Regimens and Response Assessment in Minimally Invasive Image-Guided Therapies for Vascular Malformations: Insights from a Large Cohort Study at a Tertiary-Care Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Design
2.2. Medical Record Review and Minimally Invasive Therapies
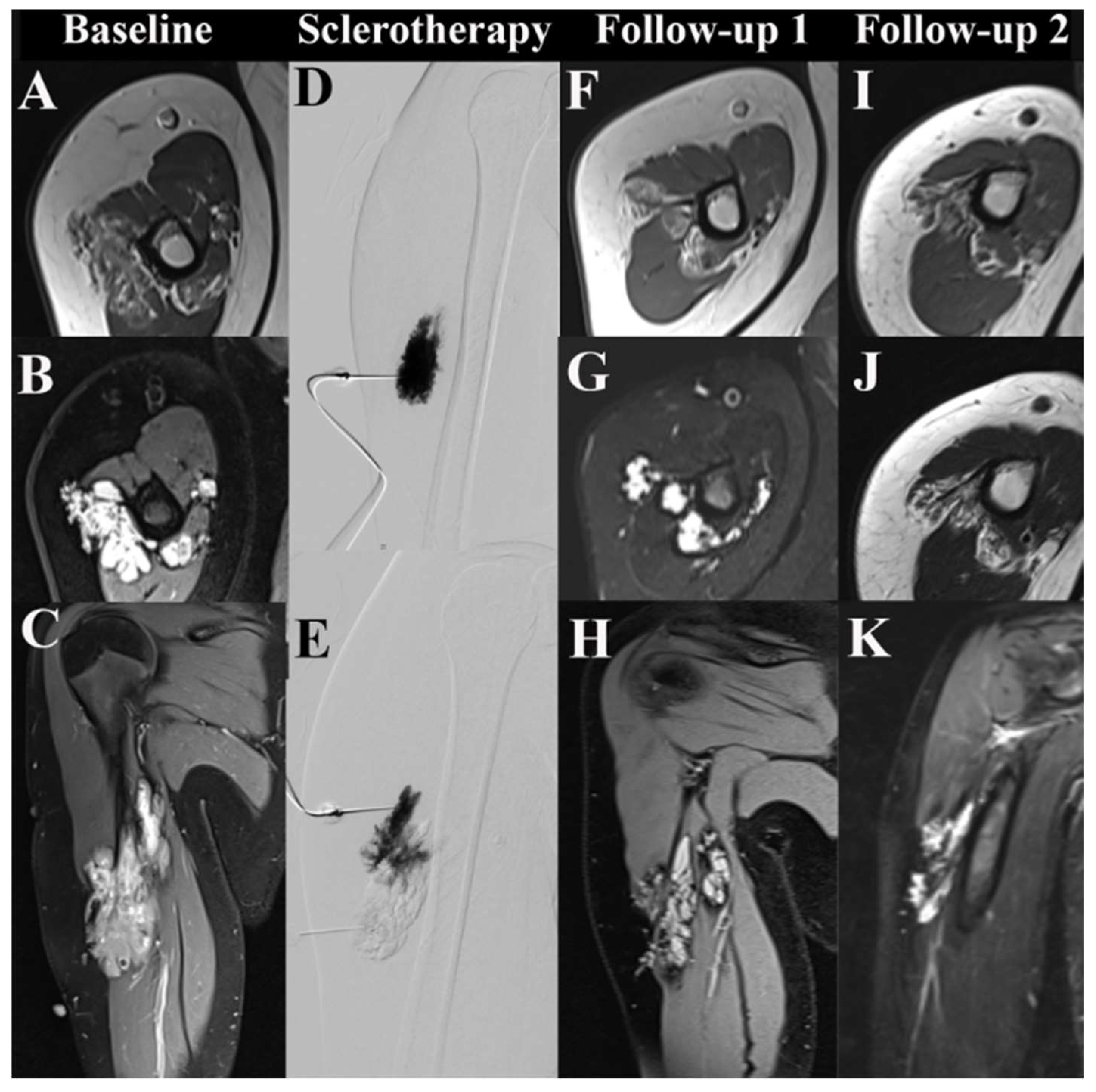
2.3. MRI Acquisition and Image Analysis
2.3.1. Volumetry
2.3.2. Mean Signal Intensities (MSI)
2.4. Statistical Analysis
3. Results
3.1. Study Cohort and Patient Characteristics
3.2. Types and Localization of VMFs
3.3. Minimally Invasive Therapies
3.4. Imaging-Based Response Assessment
3.4.1. VMF Volumes
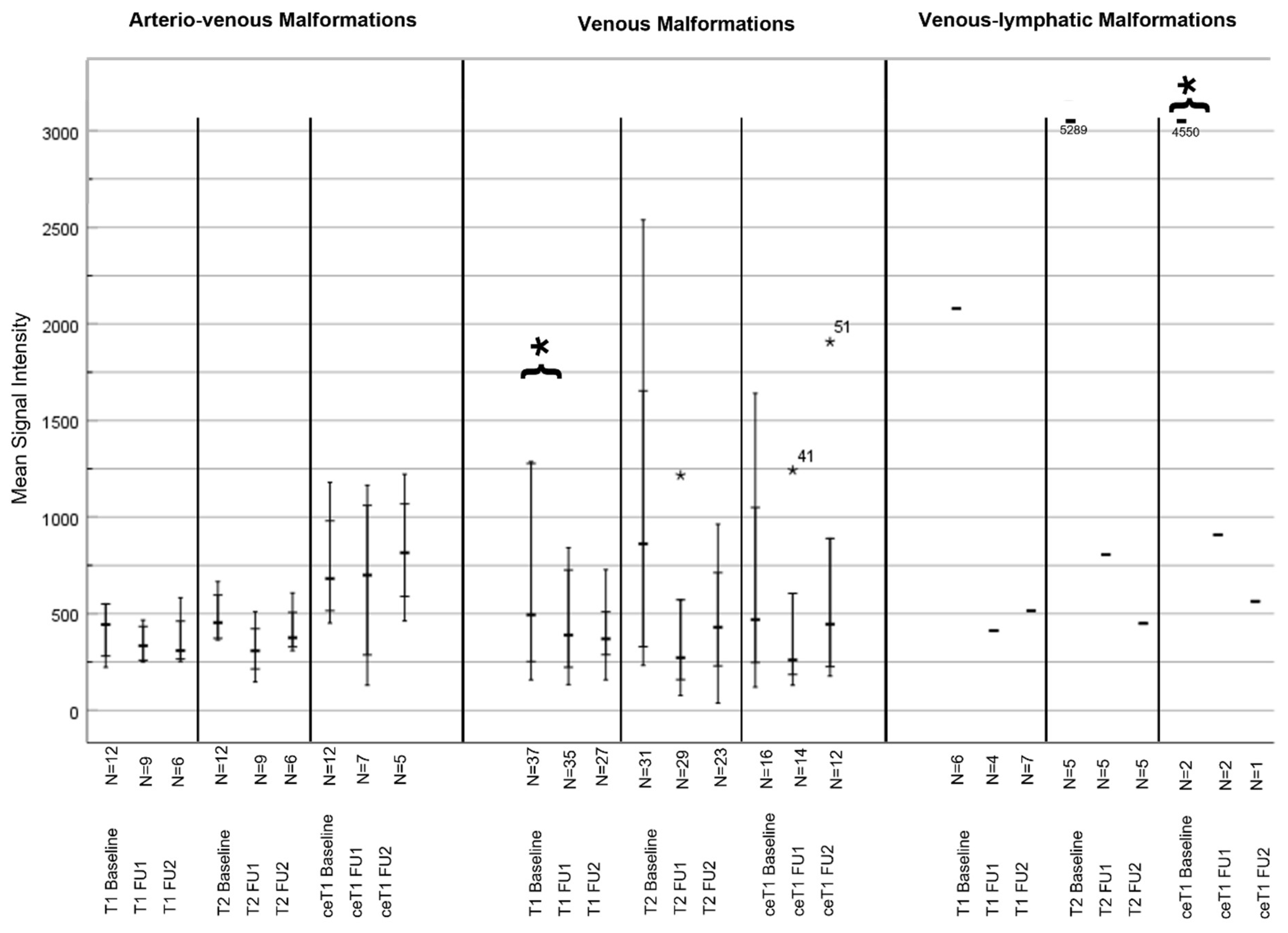
3.4.2. MSI Analysis
3.5. Symptoms and Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Pozo, J.; Gomez-Tellado, M.; Lopez-Gutierrez, J.C. Vascular malformations in childhood. Actas Dermosifiliogr. 2012, 103, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Acord, M.; Srinivasan, A. Management of Venous Malformations. Semin. Intervent. Radiol. 2021, 38, 215–225. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, I. Management of Low-Flow Vascular Malformations: Clinical Presentation, Classification, Patient Selection, Imaging and Treatment. Cardiovasc. Intervent. Radiol. 2015, 38, 1082–1104. [Google Scholar] [CrossRef] [PubMed]
- Sadick, M.; Muller-Wille, R.; Wildgruber, M.; Wohlgemuth, W.A. Vascular Anomalies (Part I): Classification and Diagnostics of Vascular Anomalies. Rofo 2018, 190, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Flors, L.; Hagspiel, K.D.; Park, A.W.; Norton, P.T.; Leiva-Salinas, C. Soft-tissue vascular malformations and tumors. Part 2: Low-flow lesions. Radiologia (Engl. Ed.) 2019, 61, 124–133. [Google Scholar] [CrossRef] [PubMed]
- International Society for the Study of Vascular Anomalies. Available online: https://www.issva.org/ (accessed on 2 February 2023).
- Janardhan, H.P.; Saheera, S.; Jung, R.; Trivedi, C.M. Vascular and Lymphatic Malformations: Perspectives From Human and Vertebrate Studies. Circ. Res. 2021, 129, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Compendium Vascular Anomalies. Available online: https://www.compva.com/ (accessed on 2 February 2023).
- Judith, N.; Ulrike, E.; Siegmar, R.; Matthias, N.; Jurgen, H. Current concepts in diagnosis and treatment of venous malformations. J. Craniomaxillofac. Surg. 2014, 42, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Wojcicki, P.; Wojcicka, K. Epidemiology, diagnostics and treatment of vascular tumours and malformations. Adv. Clin. Exp. Med. 2014, 23, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.B.; Baumgartner, I.; Berlien, P.; Bianchini, G.; Burrows, P.; Gloviczki, P.; Huang, Y.; Laredo, J.; Loose, D.A.; Markovic, J.; et al. Diagnosis and Treatment of Venous Malformations. Consensus Document of the International Union of Phlebology (IUP): Updated 2013. Int. Angiol. 2015, 34, 97–149. [Google Scholar] [PubMed]
- Choi, W.K.; Bailey, C.R.; Fritz, J.; Weiss, C.R. MR-Guided Sclerotherapy for the Treatment of Low-Flow Vascular Malformations. Top. Magn. Reson. Imaging 2018, 27, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bern, I.U. Universitätsinstitut für Diagnostische und Interventionelle Neuroradiologie. Available online: http://www.neurorad.insel.ch/de/unser-angebot/neu-neuro-interventionen/sklerotherapie-von-vaskulaeren-malformationen (accessed on 7 February 2024).
