Genome-Wide Identification of the Remorin Gene Family in Poplar and Their Responses to Abiotic Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of the PtREM Gene in P. trichocarpa
2.2. Bioinformatics Analysis
2.3. Phylogenetic and Sequence Analysis
2.4. Analysis of Cis-Acting Element
2.5. Gene Duplication Events Analysis
2.6. Analysis of Gene Expression
2.7. Co-Expression Network Analysis
3. Results
3.1. Identification and Physicochemical Properties of PtREM Members
3.2. Structural and Phylogenetic Analysis of PtREMs
3.3. Analysis of Gene Duplication Events
3.4. Tissue Specific Expression Analysis of PtREM Genes in P. trichocarpa
3.5. Cis-Acting Element Analysis
3.6. Co-Expression Network Analysis of PtREMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raffaele, S.; Mongrand, S.; Gamas, P.; Niebel, A.; Ott, T. Genome-wide annotation of remorins, a plant-specific protein family: Evolutionary and functional perspectives. Plant Physiol. 2007, 145, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Peskan, T.; Westermann, M.; Oelmüller, R. Identification of low-density Triton X-100-insoluble plasma membrane microdomains in higher plants. Eur. J. Biochem. 2000, 267, 6989–6995. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P.; Kunz, B.; PaulPletzer, K.; Grimm, R.; Eckerskorn, C.; Farmer, E.E. Cloning of a cDNA encoding a plasma membrane-associated, uronide binding phosphoprotein with physical properties similar to viral movement proteins. Plant Cell 1996, 8, 2265–2276. [Google Scholar] [PubMed]
- Bariola, P.A.; Retelska, D.; Stasiak, A.; Kammerer, R.A.; Fleming, A.; Hijri, M.; Franks, S.; Farmer, E.E. Remorins form a novel family of coiled coil-forming oligomeric and filamentous proteins associated with apical, vascular and embryonic tissues in plants. Plant Mol. Biol. 2004, 55, 579–594. [Google Scholar] [CrossRef]
- Cai, J.; Qin, G.; Chen, T.; Tian, S. The mode of action of remorin1 in regulating fruit ripening at transcriptional and post-transcriptional levels. New Phytol. 2018, 219, 1406–1420. [Google Scholar] [CrossRef]
- Raffaele, S.; Bayer, E.; Mongrand, S. Upregulation of the plant protein remorin correlates with dehiscence and cell maturation A link with the maturation of plasmodesmata? Plant Signal. Behav. 2009, 4, 915–919. [Google Scholar] [CrossRef]
