Effects of Repeated Exposure to Ambient Cold on the Development of Inflammatory Pain in a Rat Model of Knee Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
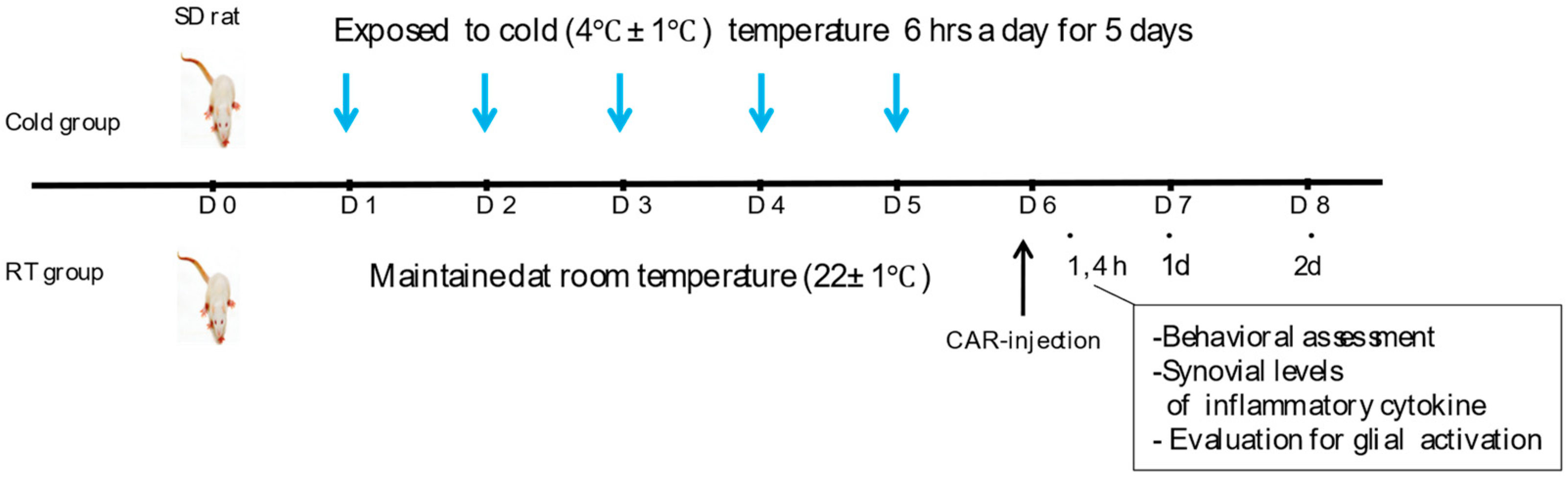
2.2. Exposure to Ambient Cold and Induction of Inflammatory Pain
2.3. Behavioural Test
2.4. Cytokine Analysis of Synovial Fluid
2.5. Immunohistochemistry
2.6. Statistical Analyses
3. Results
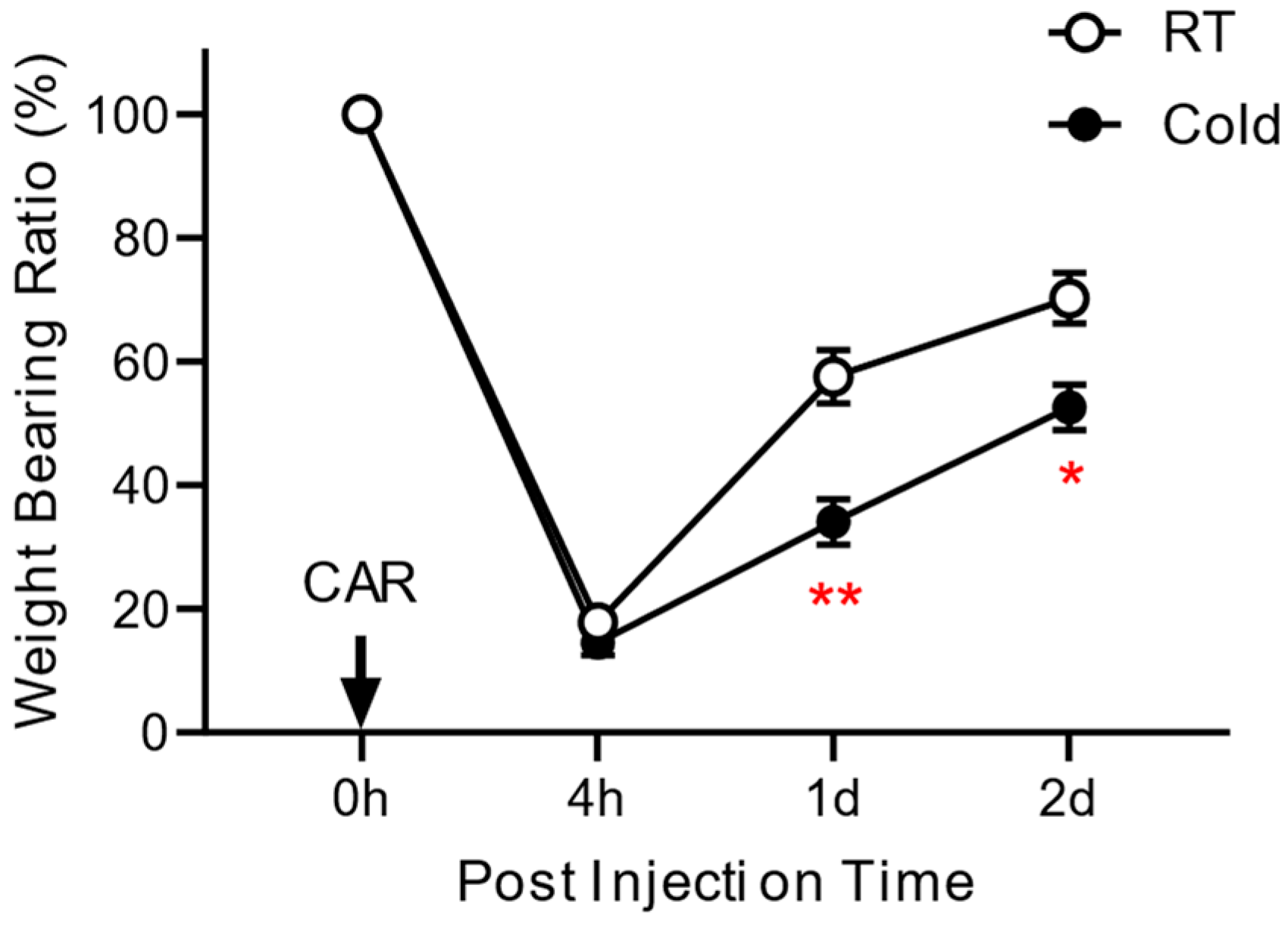
3.1. Effect of Cold Exposure on the Development of Inflammatory Pain
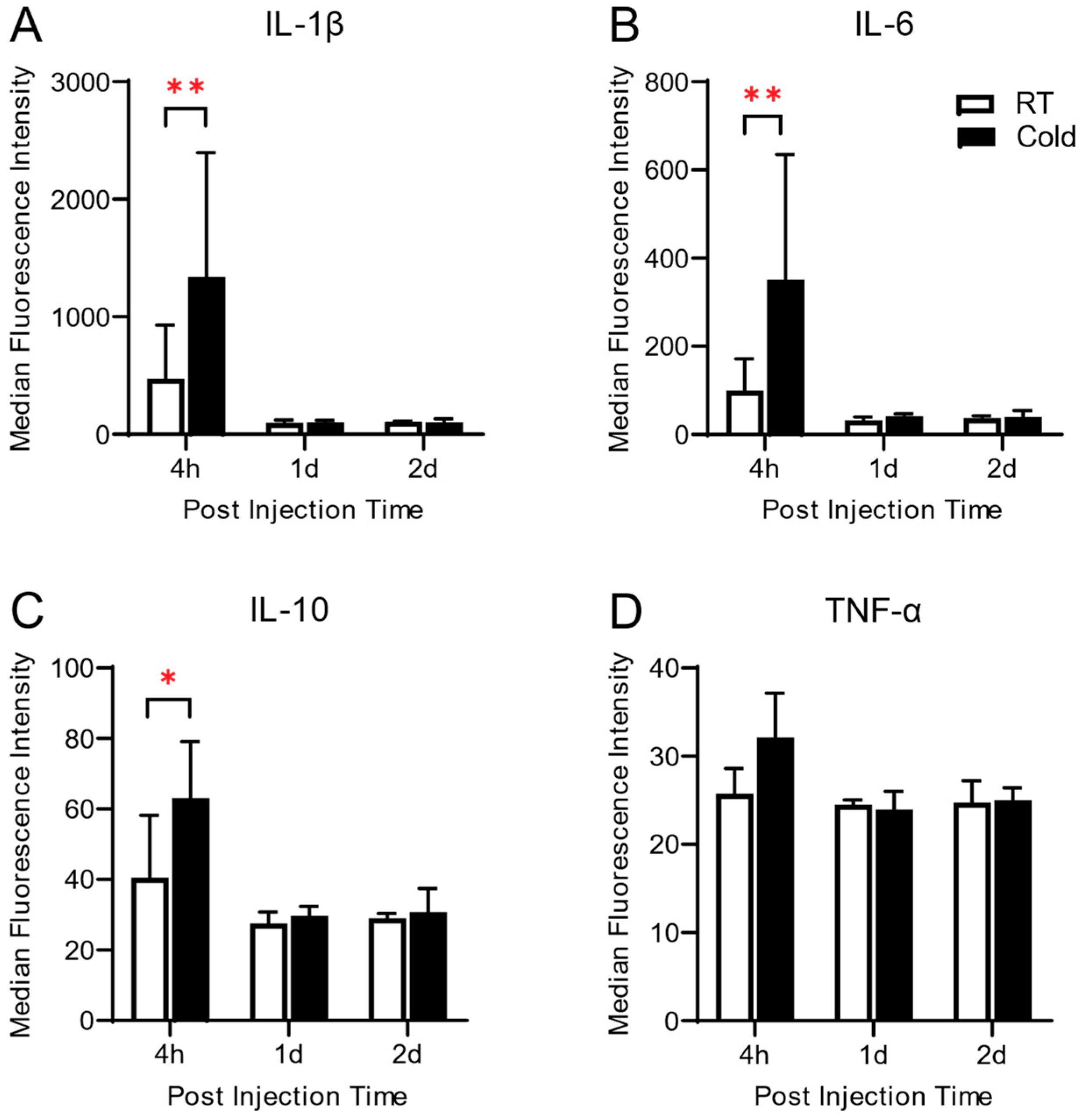
3.2. Synovial Levels of Inflammatory Cytokines Altered by Cold Exposure in Carrageenan-Induced Knee Arthritis
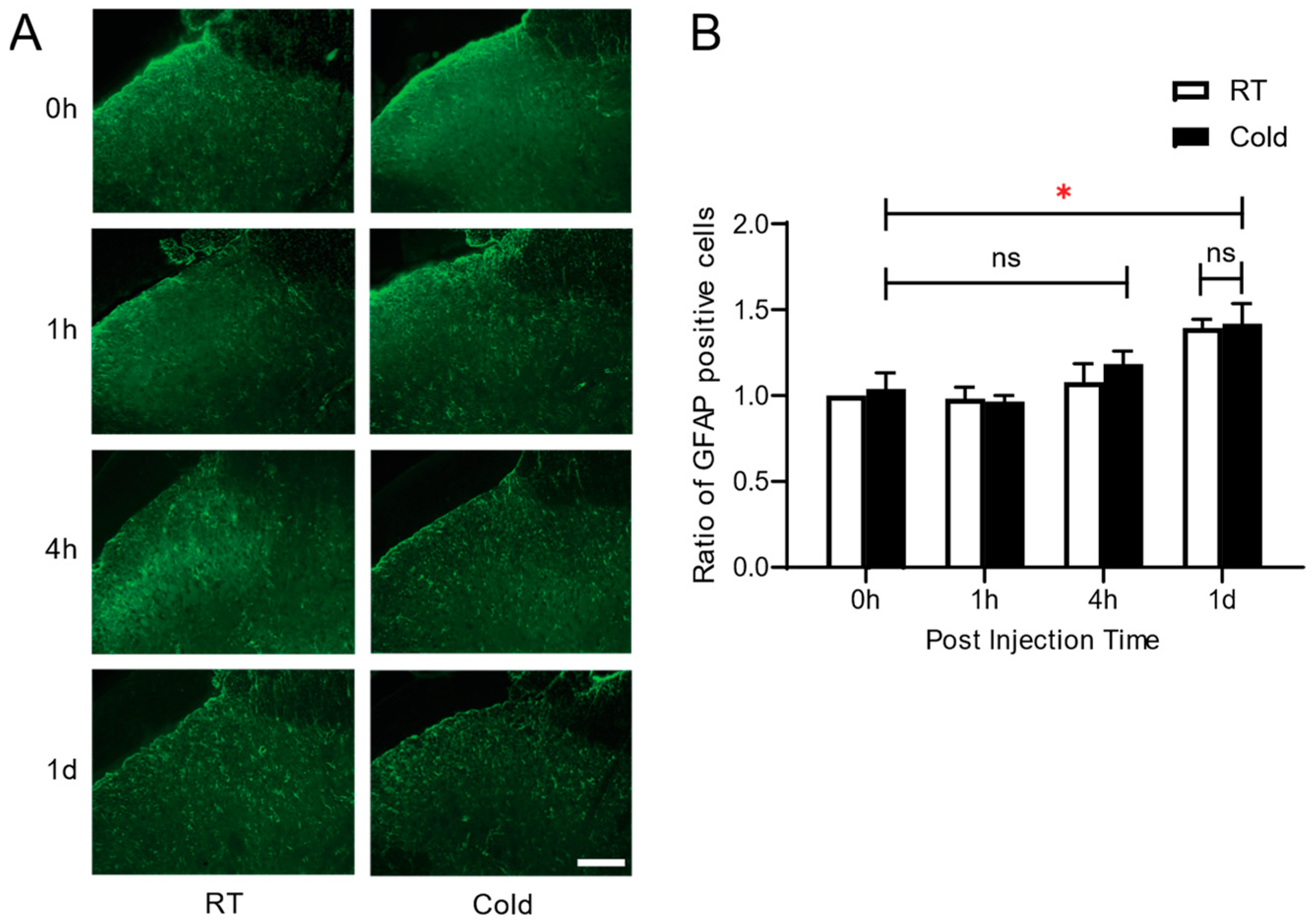
3.3. Effect of Cold Exposure on Spinal Glial Activation in Carrageenan-Induced Knee Arthritis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, D.; Lo, Y.T.E.; Ball, E.; Godwin, J.L.; Andrews, O.; Barciela, R.; Ford, L.B.; Di Napoli, C.; Ebi, K.L.; Fučkar, N.S.; et al. Expert judgement reveals current and emerging UK climate-mortality burden. Lancet Planet. Health 2024, 8, e684–e694. [Google Scholar] [CrossRef] [PubMed]
- Salek, K.M.; Mamun, M.A.; Parvin, N.; Ahmed, S.M.; Khan, M.M.; Rijvi, A.N.; Rahman, M.H.; Khasru, M.R.; Akther, A.; Rahman, M.; et al. Fluctuation of pain by weather change in musculoskeletal disorders. Mymensingh Med. J. 2011, 20, 645–651. [Google Scholar] [PubMed]
- Pienimäki, T. Cold exposure and musculoskeletal disorders and diseases. A review. Int. J. Circumpolar Health 2002, 61, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Raatikka, V.-P.; Rytkönen, M.; Näyhä, S.; Hassi, J. Prevalence of cold-related complaints, symptoms and injuries in the general population: The FINRISK 2002 cold substudy. Int. J. Biometeorol. 2007, 51, 441–448. [Google Scholar] [CrossRef]