- Mulligan, P.R.; Prajapati, H.J.; Martin, L.G.; Patel, T.H. Vascular anomalies: Classification, imaging characteristics and implications for interventional radiology treatment approaches. Br. J. Radiol. 2014, 87, 20130392. [Google Scholar] [CrossRef] [PubMed]
- Muir, T.; Bertino, G.; Groselj, A.; Ratnam, L.; Kis, E.; Odili, J.; Mccafferty, I.; Wohlgemouth, W.A.; Cemazar, M.; Krt, A.; et al. Bleomycin electrosclerotherapy (BEST) for the treatment of vascular malformations. An International Network for Sharing Practices on Electrochemotherapy (InspECT) study group report. Radiol. Oncol. 2023, 57, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kostusiak, M.; Murugan, S.; Muir, T. Bleomycin Electrosclerotherapy Treatment in the Management of Vascular Malformations. Dermatol. Surg. 2022, 48, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Taghinia, A.H.; Upton, J. Vascular Anomalies. J. Hand Surg. Am. 2018, 43, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Evans, N.; Kaur, I.; Papadopoulou, A.; Khalifa, M.; Tsui, J.; Hamilton, G.; Brookes, J. Incidence of major complication following embolo-sclerotherapy for upper and lower extremity vascular malformations. Vascular 2021, 29, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, H.; Lin, X.; Hu, X.; Jin, Y.; Ma, G. Outcomes and complications of sclerotherapy for venous malformations. Vasc. Endovascular. Surg. 2013, 47, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Kannath, S.K.; Rajendran, A.; Thomas, B.; Rajan, J.E. Volumetric T2-weighted MRI improves the diagnostic accuracy of spinal vascular malformations: Comparative analysis with a conventional MR study. J. Neurointerv. Surg. 2019, 11, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Kannath, S.K.; Mandapalu, S.; Thomas, B.; Rajan, J.E.; Kesavadas, C. Comparative Analysis of Volumetric High-Resolution Heavily T2-Weighted MRI and Time-Resolved Contrast-Enhanced MRA in the Evaluation of Spinal Vascular Malformations. AJNR Am. J. Neuroradiol. 2019, 40, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Moukaddam, H.; Pollak, J.; Haims, A.H. MRI characteristics and classification of peripheral vascular malformations and tumors. Skelet. Radiol. 2009, 38, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Leal, B.A.N.; Procopio, R.J.; Dardik, A.; Navarro, T.P. Sclerotherapy Improves Symptoms in Patients with Small and Moderate Diameter Low-Flow Vascular Malformations: A Prospective Cohort Study. Ann. Vasc. Surg. 2023, 89, 68–77. [Google Scholar] [CrossRef] [PubMed]


| All | Female | Male | N/A | p-Value | |
|---|---|---|---|---|---|
| N (%) | 217 (100%) | 134 (61.8%) | 78 (35.9%) | 5 (2.3%) | |
| Age ø in years | 29.68 ± 18.40 | 28.01 ± 17.84 | 32.63 ± 19.14 | 0.088 | |