- Son, S.; Oh, C.J.; Bae, J.-H.; Lee, H.; An, C.S. GmREM1.1 and GmREM2.1, which encode the remorin proteins in soybean, have distinct roles during root nodule development. J. Plant Biol. 2015, 58, 17–25. [Google Scholar] [CrossRef]
- Gui, J.; Zheng, S.; Liu, C.; Shen, J.; Li, J.; Li, L. OsREM4.1 Interacts with OsSERK1 to coordinate the interlinking between abscisic acid and brassinosteroid signaling in rice. Dev. Cell 2016, 38, 202–213. [Google Scholar] [CrossRef]
- Lefebvre, B.; Timmers, T.; Mbengue, M.; Moreau, S.; Herve, C.; Toth, K.; Bittencourt-Silvestre, J.; Klaus, D.; Deslandes, L.; Godiard, L.; et al. A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc. Natl. Acad. Sci. USA 2010, 107, 2343–2348. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, M.; Li, Y.; Zhao, Z.; Li, C.; Yue, J. Genome-wide characterization of Remorin genes in terms of their evolution and expression in response to hormone signals and abiotic stresses in foxtail millet (Setaria italica). Diversity 2022, 14, 711. [Google Scholar] [CrossRef]
- Checker, V.G.; Khurana, P. Molecular and functional characterization of mulberry EST encoding remorin (MiREM) involved in abiotic stress. Plant Cell Rep. 2013, 32, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Retelska, D.; Framer, E.E.; Fleming, A. Overexpression of remorin leads to cell defense and cell death in tobacco. Plant Biol. 2000, 2000, 32. [Google Scholar]
- Jamann, T.M.; Luo, X.; Morales, L.; Kolkman, J.M.; Chung, C.-L.; Nelson, R.J. A remorin gene is implicated in quantitative disease resistance in maize. Theor. Appl. Genet. 2016, 129, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Perraki, A.; Mongrand, S. The Remorin C-terminal Anchor was shaped by convergent evolution among membrane binding domains. Plant Signal. Behav. 2013, 8, e23207. [Google Scholar] [CrossRef] [PubMed]