- Aléx, J.; Lundgren, P.; Henriksson, O.; Saveman, B.I. Being cold when injured in a cold environment—Patients’ experiences. Int. Emerg. Nurs. 2013, 21, 42–49. [Google Scholar] [CrossRef]
- Inaba, R.; Mirbod, S.M. Subjective musculoskeletal symptoms in winter and summer among Indoor working construction electricians. Ind. Health 2010, 48, 29–37. [Google Scholar] [CrossRef]
- Aikman, H. The association between arthritis and the weather. Int. J. Biometeorol. 1997, 40, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Laborde, J.M.; Dando, W.A.; Powers, M.J. Powers, Influence of weather on osteoarthritics. Soc. Sci. Med. 1986, 23, 549–554. [Google Scholar] [CrossRef]
- Pope, D.P.; Croft, P.R.; Pritchard, C.M.; Silman, A.J.; Macfarlane, G.J. Occupational factors related to shoulder pain and disability. Occup. Environ. Med. 1997, 54, 316–321. [Google Scholar] [CrossRef]
- Devereux, J.J.; Vlachonikolis, I.G.; Buckle, P.W. Epidemiological study to investigate potential interaction between physical and psychosocial factors at work that may increase the risk of symptoms of musculoskeletal disorder of the neck and upper limb. Occup. Environ. Med. 2002, 59, 269–277. [Google Scholar] [CrossRef]
- Burström, L.; Järvholm, B.; Nilsson, T.; Wahlström, J. Back and neck pain due to working in a cold environment: A cross-sectional study of male construction workers. Int. Arch. Occup. Environ. Health 2012, 86, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Aasmoe, L.; Bang, B.; Egeness, C.; Løchen, M.