
3.3. Reversed Effects of Psychiatric Disorders on Morning Diurnal Preference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, V.; Saxena, S.; Lund, C.; Thornicroft, G.; Baingana, F.; Bolton, P.; Chisholm, D.; Collins, P.Y.; Cooper, J.L.; Eaton, J.; et al. The Lancet Commission on Global Mental Health and Sustainable Development. Lancet 2018, 392, 1553–1598. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Arias, D.; Saxena, S.; Verguet, S. Quantifying the Global Burden of Mental Disorders and Their Economic Value. EClinicalMedicine 2022, 54, 101675. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, M.A.; Azarkolah, A.; Ghanavati, E.; Nitsche, M.A. Circadian Disturbances, Sleep Difficulties and the COVID-19 Pandemic. Sleep. Med. 2022, 91, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Cosco, T.D.; Fortuna, K.; Wister, A.; Riadi, I.; Wagner, K.; Sixsmith, A. COVID-19, Social Isolation, and Mental Health Among Older Adults: A Digital Catch-22. J. Med. Internet Res. 2021, 23, e21864. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H.; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian Rhythm Disruption and Mental Health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
- Merikanto, I.; Kortesoja, L.; Benedict, C.; Chung, F.; Cedernaes, J.; Espie, C.A.; Morin, C.M.; Dauvilliers, Y.; Partinen, M.; De Gennaro, L.; et al. Evening-Types Show Highest Increase of Sleep and Mental Health Problems during the COVID-19 Pandemic—Multinational Study on 19 267 Adults. Sleep 2022, 45, zsab216. [Google Scholar] [CrossRef]
- Tao, S.; Wu, X.; Li, S.; Ma, L.; Yu, Y.; Sun, G.; Zhang, Y.; Li, T.; Tao, F. Circadian Rhythm Abnormalities during the COVID-19 Outbreak Related to Mental Health in China: A Nationwide University-Based Survey. Sleep Med. 2021, 84, 165–172. [Google Scholar] [CrossRef]
- Walsh, N.A.; Repa, L.M.; Garland, S.N. Mindful Larks and Lonely Owls: The Relationship between Chronotype, Mental Health, Sleep Quality, and Social Support in Young Adults. J. Sleep Res. 2022, 31, e13442. [Google Scholar] [CrossRef]
- Holmes, A.; Al-Bayat, S.; Hilditch, C.; Bourgeois-Bougrine, S. Sleep and Sleepiness during an Ultra Long-Range Flight Operation between the Middle East and United States. Accid. Anal. Prev. 2012, 45, 27–31. [Google Scholar] [CrossRef]
- García, A.; Papior, N.; Akhtar, A.; Artacho, E.; Blum, V.; Bosoni, E.; Brandimarte, P.; Brandbyge, M.; Cerdá, J.I.; Corsetti, F.; et al. Siesta: Recent Developments and Applications. J. Chem. Phys. 2020, 152, 204108. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Morosoli, J.J.; Madrid, J.A.; Garaulet, M.; Ordoñana, J.R. Heritability of Siesta and Night-Time Sleep as Continuously Assessed by a Circadian-Related Integrated Measure. Sci. Rep. 2017, 7, 12340. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Lane, J.M.; Wood, A.R.; van Hees, V.T.; Tyrrell, J.; Beaumont, R.N.; Jeffries, A.R.; Dashti, H.S.; Hillsdon, M.; Ruth, K.S.; et al. Genome-Wide Association Analyses of Chronotype in 697,828 Individuals Provides Insights into Circadian Rhythms. Nat. Commun. 2019, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, J.; Casanova, F.; Jones, S.E.; Hagenaars, S.P.; Beaumont, R.N.; Freathy, R.M.; Watkins, E.R.; Vetter, C.; Rutter, M.K.; Cain, S.W.; et al. Using Mendelian Randomisation Methods to Understand Whether Diurnal Preference Is Causally Related to Mental Health. Mol. Psychiatry 2021, 26, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Lee, J.; Asthana, K.; Vakil Monfared, R.; Chen, J.; Alhassen, S.; Samad, M.; Wood, M.; Mayer, E.A.; Baldi, P. The Hidden Link between Circadian Entropy and Mental Health Disorders. Transl. Psychiatry 2022, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Wang, H.; Liu, Z.; Yu, X.; Yan, J.; Yu, Y.; Kou, C.; Xu, X.; Lu, J.; et al. Prevalence of Mental Disorders in China: A Cross-Sectional Epidemiological Study. Lancet Psychiatry 2019, 6, 211–224. [Google Scholar] [CrossRef]