- Perraki, A.; Cacas, J.-L.; Crowet, J.-M.; Lins, L.; Castroviejo, M.; German-Retana, S.; Mongrand, S.; Raffaele, S. Plasma membrane localization of Solanum tuberosum Remorin from Group 1, homolog 3 is mediated by conformational changes in a novel C-terminal anchor and required for the restriction of potato virus X movement. Plant Physiol. 2012, 160, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Legrand, A.; Gronnier, J.; Decossas, M.; Gouguet, P.; Lambert, O.; Berbon, M.; Verron, L.; Grelard, A.; Germain, V.; et al. Coiled-coil oligomerization controls localization of the plasma membrane REMORINs. J. Struct. Biol. 2019, 206, 12–19. [Google Scholar] [CrossRef]
- Gronnier, J.; Crowet, J.-M.; Habenstein, B.; Nasir, M.N.; Bayle, V.; Hosy, E.; Platre, M.P.; Gouguet, P.; Raffaele, S.; Martinez, D.; et al. Structural basis for plant plasma membrane protein dynamics and organization into functional nanodomains. Elife 2017, 6, e26404. [Google Scholar] [CrossRef]
- Toth, K.; Stratil, T.F.; Madsen, E.B.; Ye, J.; Popp, C.; Antolin-Llovera, M.; Grossmann, C.; Jensen, O.N.; Schuessler, A.; Parniske, M.; et al. Functional domain analysis of the remorin protein LjSYMREM1 in Lotus japonicus. PLoS ONE 2012, 7, e30817. [Google Scholar] [CrossRef]
- Marin, M.; Ott, T. Phosphorylation of intrinsically disordered regions in remorin proteins. Front. Plant Sci. 2012, 3, 86. [Google Scholar] [CrossRef]
- Konrad, S.S.A.; Popp, C.; Stratil, T.F.; Jarsch, I.K.; Thallmair, V.; Folgmann, J.; Marin, M.; Ott, T. S-acylation anchors remorin proteins to the plasma membrane but does not primarily determine their localization in membrane microdomains. New Phytol. 2014, 203, 758–769. [Google Scholar] [CrossRef]
- Legrand, A.; G-Cava, D.; Jolivet, M.-D.; Decossas, M.; Lambert, O.; Bayle, V.; Jaillais, Y.; Loquet, A.; Germain, V.; Boudsocq, M.; et al. Structural determinants of REMORIN nanodomain formation in anionic membranes. Biophys. J. 2023, 122, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Liu, C.; Shen, J.; Li, L. Grain setting defect1, encoding a remorin protein, affects the grain setting in rice through regulating plasmodesmatal conductance. Plant Physiol. 2014, 166, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Zheng, S.; Shen, J.; Li, L. Grain setting defect1 (GSD1) function in rice depends on S-acylation and interacts with actin 1 (OsACT1) at its C-terminal. Front. Plant Sci. 2015, 6, 804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Yang, Z.; Zhang, H.; Zhang, J.; Xu, J. Remorin interacting with PCaP1 impairs Turnip mosaic virus intercellular movement but is antagonised by VPg. New Phytol. 2020, 225, 2122–2139. [Google Scholar] [CrossRef]
