Bojungikki-Tang Augments Pembrolizumab Efficacy in Human PBMC-Injected H460 Tumor-Bearing Mice
Abstract
:1. Introduction
2. Materials and Methods
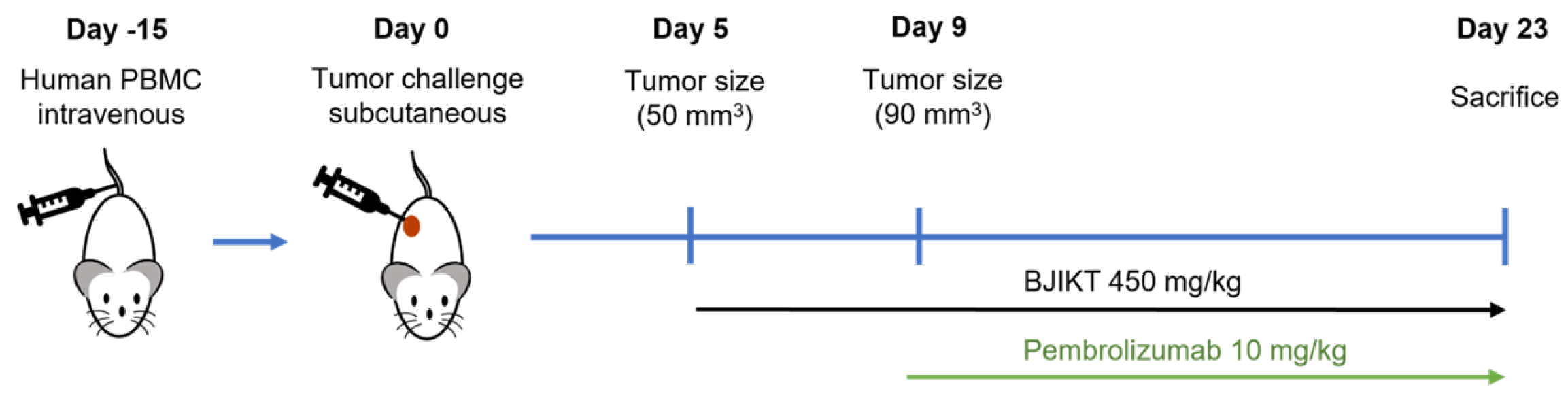
2.1. Animals
2.2. Chemicals and Reagents
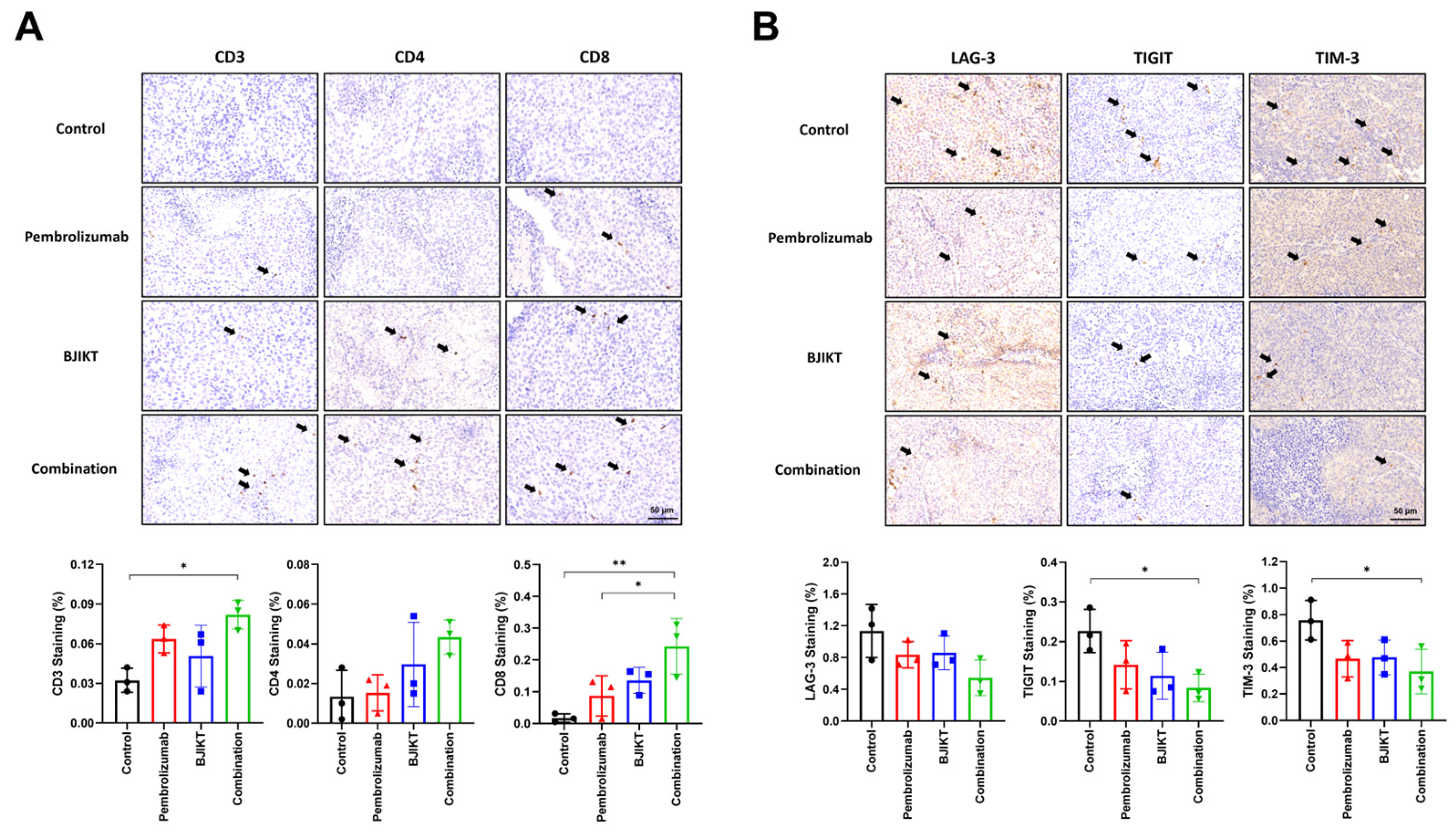
2.3. Immunohistochemistry (IHC) and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining
2.4. RNA Isolation for the RNA-seq Data
2.5. Library Preparation and Sequencing of the RNA-seq Data
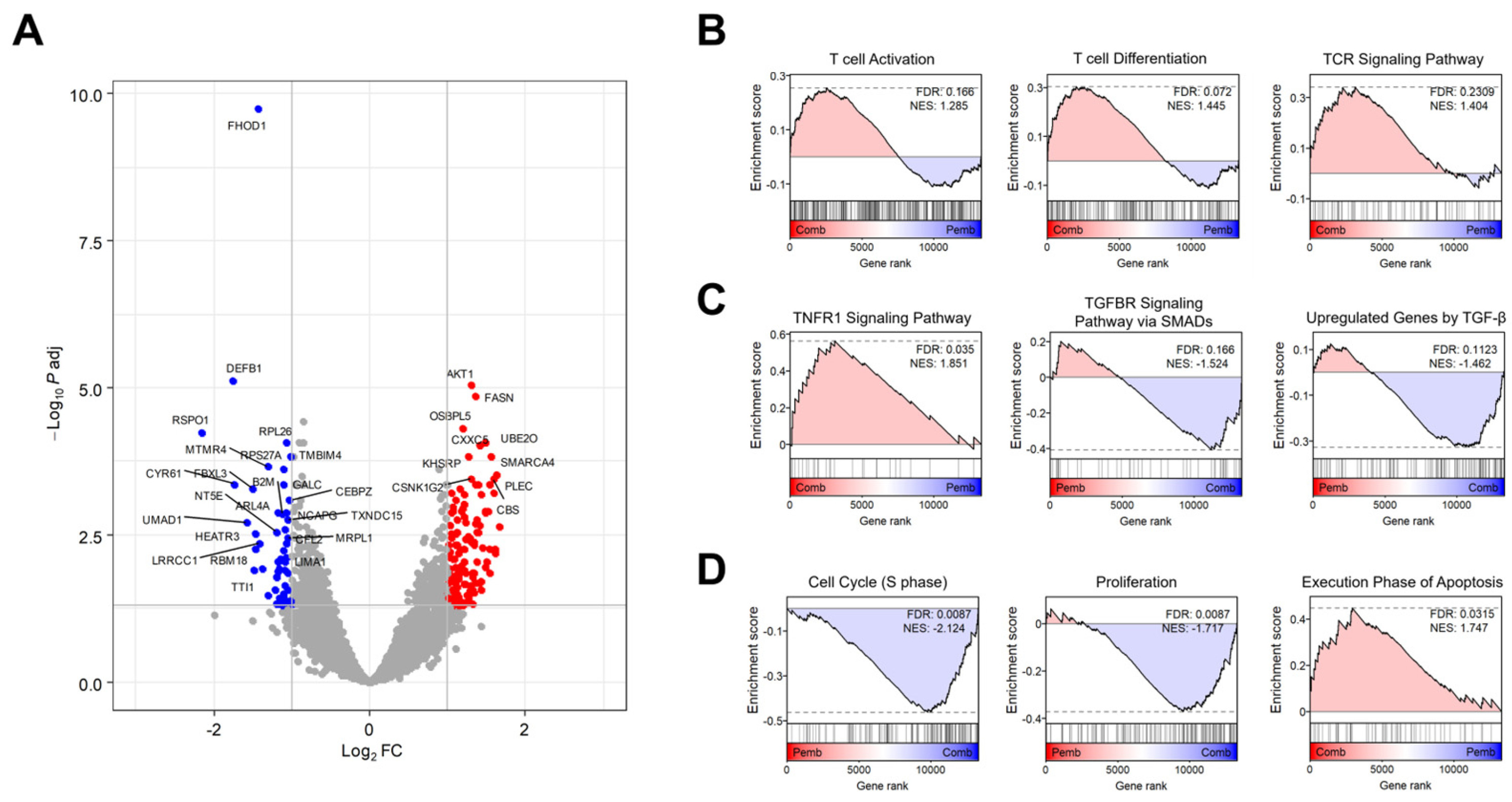
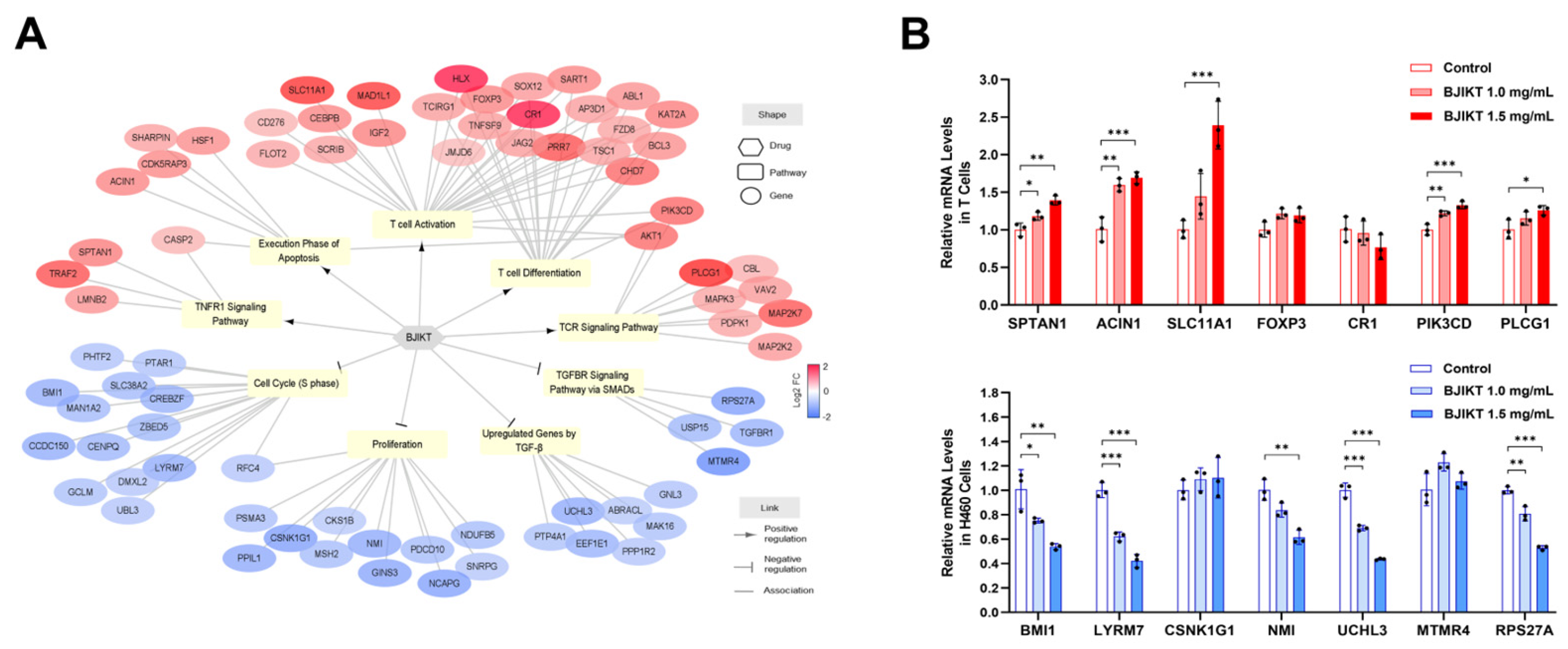
2.6. Transcriptome Analysis of the RNA-seq Data
2.7. Cell Culture
2.8. Quantitative PCR
2.9. Statistical Analysis
3. Results
3.1. Combination of BJIKT and Pembrolizumab Inhibited Tumor Growth in Human PBMC-Injected H460 Tumor-Bearing MHC I/II dKO NSG Mice
3.2. BJIKT and Pembrolizumab Combination Enhanced Immune Cell Infiltration and Reduced T Cell Exhaustion Markers
3.3. Transcriptome Analysis Revealed the Target Signaling Pathways of BJIKT in NSCLC Treatment
3.4. Network Analysis Regarding the Molecular Target of BJIKT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BJIKT | Bojungikki-Tang |
| cDNA | Complementary DNA |
| DEGs | Differentially expressed genes |
| dKO | double knockout |
| FC | Fold-change |
| GSEA | Gene Set Enrichment Analysis |
| ICIs | Immune checkpoint inhibitors |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| LAG-3 | Lymphocyte activation gene-3 |
| NES | Normalized enrichment scores |
| NSCLC | Non-small-cell lung cancer |
| PBMC | Peripheral blood mononuclear cells |
| PD-1 | Programmed death 1 |
| SD | Standard deviation |