-L. Musculoskeletal symptoms among seafood production workers in North Norway. Occup. Med. 2008, 58, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Sorock, G.S.; Courtney, T.; Liang, Y.; Yao, Z.; Matz, S.; Ge, L. Risk factors for work-related low back pain in the people’s republic of China. Int. J. Occup. Environ. Health 2000, 6, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Oksa, J.; Ducharme, M.B.; Rintamäki, H. Combined effect of repetitive work and cold on muscle function and fatigue. J. Appl. Physiol. 2002, 92, 354–361. [Google Scholar] [CrossRef]
- Alba, B.K.; Castellani, J.W.; Charkoudian, N. Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp. Physiol. 2019, 104, 1202–1214. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef]
- Fiore, N.T.; Debs, S.R.; Hayes, J.P.; Duffy, S.S.; Moalem-Taylor, G. Pain-resolving immune mechanisms in neuropathic pain. Nat. Rev. Neurol. 2023, 19, 199–220. [Google Scholar] [CrossRef]
- Sideris-Lampretsas, G.; Malcangio, M. Pain-resolving microglia. Science 2022, 376, 33–34. [Google Scholar] [CrossRef]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Sato, J. Weather change and pain: A behavioral animal study of the influences of simulated meteorological changes on chronic pain. Int. J. Biometeorol. 2003, 47, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Christy, M.R.; Peterson, W.L.; Rudd, S.L.; Troy, S.M. Reduction of pain-related behaviors with either cold or heat treatment in an animal model of acute arthritis. Arch. Phys. Med. Rehabil. 