- Ma, T.; Fu, S.; Wang, K.; Wang, Y.; Wu, J.; Zhou, X. Palmitoylation is indispensable for remorin to restrict tobacco mosaic virus cell-to-cell movement in Nicotiana benthamiana. Viruses 2022, 14, 1324. [Google Scholar] [CrossRef]
- Huang, D.; Sun, Y.; Ma, Z.; Ke, M.; Cui, Y.; Chen, Z.; Chen, C.; Ji, C.; Tuan Minh, T.; Yang, L.; et al. Salicylic acid-mediated plasmodesmal closure via Remorin-dependent lipid organization. Proc. Natl. Acad. Sci. USA 2020, 117, 8659. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Zhang, F.; Gan, P.; Li, M.; Wang, C.; Li, H.; Gao, G.; Wang, X.; Kang, Z.; et al. CaREM1.4 interacts with CaRIN4 to regulate Ralstonia solanacearum tolerance by triggering cell death in pepper. Hortic. Res. 2023, 10, uhad053. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Wang, Y.; Qin, G.; Tian, S. SlREM1 triggers cell death by activating an oxidative burst and other regulators. Plant Physiol. 2020, 183, 717–732. [Google Scholar] [CrossRef]
- Yu, Y. Remorins: Essential regulators in plant-microbe interaction and cell death induction. Plant Physiol. 2020, 183, 435–436. [Google Scholar] [CrossRef]
- Su, C.; Rodriguez-Franco, M.; Lace, B.; Nebel, N.; Hernandez-Reyes, C.; Liang, P.; Schulze, E.; Mymrikov, E.V.; Gross, N.M.; Knerr, J.; et al. Stabilization of membrane topologies by proteinaceous remorin scaffolds. Nat. Commun. 2023, 14, 323. [Google Scholar] [CrossRef]
- Dimitriou, I.; Mola-Yudego, B. Impact of Populus plantations on water and soil quality. Bioenergy Res. 2017, 10, 750–759. [Google Scholar] [CrossRef]
- Wood, J.; Aryal, N.; Subedi, K. Wastewater treatment using poplar plants: Processes. Water 2023, 15, 3812. [Google Scholar] [CrossRef]
- Fengmei, D.; Fadian, L.U.; Zhanyong, H.O.U.; Yan, G.A.O.; Kezhi, L.U. Analysis on the economic mature age and economic benefit of fast- growing and high -yield plantation of poplar. J. Shandong Agric. University. Nat. Sci. 2008, 39, 233–238,250. [Google Scholar]
