Regular Physical Activity Seems to Eliminate Lower Limb Perfusion Asymmetries in Sedentary Non-Healthy Older Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Experimental Procedures and Variables
2.3. Statistical Procedures
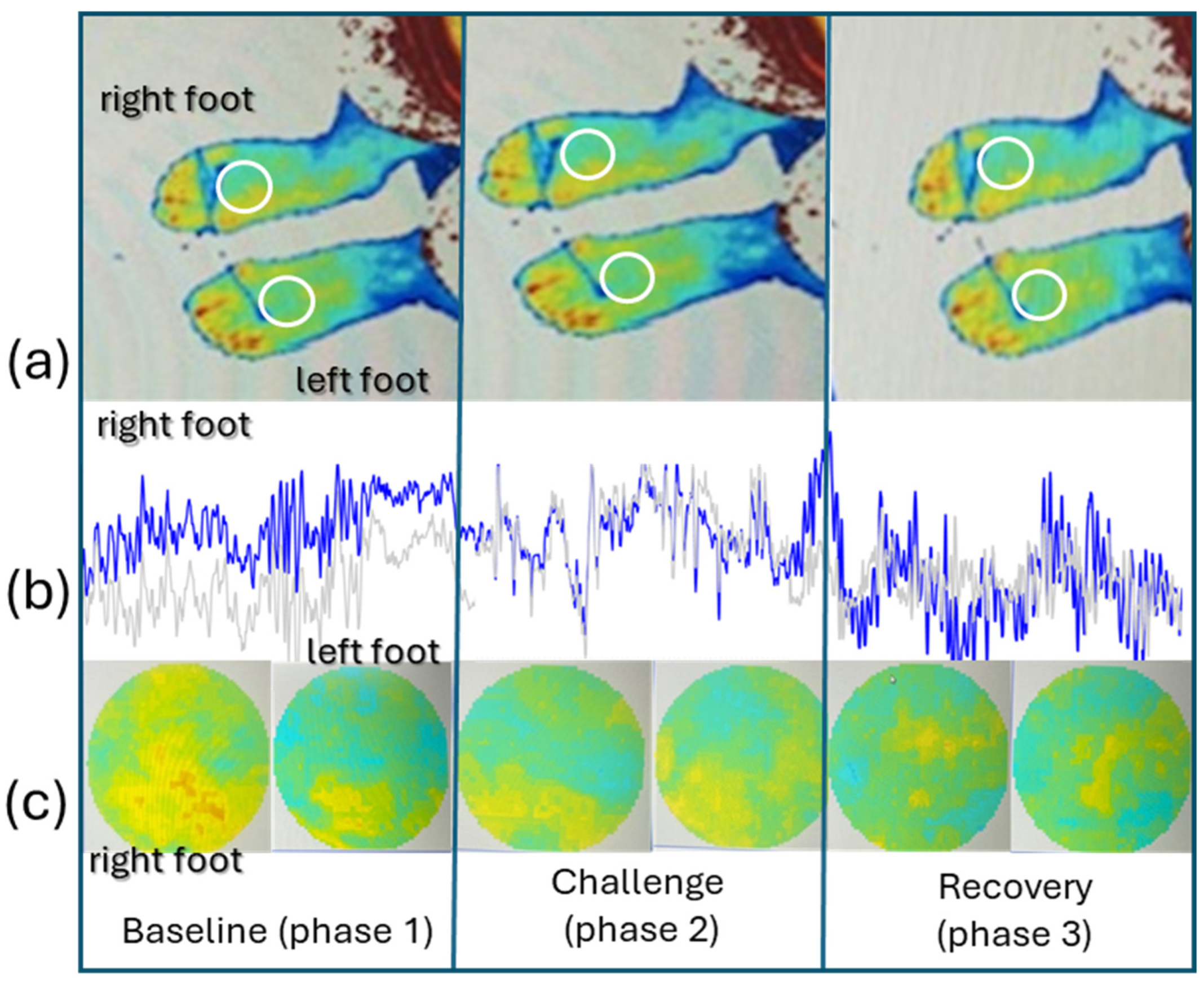
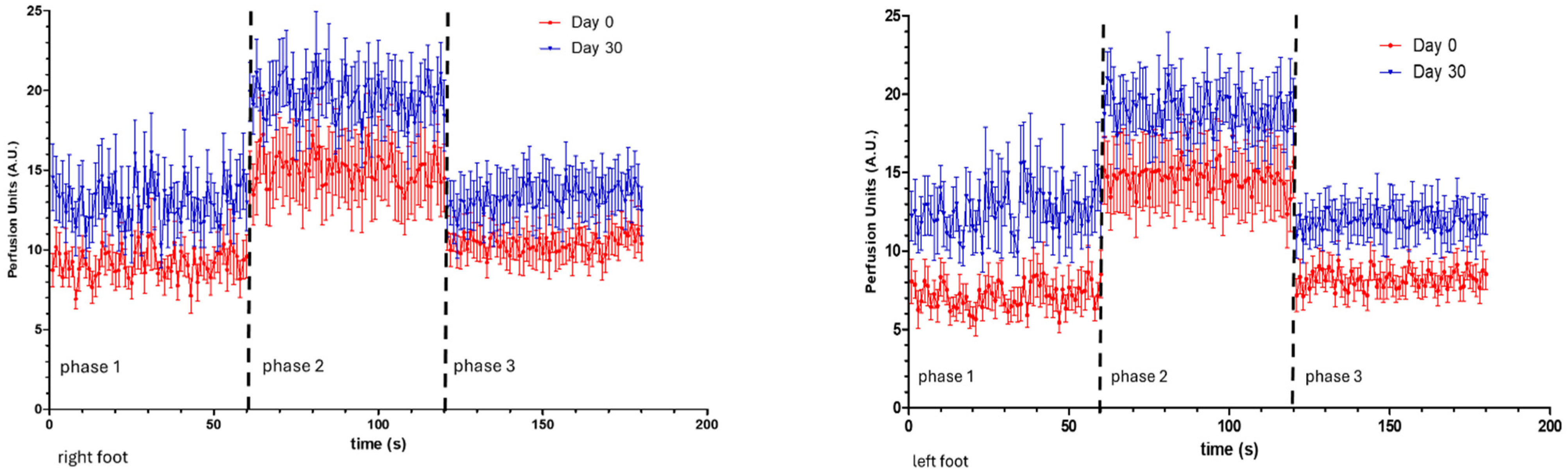
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, L.M.; Rocha, C.G.; Florindo, M.E.; Gregório, J. Lower Limb Perfusion Asymmetries in Humans at Rest and Following Activity—A Collective View. Symmetry 2021, 13, 2348. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Nuno, S.L.; Granja, T.; Florindo, M.E.; Gregório, J.; Atalaia, T. Perfusion, Stance and Plantar Pressure Asymmetries on the Human Foot in the Absence of Disease—A Pilot Study. Symmetry 2022, 14, 441. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Larsen, P.B. Pulsatile blood flow asymmetry in paired human legs. Clin. Physiol. Funct. Imaging 1996, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.A.; Minami, T.; Sekiguchi, H.; Berger, J.S.; Mayo, P.; Narasimhan, M. Ultrasound demonstration of asymmetry between the left and right femoral and radial arteries. Chest 2006, 130, 201S. [Google Scholar] [CrossRef]
- Wood, N.B.; Zhao, S.Z.; Zambanini, A.; Jackson, M.; Gedroyc, W.; Thom, S.A.; Hughes, A.D.; Xu, X.Y. Curvature and tortuosity of the superficial femoral artery: A possible risk factor for peripheral arterial disease. J. Appl. Physiol. 2006, 101, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Marcinkevics, Z.; Lukstina, Z.; Rubins, U.; Grabovskis, A.; Aivars, J.-I. Bilateral difference of superficial and deep femoral artery haemodynamic and anatomical parameters. Artery Res. 2013, 7, 201–210. [Google Scholar] [CrossRef]
- Guan, Y.; Bredin, S.S.D.; Taunton, J.; Jiang, Q.; Wu, N.; Warburton, D.E.R. Association between Inter-Limb Asymmetries in Lower-Limb Functional Performance and Sport Injury: A Systematic Review of Prospective Cohort Studies. J. Clin. Med. 2022, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.T.; Pearson, L.T.; Hicks, K.M. The effect of lower inter-limb asymmetries on athletic performance: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0286942. [Google Scholar] [CrossRef]
- Florindo, M.; Gregório, J.; Rodrigues, L.M. Short duration–low intensity isometric plantar flexion increases distal perfusion: Observations from a healthy cohort. Biomed. Biopharm. Res. J. 2022, 19, 58–71. [Google Scholar] [CrossRef]