| TGF-β | Transforming growth factor-β |
| TIGIT | T cell immunoreceptor with immunoglobulin and ITIM domain |
| TIM-3 | T cell immunoglobulin and mucin domain-containing-3 |
| TME | Tumor microenvironment |
| TNF | Tumor necrosis factor |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP Nick End Labeling |
References
- Bajbouj, K.; Al-Ali, A.; Ramakrishnan, R.K.; Saber-Ayad, M.; Hamid, Q. Histone Modification in NSCLC: Molecular Mechanisms and Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 11701. [Google Scholar] [CrossRef] [PubMed]
- Edahiro, Y.; Koike, M.; Nojiri, S.; Harada, Y.; Gotoh, A.; Fujibayashi, K.; Nishizaki, Y.; Yanagisawa, N.; Takaku, T.; Nitta, H.; et al. A pilot study examining the efficacy of hochuekkito for improving quality of life in patients with myeloproliferative neoplasms. Jpn. J. Clin. Oncol. 2022, 52, 880–886. [Google Scholar] [CrossRef]
- Yoneda, K.; Imanishi, N.; Ichiki, Y.; Tanaka, F. Immune Checkpoint Inhibitors (ICIs) in Non-Small Cell Lung Cancer (NSCLC). J. UOEH 2018, 40, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Chyuan, I.T.; Chu, C.L.; Hsu, P.N. Targeting the Tumor Microenvironment for Improving Therapeutic Effectiveness in Cancer Immunotherapy: Focusing on Immune Checkpoint Inhibitors and Combination Therapies. Cancers 2021, 13, 1188. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Kim, K.H. Effects of Bu-Zhong-Yi-Qi-Tang for the treatment of functional dyspepsia: A feasibility study protocol. Integr. Med. Res. 2017, 6, 317–324. [Google Scholar] [CrossRef]