- Lu, Y.; Zheng, B.; Zhang, C.; Yu, C.; Luo, J. Wood formation in trees responding to nitrogen availability. Ind. Crops Prod. 2024, 218, 118978. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J. Growth performance, photosynthesis, and root characteristics are associated with nitrogen use efficiency in six poplar species. Environ. Exp. Bot. 2019, 164, 40–51. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J.; Masclaux-Daubresse, C.; Wang, N.; Wang, H.; Zheng, B. Morphological and physiological responses to contrasting nitrogen regimes in Populus cathayana is linked to resources allocation and carbon/nitrogen partition. Environ. Exp. Bot. 2019, 162, 247–255. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Li, Z.; Wang, Z.; Cao, X.; Wang, N. Haplotype-resolved genome assembly of poplar line NL895 provides a valuable tree genomic resource. For. Res. 2024, 4, e015. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.; Wang, X.; Wang, S.; Zhang, T.; Zhou, Y.; He, R.; Meng, N.; Wang, Y.; Liu, W. Chromosome-level genome assembly of a triploid poplar Populus alba ‘Berolinensis’. Mol. Ecol. Resour. 2023, 23, 1092–1107. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Guan, L.; Li, Z.; Wang, H.; Luo, J. Optimization of high-efficiency tissue culture regeneration systems in gray poplar. Life 2023, 13, 1896. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kumar, P.; Parmar, N.; Shandil, R.K.; Aggarwal, G.; Gaur, A.; Srivastava, D.K. Achievements and prospects of genetic engineering in poplar: A review. New For. 2021, 52, 889–920. [Google Scholar] [CrossRef]
- Kang, X.; Wei, H. Breeding polyploid Populus: Progress and perspective. For. Res. 2022, 2, 4. [Google Scholar] [CrossRef]
- Li, S.; Su, X.; Zhang, B.; Huang, Q.; Hu, Z.; Lu, M. Molecular cloning and functional analysis of the Populus deltoides remorin gene PdREM. Tree Physiol. 2013, 33, 1111–1121. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, H.; Yao, J.; Zhang, Y.; Zhao, N.; Zhao, R.; Zhou, X.; Chen, S. Populus euphratica PeREM6.5 regulating tolerance mechanism to water stress in Arabidopsis thaliana. J. Beijing For. Univ. 2022, 44, 40–51. [Google Scholar]