- Florindo, M.; Silva, H.; Monteiro Rodrigues, L. Assessing microcirculation dynamics during gait in the lower limb—A preliminary approach. Biomed. Biopharm. Res. J. 2018, 15, 189–195. [Google Scholar] [CrossRef]
- Rocha, C.; Macedo, A.; Nuno, S.; Silva, H.; Ferreira, H.; Rodrigues, L.M. Exploring the perfusion modifications occurring with massage in the human lower limbs by non-contact polarized spectroscopy. Biomed. Biopharm. Res. J. 2018, 15, 196–204. [Google Scholar] [CrossRef]
- Monteiro Rodrigues, L.; Rocha, C.; Ferreira, H.T.; Silva, H.N. Lower limb massage in humans increases local perfusion and impacts systemic hemodynamics. J. Appl. Physiol. 2020, 128, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Nuno, S.; Florindo, M.; Silva, H.; Rodrigues, L.M. Studying the impact of different body positioning, squatting, and unipodal flexion on perfusion in the lower limb—An exploratory approach complemented with optical spectroscopy (TiVi). Biomed. Biopharm. Res. J. 2020, 17, 187–196. [Google Scholar] [CrossRef]
- Mei, C.C.; Zhang, J.; Jing, H.X. Fluid mechanics of Windkessel effect. Med. Biol. Eng. Comput. 2018, 56, 1357–1366. [Google Scholar] [CrossRef]
- Climie, R.E.; Gallo, A.; Picone, D.S.; Di Lascio, N.; van Sloten, T.T.; Guala, A.; Mayer, C.C.; Hametner, B.; Bruno, R.M. Measuring the Interaction Between the Macro- and Micro-Vasculature. Front. Cardiovasc. Med. 2019, 6, 169. [Google Scholar] [CrossRef]
- Cracowski, J.L.; Roustit, M. Human Skin Microcirculation. Compr Physiol. 2020, 10, 1105–1154. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2018; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Ciolac, E.G.; Babjakova, J.; de Abreu, R.M.; Mao, S.-J.; Qian, G.; Amaral, V.T.D.; Wrzesinski, B.; Ferron, A.J.T.; Ossowski, Z.; Francisqueti-Ferron, F.V.; et al. Role of sex and training characteristics on exercise effects on cardiovascular aging: Protocol for a systematic review with meta-analysis of randomized trials. Syst. Rev. 2024, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and Interpretation of the Ankle-Brachial Index: A Scientific Statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- Alzamora, M.T.; Forés, R.; Pera, G.; Baena-Díez, J.M.; Valverde, M.; Torán, P. Low, borderline and normal ankle-brachial index as a predictor of incidents outcomes in the Mediterranean based-population ARTPER cohort after 9 years follow-up. PLoS ONE 2019, 14, e0209163. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Bergkvist, M.; Henricson, J.; Iredahl, F.; Tesselaar, E.; Sjöberg, F.; Farnebo, S. Assessment of microcirculation of the skin using Tissue Viability Imaging: A promising technique for detecting venous stasis in the skin. Microvasc. Res. 2015, 101, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Rocha, C.; Ferreira, H.; Silva, H. Different lasers reveal different skin microcirculatory flowmotion-data from the wavelet transform analysis of human hindlimb perfusion. Sci. Rep. 2019, 9, 16951. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.; Henricson, J.; Anderson, C.; Leahy, M.J.; Nilsson, G.; Sjoberg, F. Sub-epidermal imaging using polarized light spec-troscopy for assessment of skin microcirculation. Ski. Res. Technol. 2007, 13, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Zhai, H.; Chan, H.P.; Farahmand, S.; Maibach, H.I. Cutaneous bioengineering instrumentation standardization: The Tissue Viability Imager. Ski. Res. Technol. 2009, 15, 6–13. [Google Scholar] [CrossRef]
- Karvonen, M.; Kentala, K.; Mustala, O. The effects of training heart rate: A longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar]
- Bouley, J.F. Claudication Intermittent des Membres Posterieurs, determinee par L’obliteration des Arteres Femorales. Recl. Med. Vet. EcAlfort. 1831, 8, 517–527. [Google Scholar]
- Siegel, M.; Siemsen, J.; Siemsen, M.S.A.J.; Coel, M.; Dodge, W.; Freeny, P.; Weinstein, C.; Taft, D.; Allen, F.; Korobkin, M.; et al. A new noninvasive approach to peripheral vascular disease: 379 thallium-201 leg scans. AJR Am. J. Roentgenol. 1978, 131, 827–830. [Google Scholar] [CrossRef][Green Version]
- Mahrous, S.A.; Sidik, N.A.C.; Saqr, K.M. Numerical study on the energy cascade of pulsatile Newtonian and power-law flow models in an ICA bifurcation. PLoS ONE 2021, 16, e0245775. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Burch, J.; Cranny, G.; Aguiar-Ibáñez, R.; Craig, D.; Wright, K.; Berry, E.; Gough, M.; Kleijnen, J.; Westwood, M. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: Systematic review. BMJ 2007, 334, 1257. [Google Scholar] [CrossRef]