- Staley, J.R.; Blackshaw, J.; Kamat, M.A.; Ellis, S.; Surendran, P.; Sun, B.B.; Paul, D.S.; Freitag, D.; Burgess, S.; Danesh, J.; et al. PhenoScanner: A Database of Human Genotype-Phenotype Associations. Bioinformatics 2016, 32, 3207–3209. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An Expanded Tool for Searching Human Genotype-Phenotype Associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Davey Smith, G. Mendelian Randomization: Using Genes as Instruments for Making Causal Inferences in Epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Papadimitriou, N.; Dimou, N.; Tsilidis, K.K.; Banbury, B.; Martin, R.M.; Lewis, S.J.; Kazmi, N.; Robinson, T.M.; Albanes, D.; Aleksandrova, K.; et al. Physical Activity and Risks of Breast and Colorectal Cancer: A Mendelian Randomisation Analysis. Nat. Commun. 2020, 11, 597. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Avoiding Bias from Weak Instruments in Mendelian Randomization Studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust Inference in Summary Data Mendelian Randomization via the Zero Modal Pleiotropy Assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, S.; Tian, Y.; Si, H.; Zeng, Y.; Wu, Y.; Liu, Y.; Li, M.; Sun, K.; Wu, L.; et al. Genetic Causal Association between Iron Status and Osteoarthritis: A Two-Sample Mendelian Randomization. Nutrients 2022, 14, 3683. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the Causal Relationship between Imprecisely Measured Traits Using GWAS Summary Data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco M, F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A Framework for the Investigation of Pleiotropy in Two-sample Summary Data Mendelian Randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Brion, M.-J.A.; Shakhbazov, K.; Visscher, P.M. Calculating Statistical Power in Mendelian Randomization Studies. Int. J. Epidemiol. 2013, 42, 1497–1501. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base Platform Supports Systematic Causal Inference across the Human Phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Daghlas, I.; Lane, J.M.; Saxena, R.; Vetter, C. Genetically Proxied Diurnal Preference, Sleep Timing, and Risk of Major Depressive Disorder. JAMA Psychiatry 2021, 78, 903. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.L.; Norbury, R. Associations between Diurnal Preference, Impulsivity and Substance Use in a Young-Adult Student Sample. Chronobiol. Int. 2021, 38, 79–89. [Google Scholar] [CrossRef]
- Burgess, H.J.; Rizvydeen, M.; Kikyo, F.; Kebbeh, N.; Tan, M.; Roecklein, K.A.; Hasler, B.P.; King, A.C.; Cao, D. Sleep and Circadian Differences between Light and Heavy Adult Alcohol Drinkers. Alcohol. Clin. Exp. Res. 2022, 46, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Obayashi, K.; Saeki, K.; Fujita, K.; Iwata, N.; Kitajima, T. Higher Prevalence of Intentional Self-Harm in Bipolar Disorder with Evening Chronotype: A Finding from the APPLE Cohort Study. J. Affect. Disord. 2020, 277, 727–732. [Google Scholar] [CrossRef]
- Gonzalez, D.; Justin, H.; Reiss, S.; Faulkner, J.; Mahoney, H.; Yunus, A.; Gamsby, J.; Gulick, D. Circadian Rhythm Shifts and Alcohol Access in Adolescence Synergistically Increase Alcohol Preference and Intake in Adulthood in Male C57BL/6 Mice. Behav. Brain Res. 2023, 438, 114216. [Google Scholar] [CrossRef]
- Ozen, F.; Yegin, Z.; Saglam, Z.A.; Yavlal, F.; Koc, H.; Ulasoglu, C. Association Analysis of Epworth Sleepiness Scale (ESS) Scores with Serotonin Transporter (5-HTT-LPR, 5-HTT-VNTR) and Circadian (PER3-VNTR) Genes. Sleep Sci. 2022, 15, 110–115. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Zou, M.; Liu, J.; Zhao, H.; Wang, Y. Antidepressant Actions of Melatonin and Melatonin Receptor Agonist: Focus on Pathophysiology and Treatment. Behav. Brain Res. 2022, 420, 113724. [Google Scholar] [CrossRef]