- Amitani, M.; Amitani, H.; Sloan, R.A.; Suzuki, H.; Sameshima, N.; Asakawa, A.; Nerome, Y.; Owaki, T.; Inui, A.; Hoshino, E. The translational aspect of complementary and alternative medicine for cancer with particular emphasis on Kampo. Front. Pharmacol. 2015, 6, 150. [Google Scholar] [CrossRef]
- Qi, F.; Zhao, L.; Zhou, A.; Zhang, B.; Li, A.; Wang, Z.; Han, J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci. Trends 2015, 9, 16–34. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, H.; Li, C.; Cai, P.; Qi, F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci. Trends 2021, 15, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Liu, Y.; Tan, W.; Xiao, Y.; Yu, K.; Sun, X.; Huang, Y.; Cheng, J.; Luo, R.; Zhao, X. Bu-zhong-yi-qi pill alleviate the chemotherapy-related fatigue in 4 T1 murine breast cancer model. BMC Complement. Altern. Med. 2014, 14, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Fukasawa, I.; Furuno, M.; Inaba, F.; Yamazaki, T.; Kamemori, T.; Kousaka, N.; Ota, Y.; Hayashi, M.; Maehama, T.; et al. Inhibitory effects of herbal drugs on the growth of human ovarian cancer cell lines through the induction of apoptosis. Gynecol. Oncol. 2005, 97, 405–409. [Google Scholar] [CrossRef]
- Ahn, L.; Park, S.W.; Choi, D.J. Bojungikgi-tang for Chemotherapy-induced Leukopenia: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2024, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kang, H.N.; Yi, J.-M.; Hong, S.H.; Park, S.-M.; Jeong, M.-K. Bojungikki-Tang Enhances the Effect of PD-1 Blockade in a Syngeneic Murine Model of Lung Carcinoma. Processes 2022, 10, 1683. [Google Scholar] [CrossRef]