1999, 80, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Ward, M.M. Advances in the treatment of inflammatory arthritis. Best Pract. Res. Clin. Rheumatol. 2012, 26, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, E.J.; Schaap, L.A.; Herbolsheimer, F.; Dennison, E.M.; Maggi, S.; Pedersen, N.L.; Castell, M.V.; Denkinger, M.D.; Edwards, M.H.; Limongi, F.; et al. The Influence of Weather Conditions on Joint Pain in Older People with Osteoarthritis: Results from the European Project on OSteoArthritis. J. Rheumatol. 2015, 42, 1885–1892. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.S.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef]
- Sesti-Costa, R.; Ignacchiti, M.D.C.; Chedraoui-Silva, S.; Marchi, L.F.; Mantovani, B. Chronic cold stress in mice induces a regulatory phenotype in macrophages: Correlation with increased 11beta-hydroxysteroid dehydrogenase expression. Brain Behav. Immun. 2012, 26, 50–60. [Google Scholar] [CrossRef]
- Min, S.S.; Han, J.S.; Kim, Y.I.; Na, H.S.; Yoon, Y.W.; Kil Hong, S.; Han, H.C. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci. Lett. 2001, 308, 95–98. [Google Scholar] [CrossRef]
- Koo, S.T.; Lim, K.S.; Chung, K.; Ju, H.; Chung, J.M. Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal α-adrenoceptors. Pain 2008, 135, 11–19. [Google Scholar] [CrossRef]
- Koo, S.T.; Lee, C.-H.; Shin, Y.I.; Ko, H.Y.; Lee, D.G.; Jeong, H.-S. Acquiring the optimal time for hyperbaric therapy in the rat model of CFA induced arthritis. Pain Physician 2014, 17, 197–202. [Google Scholar]
- Koo, S.T.; Park, Y.I.; Lim, K.S.; Chung, K.; Chung, J.M. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain 2002, 99, 423–431. [Google Scholar] [CrossRef]