| Type of Malformation | |||||
| AVM | 44 (20.3%) | 31 (70.5%) | 13 (29.5%) | 0.173 | |
| VM | 102 (47%) | 67 (65.7%) | 35 (34.3%) | 0.282 | |
| VLM | 12 (5.5%) | 7 (58.3%) | 5 (41.7%) | 0.469 | |
| LM | 8 (3.7%) | 3 (37.5%) | 5 (62.5%) | 0.124 | |
| Others | 17 (7.8%) | 8 (47.1%) | 9 (52.9%) | 0.150 | |
| N/A | 34 (15.7%) | 18 (52.9%) | 11 (32.4%) | 5 (14.7%) |
| Head Neck Mouth Tongue | Eye | Upper/ Lower Jaw | Shoulder | Arm | Hand | Thorax | Abdomen Pelvis Visceral Organs | Back Sacrum Flank | Genitals Groin Urinary Tract | Buttock | Upper Leg | Lower Leg | Knee | Foot | Bone | Multi- Focal | Unknown | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVM | 8 (22.22%) | 2 (28.6%) | 2 (25%) | 1 (10%) | 3 (15%) | 3 (33.3%) | 2 (20%) | 1 (12.5%) | 5 (41.7%) | 2 (40%) | 7 (24.1%) | 3 (14.3%) | 1 (7.1%) | 3 (23.1%) | 1 (33.3%) | |||
| VM | 13 (36.11%) | 4 (57.2%) | 2 (25%) | 8 (80%) | 12 (60%) | 5 (55.6%) | 2 (33.3%) | 2 (20%) | 4 (50%) | 4 (33.3%) | 1 (20%) | 14 (48.3%) | 12 (57.1%) | 11 (78.6%) | 6 (46.2%) | 1 (50%) | 1 (33.3%) | |
| VLM | 5 (13.88%) | 1 (14.2%) | 2 (25%) | 1 (8.3%) | 1 (4.8%) | 1 (7.7%) | 1 (50%) | |||||||||||
| LM | 2 (5.55%) | 1 (12.5%) | 1 (16.7%) | 1 (12.5%) | 2 (40%) | 1 (3.5%) | ||||||||||||
| Others | 1 (2.8%) | 1 (10%) | 1 (5%) | 2 (33.3%) | 4 (40%) | 2 (25%) | 1 (8.3%) | 1 (4.8%) | 2 (14.3%) | 1 (7.7%) | ||||||||
| No data | 7 (19.44%) | 1 (12.5%) | 4 (20%) | 1 (11.1%) | 1 (16.7%) | 2 (20%) | 1 (8.3%) | 7 (24.1%) | 4 (19%) | 2 (15.3%) | 1 (33.3) | 4 (100%) | ||||||
| Total N = 217 | 36 (16.6%) | 7 (3.22%) | 8 (3.7%) | 10 (4.61%) | 20 (9.22%) | 9 (4.1%) | 6 (2.8%) | 10 (4.61%) | 8 (3.7%) | 12 (5.53%) | 5 (2.3%) | 29 (13.4%) | 21 (9.7%) | 14 (6.45%) | 13 (5.9%) | 2 (0.9%) | 3 (1.4%) | 4 (1.8%) |
| Type of Therapy | No Therapy | Therapy with Unknown Agents | Electro-Sclerotherapy | Embolization | Embolization + Surgery | Embolization + Cemento-Plasty | Sclero- therapy | Sclerotherapy + Surgery | Sclerotherapy + Electro-Sclerotherapy | Sclerotherapy + Embolization | Sclerotherapy + Cementoplasty | Sclerotherapy, Embolization + Surgery | Cemento- Plasty | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Type of VMF | |||||||||||||
| Male N = 78 | AVM | 4 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 2 | 0 |
| VM | 18 | 0 | 3 | 0 | 0 | 10 | 0 | 1 | 2 | 1 | 0 | 0 | ||
| VLM | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| LM | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Others | 3 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | |
| N/A | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Total | 38 (35.2%) | 2 (50%) | 1 (50%) | 6 (28.6%) | 0 | 0 | 20 (31.7%) | 1 (100%) | 1 (100%) | 5 (50%) | 1 (100%) | 2 (100%) | 1 (50%) | |