- Chun, J.; Park, S.M.; Yi, J.M.; Ha, I.J.; Kang, H.N.; Jeong, M.K. Bojungikki-Tang Improves Response to PD-L1 Immunotherapy by Regulating the Tumor Microenvironment in MC38 Tumor-Bearing Mice. Front. Pharmacol. 2022, 13, 901563. [Google Scholar] [CrossRef] [PubMed]
- Cogels, M.M.; Rouas, R.; Ghanem, G.E.; Martinive, P.; Awada, A.; Van Gestel, D.; Krayem, M. Humanized Mice as a Valuable Pre-Clinical Model for Cancer Immunotherapy Research. Front. Oncol. 2021, 11, 784947. [Google Scholar] [CrossRef]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Ishola, A.A.; Chien, C.S.; Yang, Y.P.; Chien, Y.; Yarmishyn, A.A.; Tsai, P.H.; Chen, J.C.; Hsu, P.K.; Luo, Y.H.; Chen, Y.M.; et al. Oncogenic circRNA C190 Promotes Non-Small Cell Lung Cancer via Modulation of the EGFR/ERK Pathway. Cancer Res. 2022, 82, 75–89. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Xia, H.; Yan, Y.; Zhu, X.; Sun, F.; Sun, L.; Li, S.; Li, D.; Wang, J.; et al. Tumor microenvironment remodeling after neoadjuvant immunotherapy in non-small cell lung cancer revealed by single-cell RNA sequencing. Genome Med. 2023, 15, 14. [Google Scholar] [CrossRef]
- Sun, D.; Liu, J.; Zhou, H.; Shi, M.; Sun, J.; Zhao, S.; Chen, G.; Zhang, Y.; Zhou, T.; Ma, Y.; et al. Classification of Tumor Immune Microenvironment According to Programmed Death-Ligand 1 Expression and Immune Infiltration Predicts Response to Immunotherapy Plus Chemotherapy in Advanced Patients With NSCLC. J. Thorac. Oncol. 2023, 18, 869–881. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Zhu, J.; Zhang, Y.; Yang, R.; Yan, J.; Huang, R.; Zheng, C.; Xiao, W.; Huang, C.; et al. Predicting the herbal medicine triggering innate anti-tumor immunity from a system pharmacology perspective. Biomed. Pharmacother. 