- Yang, E.J.; Koo, S.T.; Kim, Y.S.; Lee, J.E.; Hwang, H.S.; Lee, M.S.; Choi, S.-M. Contralateral electroacupuncture pretreatment suppresses carrageenan-induced inflammatory pain via the opioid-mu receptor. Rheumatol. Int. 2011, 31, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Silman, A.J. Risk factors for the development of rheumatoid arthritis. Scand. J. Rheumatol. 2006, 35, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Abasolo, L.; Tobías, A.; Leon, L.; Carmona, L.; Fernandez-Rueda, J.L.; Rodriguez, A.B.; Fernandez-Gutierrez, B.; Jover, J.A. Weather conditions may worsen symptoms in rheumatoid arthritis patients: The possible effect of temperature. Reumatol. Clin. 2013, 9, 226–228. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Russell, F.A.; Alawi, K.M.; Sand, C.; Liang, L.; Salamon, R.; Bodkin, J.V.; Aubdool, A.A.; Arno, M.; Gentry, C.; et al. Environmental cold exposure increases blood flow and affects pain sensitivity in the knee joints of CFA-induced arthritic mice in a TRPA1-dependent manner. Arthritis Res. Ther. 2016, 18, 7. [Google Scholar] [CrossRef]
- Moss, P.; Knight, E.; Wright, A. Wright, Subjects with knee osteoarthritis exhibit widespread hyperalgesia to pressure and cold. PLoS ONE 2016, 11, e0147526. [Google Scholar] [CrossRef] [PubMed]
- Daniela Guedj, A.W. Effect of weather conditions on rheumatic patients. Ann. Ofthe Rheum. Dis. 1990, 49, 158–159. [Google Scholar] [CrossRef]