- Sahbaz, C.; Özer, O.F.; Kurtulmus, A.; Kırpınar, I.; Sahin, F.; Guloksuz, S. Evidence for an Association of Serum Melatonin Concentrations with Recognition and Circadian Preferences in Patients with Schizophrenia. Metab. Brain Dis. 2019, 34, 865–874. [Google Scholar] [CrossRef]
- Melhuish Beaupre, L.M.; Brown, G.M.; Gonçalves, V.F.; Kennedy, J.L. Melatonin’s Neuroprotective Role in Mitochondria and Its Potential as a Biomarker in Aging, Cognition and Psychiatric Disorders. Transl. Psychiatry 2021, 11, 339. [Google Scholar] [CrossRef]
- Moon, E.; Partonen, T.; Beaulieu, S.; Linnaranta, O. Melatonergic Agents Influence the Sleep-Wake and Circadian Rhythms in Healthy and Psychiatric Participants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Neuropsychopharmacology 2022, 47, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Wehr, T.A.; Wirz-Justice, A.; Goodwin, F.K.; Duncan, W.; Gillin, J.C. Phase Advance of the Circadian Sleep-Wake Cycle as an Antidepressant. Science 1979, 206, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Morris, C.J.; Phillips, A.J.K.; Li, P.; Rahman, S.A.; Wang, W.; Hu, K.; Arendt, J.; Czeisler, C.A.; Scheer, F.A.J.L. Unanticipated Daytime Melatonin Secretion on a Simulated Night Shift Schedule Generates a Distinctive 24-h Melatonin Rhythm with Antiphasic Daytime and Nighttime Peaks. J. Pineal Res. 2022, 72, e12791. [Google Scholar] [CrossRef] [PubMed]
- Kholghi, G.; Eskandari, M.; Shokouhi Qare Saadlou, M.-S.; Zarrindast, M.-R.; Vaseghi, S. Night Shift Hormone: How Does Melatonin Affect Depression? Physiol. Behav. 2022, 252, 113835. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.; Kim, K.; Partonen, T.; Linnaranta, O. Role of Melatonin in the Management of Sleep and Circadian Disorders in the Context of Psychiatric Illness. Curr. Psychiatry Rep. 2022, 24, 623–634. [Google Scholar] [CrossRef]
- Evans, S.; Alkan, E.; Bhangoo, J.K.; Tenenbaum, H.; Ng-Knight, T. Effects of the COVID-19 Lockdown on Mental Health, Wellbeing, Sleep, and Alcohol Use in a UK Student Sample. Psychiatry Res. 2021, 298, 113819. [Google Scholar] [CrossRef]
- Chellappa, S.L. Circadian Misalignment: A Biological Basis for Mood Vulnerability in Shift Work. Eur. J. Neurosci. 2020, 52, 3846–3850. [Google Scholar] [CrossRef]
- Norbury, R. Diurnal Preference and Grey Matter Volume in a Large Population of Older Adults: Data from the UK Biobank. J. Circadian Rhythm. 2020, 18, 3. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Tan, D.-S.; Wang, X.; Ye, Z.; Xie, Z.; Zhang, D.; Wu, D.; Zhao, Y.; Qu, Y.; Jiang, Y. Exploring the Causal Association between Morning Diurnal Preference and Psychiatric Disorders: A Bidirectional Two-Sample Mendelian Randomization Analysis. Life 2024, 14, 1225. https://doi.org/10.3390/life14101225
Chen M, Tan D-S, Wang X, Ye Z, Xie Z, Zhang D, Wu D, Zhao Y, Qu Y, Jiang Y. Exploring the Causal Association between Morning Diurnal Preference and Psychiatric Disorders: A Bidirectional Two-Sample Mendelian Randomization Analysis. Life. 2024; 14(10):1225. https://doi.org/10.3390/life14101225
Chicago/Turabian StyleChen, Manman, Din-Son Tan, Xijie Wang, Zichen Ye, Zhilan Xie, Daqian Zhang, Dandan Wu, Yuankai Zhao, Yimin Qu, and Yu Jiang. 2024. "Exploring the Causal Association between Morning Diurnal Preference and Psychiatric Disorders: A Bidirectional Two-Sample Mendelian Randomization Analysis" Life 14, no. 10: 1225. https://doi.org/10.3390/life14101225
APA StyleChen, M., Tan, D.-S., Wang, X., Ye, Z., Xie, Z., Zhang, D., Wu, D., Zhao, Y., Qu, Y., & Jiang, Y. (2024). Exploring the Causal Association between Morning Diurnal Preference and Psychiatric Disorders: A Bidirectional Two-Sample Mendelian Randomization Analysis. Life, 14(10), 1225. https://doi.org/10.3390/life14101225