- Luo, J.; Liang, Z.; Wu, M.; Mei, L. Genome-wide identification of BOR genes in poplar and their roles in response to various environmental stimuli. Environ. Exp. Bot. 2019, 164, 101–113. [Google Scholar] [CrossRef]
- Mu, X.; Luo, J. Evolutionary analyses of NIN-like proteins in plants and their roles in nitrate signaling. Cell. Mol. Life Sci. 2019, 76, 3753–3764. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, 11335. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–12029. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Filichkin, S.A.; Hamilton, M.; Dharmawardhana, P.D.; Singh, S.K.; Sullivan, C.; Ben-Hur, A.; Reddy, A.S.; Jaiswal, P. Abiotic stresses modulate landscape of poplar transcriptome via alternative splicing, differential intron retention, and isoform ratio switching. Front. Plant Sci. 2018, 9, 5. [Google Scholar] [CrossRef]
- Li, Z.; Guan, L.; Zhang, C.; Zhang, S.; Liu, Y.; Lu, Y.; Luo, J. Nitrogen assimilation genes in poplar: Potential targets for improving tree nitrogen use efficiency. Ind. Crops Prod. 2024, 216, 118705. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R package 2015, 1, 790. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xia, W.; Cao, P.; Xiao, Z.a.; Zhang, Y.; Liu, M.; Zhan, C.; Wang, N. Integrated transcriptome analysis reveals plant hormones jasmonic acid and salicylic acid coordinate growth and defense responses upon fungal infection in poplar. Biomolecules 2019, 9, 12. [Google Scholar] [CrossRef]
- Luo, J.; Nvsvrot, T.; Wang, N. Comparative transcriptomic analysis uncovers conserved pathways involved in adventitious root formation in poplar. Physiol. Mol. Biol. Plants 2021, 27, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, J.; Liu, Y.; Li, D.; Xu, X.; Zhu, Z.; Xu, X.; Zhan, R.; Zhang, H.; Wang, L. Genome-wide characterization of Remorin gene family and their responsive expression to abiotic stresses and plant hormone in Brassica napus. Plant Cell Rep. 2024, 43, 155. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, G.; Yu, Q.; Jiao, W.; Zeng, K.; Luo, T.; Zhang, H.; Shang, H.; Huang, G.; Wang, F.; et al. Identification and characterization of the Remorin gene family in Saccharum and the involvement of ScREM1.5e-1/-2 in SCMV infection on sugarcane. Front. Plant Sci. 2024, 15, 1365995. [Google Scholar] [CrossRef]