- Sugama, S.; Takenouchi, T.; Fujita, M.; Kitani, H.; Hashimoto, M. Cold stress induced morphological microglial activation and increased IL-1β expression in astroglial cells in rat brain. J. Neuroimmunol. 2011, 233, 29–36. [Google Scholar] [CrossRef]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial proinflammatory cytokines mediate exaggerated pain states: Implications for clinical pain. Adv. Exp. Med. Biol. 2003, 521, 1–21. [Google Scholar]
- Romano, M.; Faggioni, R.; Sironi, M.; Sacco, S.; Echtenacher, B.; Di Santo, E.; Salmona, M.; Ghezzi, P. Carrageenan-induced acute inflammation in the mouse air pouch synovial model. Role Tumour Necrosis Factor. Mediat. Inflamm. 1997, 6, 32–38. [Google Scholar]
- Sekut, L.; Menius, J.A.; Brackeen, M.F.; Connolly, K.M. Evaluation of the significance of elevated levels of systemic and localized tumor necrosis factor in different animal models of inflammation. J. Lab. Clin. Med. 1994, 124, 813–820. [Google Scholar]
- Smith-Oliver, T.; Noel, L.S.; Stimpson, S.S.; Yarnall, D.P.; Connolly, K.M. Elevated levels of TNF in the joints of adjuvant arthritic rats. Cytokine 1993, 5, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.J.; Meager, A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin. Exp. Immunol. 1988, 73, 88–92. [Google Scholar] [PubMed]
- Husby, G.; Williams, R.C., Jr. Synovial localization of tumor necrosis factor in patients with rheumatoid arthritis. J. Autoimmun. 1988, 1, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Mir, A.R.; Abidi, M.; Talha, M.; Zafar, A.; Habib, S. Methylglyoxal modified IgG generates autoimmune response in rheumatoid arthritis. Int. J. Biol. Macromol. 2018, 118 Pt A, 15–23. [Google Scholar] [CrossRef]