- Manevska, N.; Gjorceva, D.P.; Ahmeti, I.; Todorovska, L.; Stojanoski, S.; Kocovska, M.Z. Tissue-Muscle Perfusion Scintigraphy of the Lower Limbs in a Patient with Type 2 Diabetes Mellitus and Peripheral Arterial Disease. Mol. Imaging Radionucl. Ther. 2016, 25, 42–46. [Google Scholar] [CrossRef]
- Hedna, V.S.; Bodhit, A.N.; Ansari, S.; Falchook, A.D.; Stead, L.; Heilman, K.M.; Waters, M.F. Hemispheric Differences in Ischemic Stroke: Is Left-Hemisphere Stroke More Common? J. Clin. Neurol. 2013, 9, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Hao, W.-L.; Zhang, D.-H.; Gao, B.-L. Asymmetrical than symmetrical cerebral arterial bifurcations are more vulnerable to aneurysm presence. Sci. Rep. 2019, 9, 17144. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, S.; Li, Y.; Zhang, D.; Li, D.; Zhang, C. Asymmetry in circulation system and cardiovascular diseases. Med. Nov. Technol. Devices 2024, 21, 100283. [Google Scholar] [CrossRef]
- Secchi, F.; Di Leo, G.; Delnevo, A.; Alì, M.M.; D’angelo, I.D.; Nardella, V.G.; Sardanelli, F. Peripheral artery disease: How much inter-leg symmetry? A contrast-enhanced magnetic resonance angiography study. Medicine 2020, 99, e19637. [Google Scholar] [CrossRef]
- Kolaszyńska, O.; Lorkowski, J. Symmetry and asymmetry in atherosclerosis. Int. J. Occup. Med. Environ. Health 2023, 36, 693–703. [Google Scholar] [CrossRef]
- Okada, H.; Fukui, M.; Tanaka, M.; Matsumoto, S.; Mineoka, Y.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Hasegawa, G.; Nakamura, N. A difference in systolic blood pressure between arms and between lower limbs is a novel risk marker for diabetic nephropathy in patients with Type 2 diabetes. Hypertens. Res. 2013, 36, 403–407. [Google Scholar] [CrossRef]
- Monteiro Rodrigues, L.; Rocha, C.; Andrade, S.; Granja, T.; Gregório, J. The acute adaptation of skin microcirculatory perfusion in vivo does not involve a local response but rather a centrally mediated adaptive reflex. Front. Physiol. 2023, 14, 1177583. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Ferrucci, L.; Gonzalez-Freire, M.; Kosmac, K.; Leeuwenburgh, C.; Peterson, C.A.; Saini, S.; Sufit, R. Skeletal Muscle Pathology in Peripheral Artery Disease: A Brief Review. Arter. Thromb. Vasc. Biol. 2020, 40, 2577–2585. [Google Scholar] [CrossRef]