2021, 143, 112105. [Google Scholar] [CrossRef]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, L.; Ni, H.; Shen, L.; Wei, J.; Xia, Y.; Yang, J.; Yang, M.; Zhao, Z. Prediction of CD3 T cells and CD8 T cells expression levels in non-small cell lung cancer based on radiomic features of CT images. Front. Oncol. 2023, 13, 1104316. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, J.; Liu, M.; Xing, H.; Wang, Z.; Huang, L.; Mellor, A.L.; Wang, W.; Wu, S. Adoptive CD8+ T cell therapy against cancer:Challenges and opportunities. Cancer Lett. 2019, 462, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, Y.; Liu, L.; Lv, Y.; Yu, W.; Liu, T.; Wang, L.; Mu, D.; Zhou, Q.; Liu, M.; et al. CD4+ T cells are required to improve the efficacy of CIK therapy in non-small cell lung cancer. Cell Death Dis. 2022, 13, 441. [Google Scholar] [CrossRef]
- Menon, A.P.; Moreno, B.; Meraviglia-Crivelli, D.; Nonatelli, F.; Villanueva, H.; Barainka, M.; Zheleva, A.; van Santen, H.M.; Pastor, F. Modulating T Cell Responses by Targeting CD3. Cancers 2023, 15, 1189. [Google Scholar] [CrossRef]
- Schulze, A.B.; Evers, G.; Gorlich, D.; Mohr, M.; Marra, A.; Hillejan, L.; Rehkamper, J.; Schmidt, L.H.; Heitkotter, B. Tumor infiltrating T cells influence prognosis in stage I-III non-small cell lung cancer. J. Thorac. Dis. 2020, 12, 1824–1842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; Zhou, N.; Wang, Y.; Zhang, X. Changes in T lymphocyte subsets predict the efficacy of atezolizumab in advanced non-small cell lung cancer: A retrospective study. J. Thorac. Dis. 2023, 15, 5669–5679. [Google Scholar] [CrossRef]