| Female N = 134 | AVM | 9 | 0 | 8 | 0 | 0 | 9 | 0 | 0 | 5 | 0 | 0 | 0 | |
| VM | 31 | 1 | 1 | 5 | 0 | 0 | 29 | 0 | 0 | 0 | 0 | 0 | 0 | |
| VLM | 3 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| LM | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Others | 5 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| N/A | 15 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Total | 65 (60.2%) | 2 (50%) | 1 (50%) | 15 (71.4%) | 1 (100%) | 1 (100%) | 43 (68.3%) | 0 | 0 | 5 (50%) | 0 | 0 | 1 (50%) | |
| T N = 5 | N/A | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 5 (4.6%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total N = 217 | 108 (49.8%) | 4 (1.8%) | 2 (0.9%) | 21 (9.7%) | 1 (0.5%) | 1 (0.5%) | 63 (29%) | 1 (0.5%) | 1 (0.5%) | 10 (4.5%) | 1 (0.5%) | 2 (0.9%) | 2 (0.9%) | |
| Type of VMF | MRI Parameter | MRI Sequence | Baseline | Follow-Up 1 | Follow-Up 2 | p-Value |
|---|---|---|---|---|---|---|
| AVM | MSI | T1 | 404.08 | 397.44 | 370.67 | 0.983 (BL vs. FU1)/0.819 (FU1 vs. FU 2) |
| T2 | 671.92 | 586.11 | 470.17 | 0.834 (BL vs. FU1)/0.120 (FU1 vs. FU 2) | ||
| ceT1 | 698.75 | 632.43 | 752.2 | 0.156 (BL vs. FU1)/0.863 (FU1 vs. FU 2) | ||
| MSI ratio | T1 | 1.23 | 1.22 | 1.17 | 0.575 (BL vs. FU1)/0.133 (FU1 vs. FU 2) | |
| T2 | 3.58 | 3.75 | 3.63 | 0.855 (BL vs. FU1)/0.409 (FU1 vs. FU 2) | ||
| ceT1 | 1.69 | 2.15 | 1.93 | 0.570 (BL vs. FU1)/0.466 (FU1 vs. FU 2) | ||
| Volume in cm3 | 59.5 | 67.2 | 58.16 | 0.557 (BL vs. FU1)/0.180 (FU1 vs. FU 2) | ||
| VM | MSI | T1 | 689.11 | 493.86 | 671.57 | 0.231 (BL vs. FU1)/0.231 (FU1 vs. FU 2) |
| T2 | 1007.9 | 465.26 | 653.2 | 0.028 (BL vs. FU1)/0.466 (FU1 vs. FU 2) | ||
| ceT1 | 832.2 | 589.1 | 942.25 | 0.797 (BL vs. FU1)/0.279 (FU1 vs. FU 2) | ||
| MSI ratio | T1 | 1.41 | 1.08 | 0.81 | 0.289 (BL vs. FU1)/0.224 (FU1 vs. FU 2) | |
| T2 | 4.24 | 3.77 | 3.60 | 0.729 (BL vs. FU1)/0.951 (FU1 vs. FU 2) | ||
| ceT1 | 3.40 | 2.50 | 3.16 | 0.011 (BL vs. FU1)/0.216 (FU1 vs. FU 2) | ||
| Volume in cm3 | 119.9 | 100.54 | 108.24 | 0.866 (BL vs. FU1)/0.733 (FU1 vs. FU 2) | ||
| VLM | MSI | T1 | 740.25 | 441.8 | 471.5 | 0.477 (BL vs. FU1)/0.166 (FU1 vs. FU 2) |
| T2 | 1330.2 | 459 | 542 | 0.309 (BL vs. FU1)/0.970 (FU1 vs. FU 2) | ||
| ceT1 | 1055 | 441.8 | 563 | 0.300 (BL vs. FU1) | ||
| MSI ratio | T1 | 0.86 | 0.99 | 0.91 | 0.980 (BL vs. FU1)/0.860 (FU1 vs. FU 2) | |
| T2 | 3.73 | 3.55 | 2.48 | 0.555 (BL vs. FU1)/0.536 (FU1 vs. FU 2) | ||
| ceT1 | 1.69 | 1.46 | 1.04 | 0.999 (BL vs. FU1) | ||
| Volume in cm3 | 78.9 | 62.32 | 71.7 | 0.271 (BL vs. FU1)/0.056 (FU1 vs. FU 2) | ||
| LM | MSI | T1 | 320 | 439.33 | - | 0.560 (BL vs. FU1) |
| T2 | 1184.7 | 676.33 | - | 0.208 (BL vs. FU1) | ||
| ceT1 | 557.33 | 285.33 | - | 0.029 (BL vs. FU1) | ||
| MSI ratio | T1 | 1.18 | 1.65 | - | 0.236 (BL vs. FU1) | |