- Islam, S.; Mir, A.R.; Arfat, M.Y.; Khan, F.; Zaman, M.; Ali, A. Structural and immunological characterization of hydroxyl radical modified human IgG: Clinical correlation in rheumatoid arthritis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 194, 194–201. [Google Scholar] [CrossRef]
- Di Carlo, M.; Farah, S.; Atzeni, F.; Alciati, A.; Di Franco, M.; Iannuccelli, C.; Bazzichi, L.; Bianchi, G.; Giovale, M.; Tirri, R.; et al. Geographical disparities in fibromyalgia severity: An Italian study. Eur. J. Pain. 2024. [Google Scholar] [CrossRef]
- Turk, D.C.; Adams, L.M. Using a biopsychosocial perspective in the treatment of fibromyalgia patients. Pain Manag. 2016, 6, 357–369. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Sindhuri, V.; Koo, M.-J.; Jeon, S.H.; Kim, S.; Koo, S. Effects of Repeated Exposure to Ambient Cold on the Development of Inflammatory Pain in a Rat Model of Knee Arthritis. Life 2024, 14, 1428. https://doi.org/10.3390/life14111428
Kim S-H, Sindhuri V, Koo M-J, Jeon SH, Kim S, Koo S. Effects of Repeated Exposure to Ambient Cold on the Development of Inflammatory Pain in a Rat Model of Knee Arthritis. Life. 2024; 14(11):1428. https://doi.org/10.3390/life14111428
Chicago/Turabian StyleKim, So-Hee, Vishnumolakala Sindhuri, Min-Jae Koo, Seung Heon Jeon, Seungtae Kim, and Sungtae Koo. 2024. "Effects of Repeated Exposure to Ambient Cold on the Development of Inflammatory Pain in a Rat Model of Knee Arthritis" Life 14, no. 11: 1428. https://doi.org/10.3390/life14111428
APA StyleKim, S.-H., Sindhuri, V., Koo, M.-J., Jeon, S. H., Kim, S., & Koo, S. (2024). Effects of Repeated Exposure to Ambient Cold on the Development of Inflammatory Pain in a Rat Model of Knee Arthritis. Life, 14(11), 1428. https://doi.org/10.3390/life14111428