- Chi, A.; He, X.; Hou, L.; Nguyen, N.P.; Zhu, G.; Cameron, R.B.; Lee, J.M. Classification of Non-Small Cell Lung Cancer’s Tumor Immune Micro-Environment and Strategies to Augment Its Response to Immune Checkpoint Blockade. Cancers 2021, 13, 2924. [Google Scholar] [CrossRef] [PubMed]
- Jackute, J.; Zemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Bajoriunas, V.; Lavinskiene, S.; Sakalauskas, R. The prognostic influence of tumor infiltrating Foxp3+CD4+, CD4+ and CD8+ T cells in resected non-small cell lung cancer. J. Inflamm. 2015, 12, 63. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef] [PubMed]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- Kim, S.A.; Kang, O.H.; Kwon, D.Y. Cryptotanshinone Induces Cell Cycle Arrest and Apoptosis of NSCLC Cells through the PI3K/Akt/GSK-3beta Pathway. Int. J. Mol. Sci. 2018, 19, 2739. [Google Scholar] [CrossRef]
- Sak, A.; Wurm, R.; Elo, B.; Grehl, S.; Pottgen, C.; Stuben, G.; Sinn, B.; Wolf, G.; Budach, V.; Stuschke, M. Increased radiation-induced apoptosis and altered cell cycle progression of human lung cancer cell lines by antisense oligodeoxynucleotides targeting p53 and p21(WAF1/CIP1). Cancer Gene Ther. 2003, 10, 926–934. [Google Scholar] [CrossRef]
- Liu, G.; Pei, F.; Yang, F.; Li, L.; Amin, A.D.; Liu, S.; Buchan, J.R.; Cho, W.C. Role of Autophagy and Apoptosis in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2017, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.J.; Moore, J.; Song, J.; Tsiani, E.L. Inhibition of Non-Small Cell Lung Cancer Proliferation and Survival by Rosemary Extract Is Associated with Activation of ERK and AMPK. Life 2021, 12, 52. [Google Scholar] [CrossRef]