- Marin, M.; Thallmair, V.; Ott, T. The intrinsically disordered N-terminal region of AtREM1.3 remorin protein mediates protein-protein interactions. J. Biol. Chem. 2012, 287, 39982–39991. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Cao, B.; Qin, G.; Wang, W.; Tian, S. Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 2012, 43, 2469–2480. [Google Scholar] [CrossRef]
- Luo, J.; Shi, W.; Li, H.; Janz, D.; Luo, Z.-B. The conserved salt-responsive genes in the roots of Populus × canescens and Arabidopsis thaliana. Environ. Exp. Bot. 2016, 129, 48–56. [Google Scholar] [CrossRef]
- Yue, J.; Li, C.; Liu, Y.; Yu, J. A remorin gene SiREM6, the target gene of SiARDP, from foxtail millet (Setaria italica) promotes high salt tolerance in transgenic Arabidopsis. PLoS ONE 2014, 9, e100772. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, C.; Wu, X.; Yao, J.; Zhang, Y.; Zhang, Y.; Deng, S.; Zhao, N.; Zhao, R.; Zhou, X.; et al. Populus euphratica remorin 6.5 activates plasma membrane H+-ATPases to mediate salt tolerance. Tree Physiol. 2020, 40, 731–745. [Google Scholar] [CrossRef]
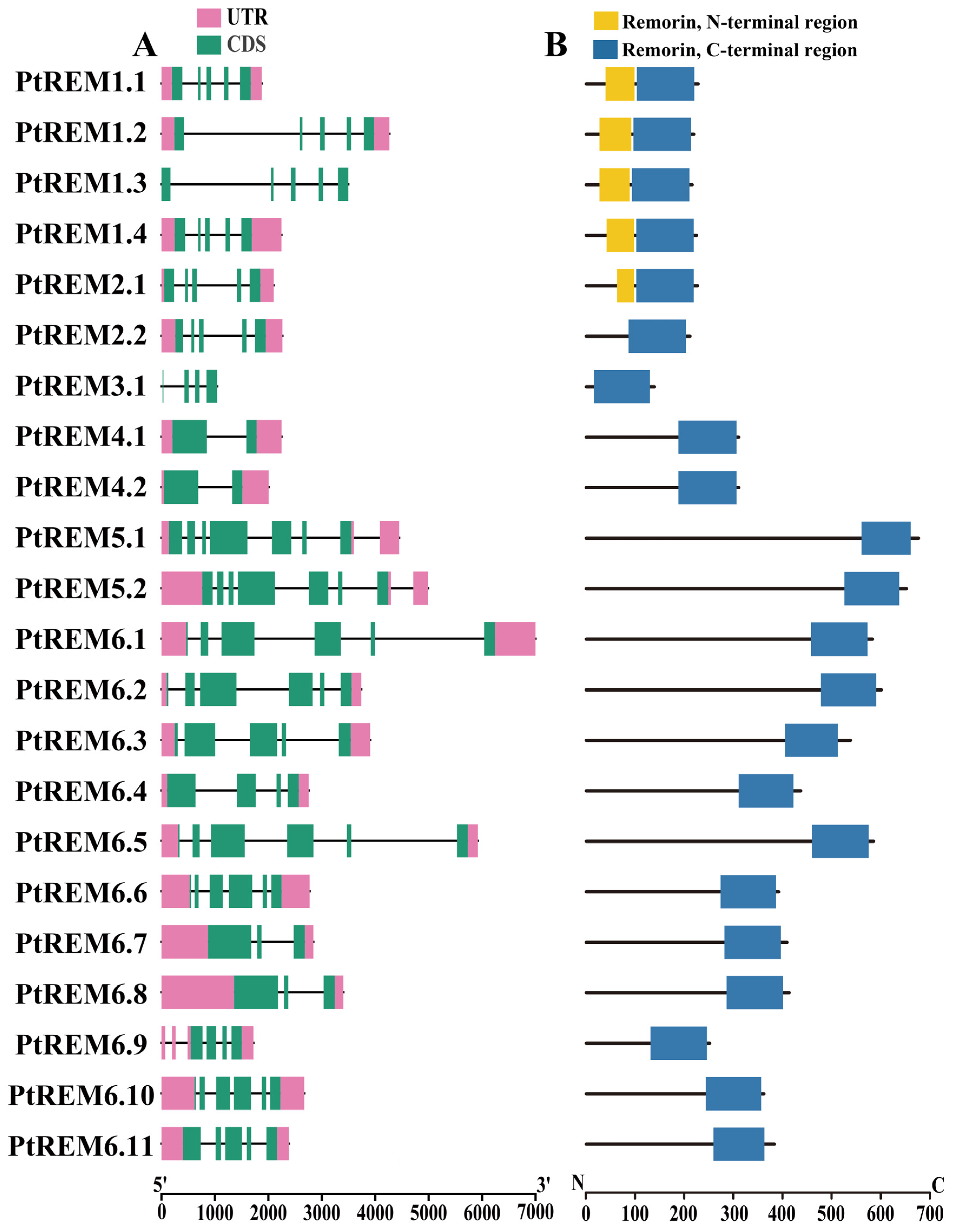


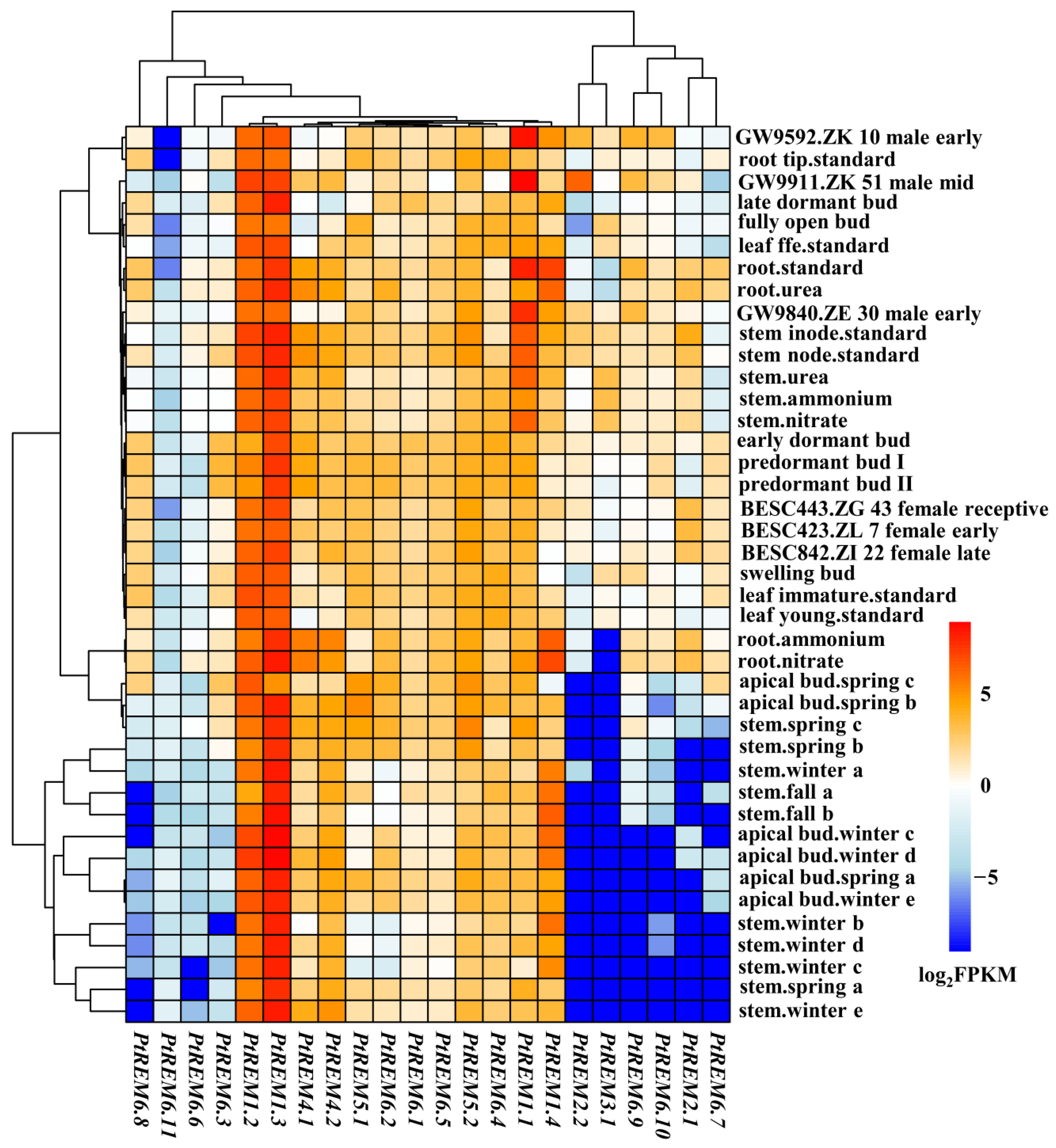
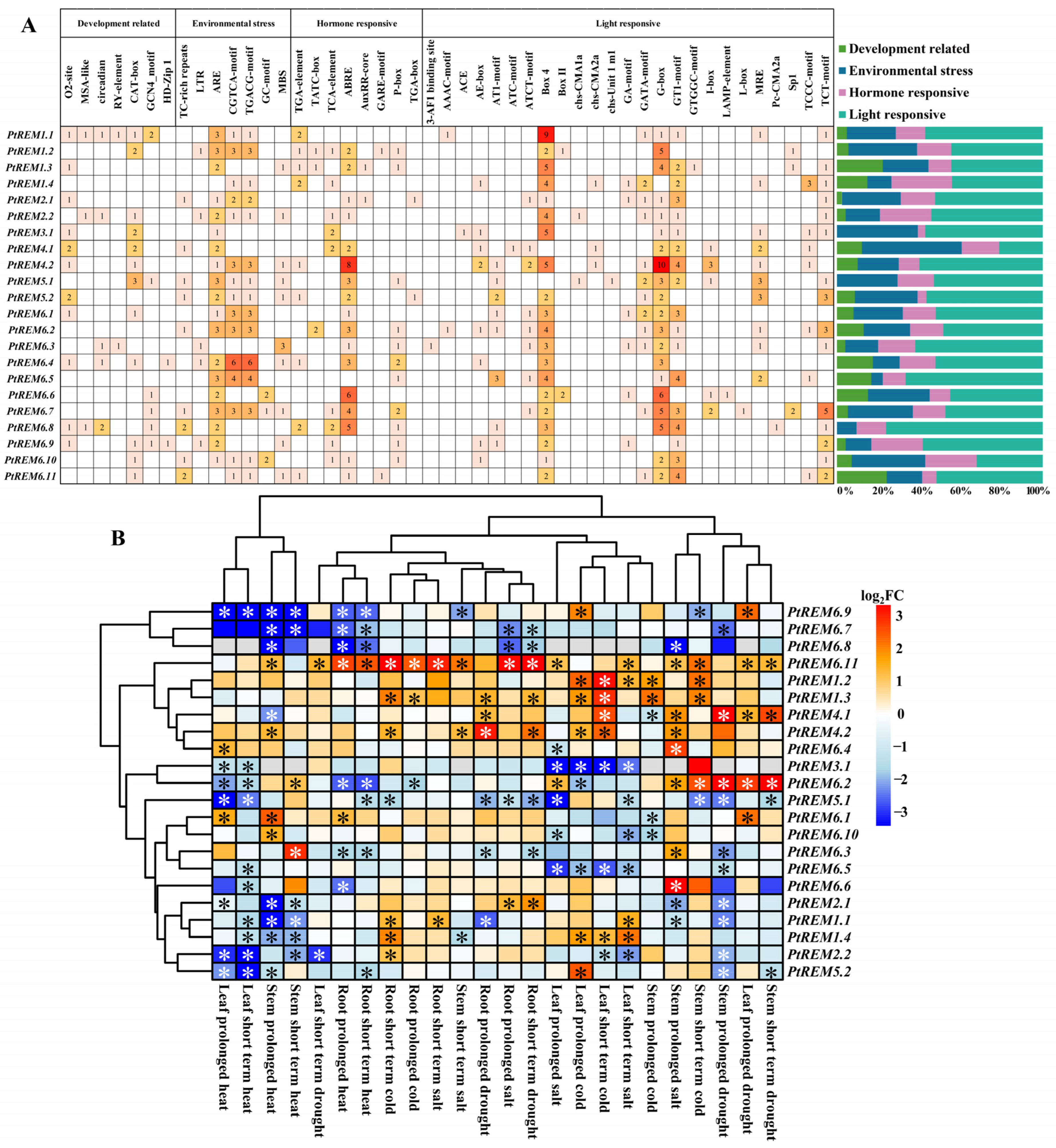

| Gene Name | Gene Model | Protein (aa) | MW (kDa) | Isoelectric Point | Subcellular Localization | Chromosome Position |
|---|---|---|---|---|---|---|
| PtREM1.1 | Potri.012G140800 | 204 | 22.71 | 6.00 | Cell membrane | Chr12:15283828-15285704 |
| PtREM1.2 | Potri.002G157700 | 196 | 21.31 | 9.13 | Cell membrane | Chr02:12028474-12032739 |
| PtREM1.3 | Potri.014G081300 | 193 | 21.49 | 6.70 | Cell membrane | Chr14:5248038-5251534 |
| PtREM1.4 | Potri.015G143600 | 201 | 22.23 | 5.09 | Cell membrane | Chr15:14900244-14902489 |
| PtREM2.1 | Potri.001G107000 | 203 | 22.71 | 8.94 | Cell membrane | Chr01:8599505-8601605 |
| PtREM2.2 | Potri.003G124400 | 189 | 21.05 | 7.69 | Cell membrane | Chr03:14441701-14443964 |
| PtREM3.1 | Potri.012G140900 | 124 | 14.47 | 9.15 | Cell membrane, Nucleus | Chr12:15289364-15290406 |