| T2 | 4.22 | 5.13 | - | 0.233 (BL vs. FU1) | ||
| ceT1 | 1.19 | 1.56 | - | 0.647 (BL vs. FU1) | ||
| Volume in cm3 | 270.25 | 376.15 | - | 0.449 (BL vs. FU1) |
| Type of VMF | MRI Parameter | MRI Sequence | Baseline | Follow-Up 1 | Follow-Up 2 | p-Value |
|---|---|---|---|---|---|---|
| AVM | MSI | T1 | 327.44 | 402.5 | 371.5 | 0.93 (BL vs. FU1) |
| T2 | 324.22 | 444.33 | 508 | 0.53 (BL vs FU1)/0.667 (FU1 vs. FU2) | ||
| ceT1 | 495.22 | 458 | 317 | 0.365 (BL vs FU1)/0.616 (FU1 vs. FU2) | ||
| MSI ratio | T1 | 1.11 | 1.03 | 0.91 | 0.376 (BL vs. FU1) | |
| T2 | 3.71 | 2.6 | 3.47 | 0.318 (BL vs FU1)/0.588 (FU1 vs. FU2) | ||
| ceT1 | 1.64 | 1.66 | 1.29 | 0.457 (BL vs FU1)/0.901 (FU1 vs. FU2) | ||
| Volume in cm3 | 180.43 | 164.23 | 79.21 | 0.135 (BL vs. FU1)/0.5 (FU1 vs. FU2) | ||
| VM | MSI | T1 | 397.6 | 293.75 | - | 0.768 (BL vs. FU1) |
| T2 | 858.13 | 348.75 | - | 0.424 (BL vs. FU1) | ||
| ceT1 | 416.17 | 428.33 | - | 0.971 (BL vs. FU1) | ||
| MSI ratio | T1 | 0.94 | 1.17 | - | 0.526 (BL vs. FU1) | |
| T2 | 5.21 | 2.87 | - | 0.419 (BL vs. FU1) | ||
| ceT1 | 1.69 | 2.53 | - | 0.068 (BL vs. FU1) | ||
| Volume in cm3 | 13.86 | 29.2 | - | 0.445 (BL vs. FU1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savic, G.D.; Torsello, G.F.; Frisch, A.; Wieners, G.; Fehrenbach, U.; Auer, T.A.; Lüdemann, W.M.; Gebauer, B.; Savic, L.J. Regimens and Response Assessment in Minimally Invasive Image-Guided Therapies for Vascular Malformations: Insights from a Large Cohort Study at a Tertiary-Care Hospital. Life 2024, 14, 1270. https://doi.org/10.3390/life14101270
Savic GD, Torsello GF, Frisch A, Wieners G, Fehrenbach U, Auer TA, Lüdemann WM, Gebauer B, Savic LJ. Regimens and Response Assessment in Minimally Invasive Image-Guided Therapies for Vascular Malformations: Insights from a Large Cohort Study at a Tertiary-Care Hospital. Life. 2024; 14(10):1270. https://doi.org/10.3390/life14101270
Chicago/Turabian StyleSavic, Gesa Doreen, Giovanni F. Torsello, Anne Frisch, Gero Wieners, Uli Fehrenbach, Timo Alexander Auer, Willie Magnus Lüdemann, Bernhard Gebauer, and Lynn Jeanette Savic. 2024. "Regimens and Response Assessment in Minimally Invasive Image-Guided Therapies for Vascular Malformations: Insights from a Large Cohort Study at a Tertiary-Care Hospital" Life 14, no. 10: 1270. https://doi.org/10.3390/life14101270
APA StyleSavic, G. D., Torsello, G. F., Frisch, A., Wieners, G., Fehrenbach, U., Auer, T. A., Lüdemann, W. M., Gebauer, B., & Savic, L. J. (2024). Regimens and Response Assessment in Minimally Invasive Image-Guided Therapies for Vascular Malformations: Insights from a Large Cohort Study at a Tertiary-Care Hospital. Life, 14(10), 1270. https://doi.org/10.3390/life14101270