- Sheng, H.; Lv, W.; Zhu, L.; Wang, L.; Wang, Z.; Han, J.; Hu, J. Liriopesides B induces apoptosis and cell cycle arrest in human non-small cell lung cancer cells. Int. J. Mol. Med. 2020, 46, 1039–1050. [Google Scholar] [CrossRef]
- Kumari, R.; Feuer, G.; Bourré, L. Humanized Mouse Models for Immuno-oncology Drug Discovery. Curr. Protoc. 2023, 3, e852. [Google Scholar] [CrossRef]
- Johnson, A.M.; Boland, J.M.; Wrobel, J.; Klezcko, E.K.; Weiser-Evans, M.; Hopp, K.; Heasley, L.; Clambey, E.T.; Jordan, K.; Nemenoff, R.A.; et al. Cancer Cell-Specific Major Histocompatibility Complex II Expression as a Determinant of the Immune Infiltrate Organization and Function in the NSCLC Tumor Microenvironment. J. Thorac. Oncol. 2021, 16, 1694–1704. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, S.W.; Yi, J.-M.; Yeo, H.; Park, S.-M.; Jeong, M.; Chun, J.; Jeong, M.-K. Bojungikki-Tang Augments Pembrolizumab Efficacy in Human PBMC-Injected H460 Tumor-Bearing Mice. Life 2024, 14, 1246. https://doi.org/10.3390/life14101246
Na SW, Yi J-M, Yeo H, Park S-M, Jeong M, Chun J, Jeong M-K. Bojungikki-Tang Augments Pembrolizumab Efficacy in Human PBMC-Injected H460 Tumor-Bearing Mice. Life. 2024; 14(10):1246. https://doi.org/10.3390/life14101246
Chicago/Turabian StyleNa, Se Won, Jin-Mu Yi, Heerim Yeo, Sang-Min Park, Mibae Jeong, Jaemoo Chun, and Mi-Kyung Jeong. 2024. "Bojungikki-Tang Augments Pembrolizumab Efficacy in Human PBMC-Injected H460 Tumor-Bearing Mice" Life 14, no. 10: 1246. https://doi.org/10.3390/life14101246