| PtREM4.1 | Potri.006G053200 | 278 | 30.55 | 8.78 | Cell membrane, Nucleus | Chr06:3708285-3710532 |
| PtREM4.2 | Potri.016G054400 | 278 | 30.86 | 6.74 | Cell membrane, Nucleus | Chr16:3577080-3579086 |
| PtREM5.1 | Potri.002G125200 | 606 | 66.83 | 9.64 | Cell membrane, Nucleus | Chr02:9533038-9537486 |
| PtREM5.2 | Potri.014G027900 | 584 | 64.13 | 9.94 | Cell membrane, Nucleus | Chr14:1748284-1753282 |
| PtREM6.1 | Potri.008G144300 | 522 | 57.73 | 9.20 | Nucleus | Chr08:9793769-9800768 |
| PtREM6.2 | Potri.001G358600 | 538 | 60.56 | 8.24 | Nucleus | Chr01:37441084-37444823 |
| PtREM6.3 | Potri.001G163000 | 482 | 53.83 | 8.20 | Cell membrane, Nucleus | Chr01:13838091-13841996 |
| PtREM6.4 | Potri.005G138500 | 391 | 42.86 | 9.66 | Cell membrane, Nucleus | Chr05:10893174-10895929 |
| PtREM6.5 | Potri.010G098000 | 524 | 58.07 | 9.20 | Nucleus | Chr10:12130727-12136648 |
| PtREM6.6 | Potri.008G093300 | 351 | 39.07 | 9.83 | Cell membrane, Nucleus | Chr08:5824430-5827202 |
| PtREM6.7 | Potri.008G178300 | 366 | 40.61 | 9.03 | Cell membrane, Nucleus | Chr08:12321723-12324565 |
| PtREM6.8 | Potri.010G056800 | 370 | 41.01 | 9.04 | Cell membrane, Nucleus | Chr10:8762054-8765457 |
| PtREM6.9 | Potri.015G049700 | 225 | 26.26 | 10.10 | Cell membrane, Nucleus | Chr15:5217249-5218970 |
| PtREM6.10 | Potri.010G160900 | 324 | 36.42 | 10.00 | Cell membrane, Nucleus | Chr10:16663990-16669562 |
| PtREM6.11 | Potri.014G058900 | 343 | 38.57 | 9.51 | Cell membrane, Nucleus | Chr14:3792409-3794792 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, H.; Li, C.; Liu, H.; Luo, J. Genome-Wide Identification of the Remorin Gene Family in Poplar and Their Responses to Abiotic Stresses. Life 2024, 14, 1239. https://doi.org/10.3390/life14101239
Li Z, Wang H, Li C, Liu H, Luo J. Genome-Wide Identification of the Remorin Gene Family in Poplar and Their Responses to Abiotic Stresses. Life. 2024; 14(10):1239. https://doi.org/10.3390/life14101239
Chicago/Turabian StyleLi, Zihui, Hang Wang, Chuanqi Li, Huimin Liu, and Jie Luo. 2024. "Genome-Wide Identification of the Remorin Gene Family in Poplar and Their Responses to Abiotic Stresses" Life 14, no. 10: 1239. https://doi.org/10.3390/life14101239