- Monteiro Rodrigues, L.; Granja, T.F.; de Andrade, S.F. Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology. Life 2022, 12, 1628. [Google Scholar] [CrossRef]
| Participants (n = 11) | Mean ± sd |
|---|---|
| Age, years old | 62.4 ± 5.6 |
| BMI, kg/m2 | 25.6 ± 2.9 |
| ABI | 1.1 ± 0.1 |
| MAP, mmHg | 95.7 ± 6.5 |
| Steps/day (number) | 3400.5 ± 826.7 |
| Physical activity/week (min) | 87.3 ± 12.7 |
| Phase 1 Baseline | Phase 2 Post-Activity | Phase 3 Recovery | |||||||
| R0 | L0 | p | R0 | L0 | p | R0 | L0 | p | |
| LDF PU | 9.2 ± 2.8 | 7.2 ± 2.6 | 0.005 * | 15.0 ± 7.1 | 14.6 ± 7.1 | ns | 10.4 ± 3.0 | 8.2 ± 2.5 | 0.017 * |
| PSp CRBC | 125.4 ± 23.7 | 114.6 ± 19.8 | 0.028 * | 118.9 ± 20.7 | 112.6 ± 16.3 | 0.022 | 119.4 ± 28.9 | 116.4 ± 21.6 | ns |
| Phase 1 Baseline | Phase 2 Post-Activity | Phase 3 Recovery | |||||||
| R30 | L30 | p | R30 | L30 | p | R30 | L30 | p | |
| LDF PU | 13.1 ± 3.9 | 12.7 ± 4.8 | ns | 19.7 ± 5.2 | 19.0 ± 5.3 | ns | 13.4 ± 5.0 | 12.0 ± 4.0 | ns |
| PSp CRBC | 112.0 ± 33.2 | 109.0 ± 32.3 | 0.007 * | 126.7 ± 32.5 | 123.1 ± 32.8 | ns | 111.2 ± 33.7 | 108.6 ± 27.4 | ns |
| Day 0 | Day 30 | p-Value | |
|---|---|---|---|
| SYS_P (mmHg) | 126.5 ± 8.4 | 119.3 ± 7.9 | 0.008 * |
| DIAS_P (mmHg) | 80.3 ± 6.4 | 77.9 ± 6.7 | 0.137 |
| MAP (mmHg) | 95.7 ± 6.8 | 91.7 ± 6.1 | 0.037 * |
| PR (bpm) | 71.7 ± 7.4 | 71.0 ± 8.8 | 0.888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florindo, M.; Gregório, J.; Rodrigues, L.M. Regular Physical Activity Seems to Eliminate Lower Limb Perfusion Asymmetries in Sedentary Non-Healthy Older Individuals. Life 2024, 14, 1258. https://doi.org/10.3390/life14101258
Florindo M, Gregório J, Rodrigues LM. Regular Physical Activity Seems to Eliminate Lower Limb Perfusion Asymmetries in Sedentary Non-Healthy Older Individuals. Life. 2024; 14(10):1258. https://doi.org/10.3390/life14101258
Chicago/Turabian StyleFlorindo, Margarida, João Gregório, and Luís Monteiro Rodrigues. 2024. "Regular Physical Activity Seems to Eliminate Lower Limb Perfusion Asymmetries in Sedentary Non-Healthy Older Individuals" Life 14, no. 10: 1258. https://doi.org/10.3390/life14101258
APA StyleFlorindo, M., Gregório, J., & Rodrigues, L. M. (2024). Regular Physical Activity Seems to Eliminate Lower Limb Perfusion Asymmetries in Sedentary Non-Healthy Older Individuals. Life, 14(10), 1258. https://doi.org/10.3390/life14101258









