Influence of Lifestyle Factors on Ocular Surface Parameters in Relation to Age
Abstract
1. Introduction
2. Materials and Methods
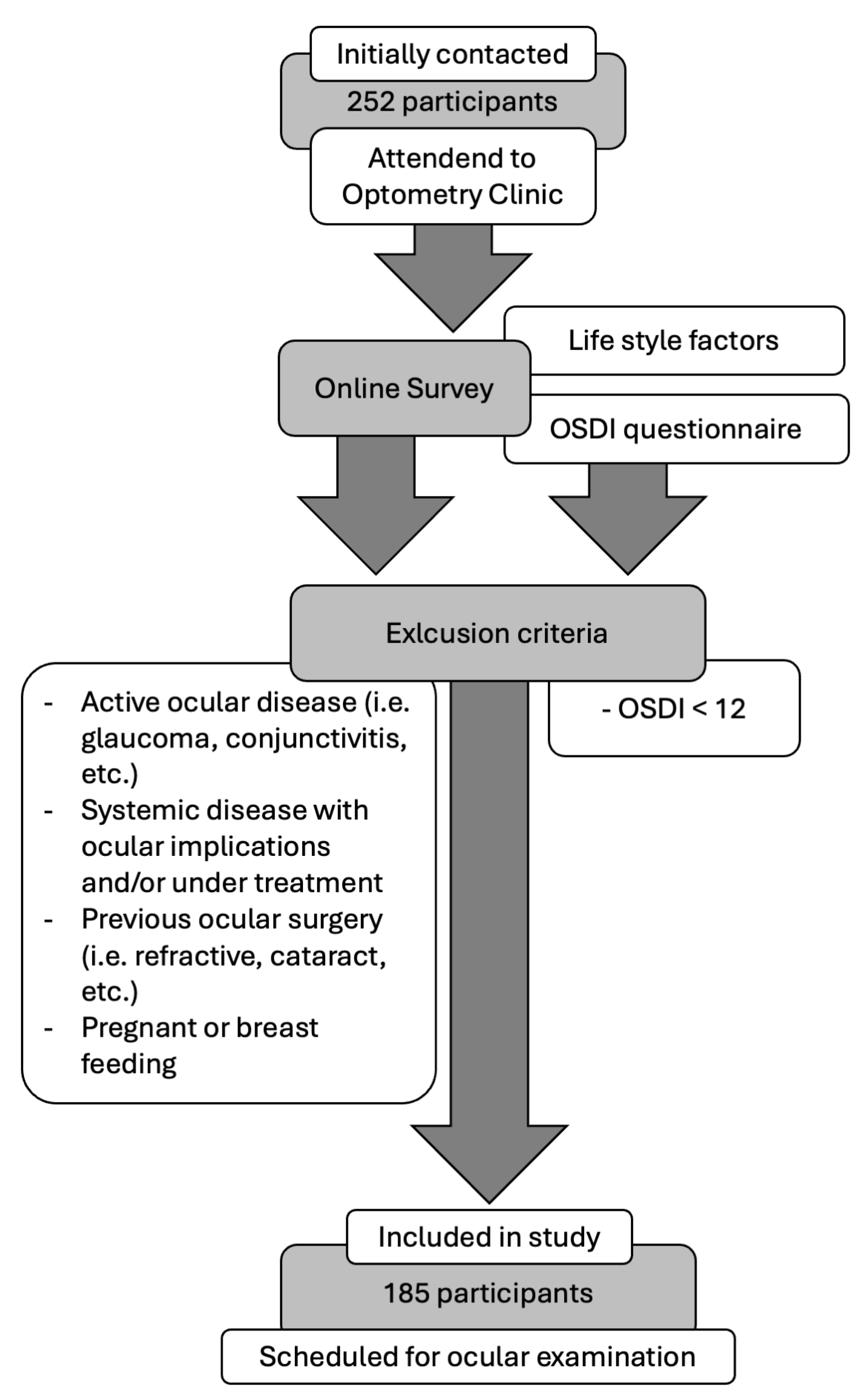
2.1. Sample
2.2. Protocol and Procedures
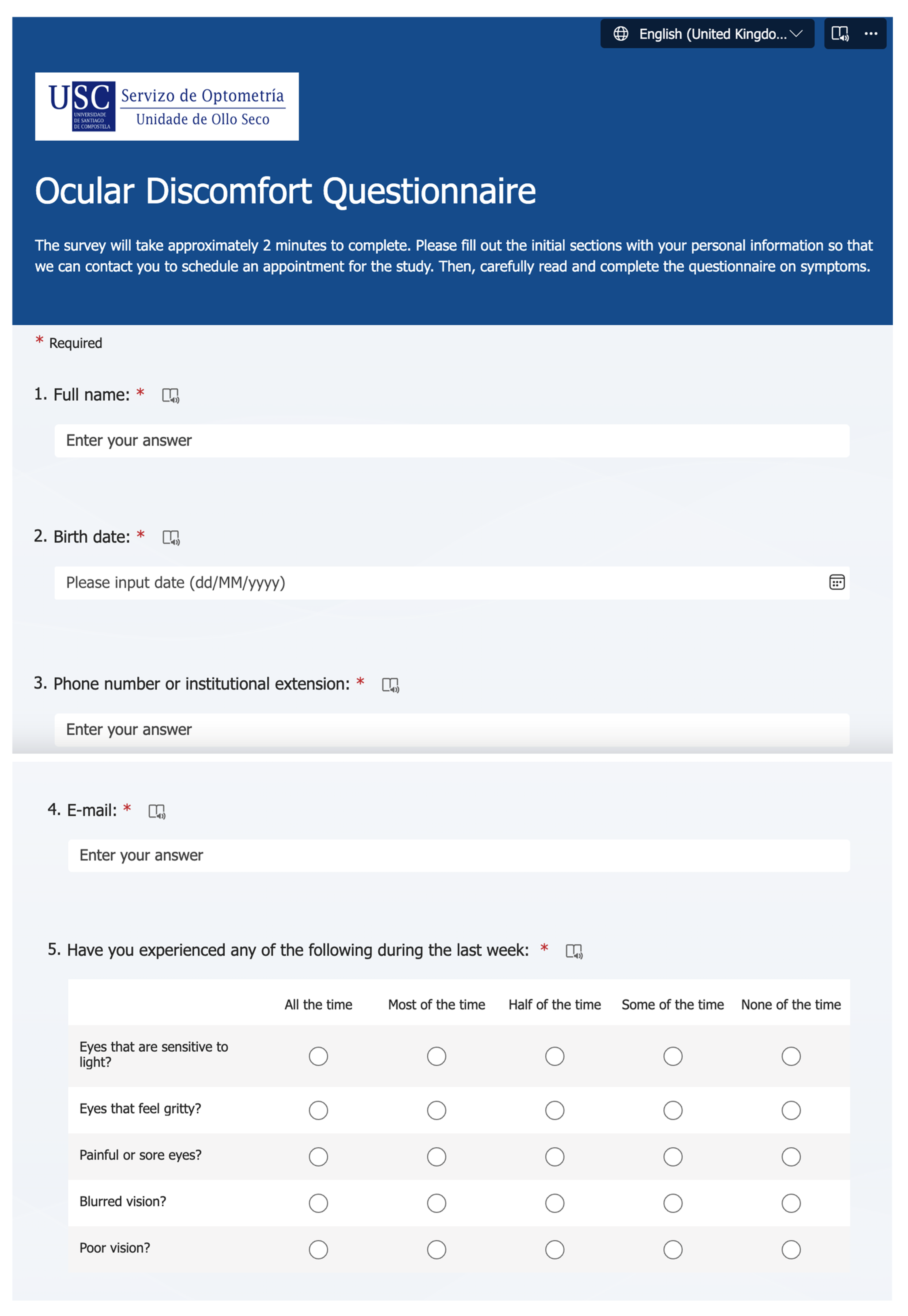
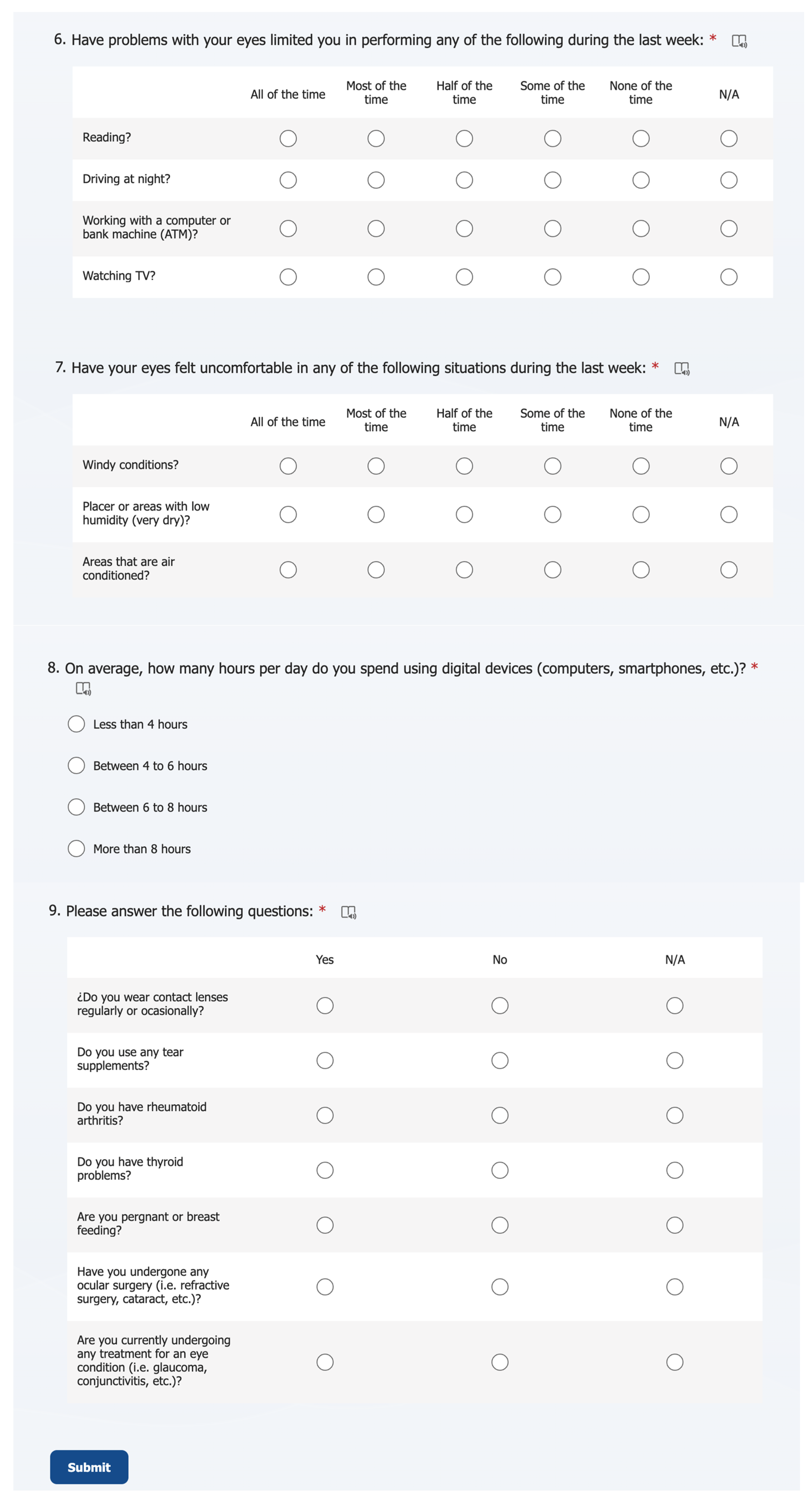
2.2.1. Ocular Discomfort and Lifestyle Factors Online Survey
2.2.2. Tear Film Osmolarity
2.2.3. Tear Film Break-Up Time and Maximum Blink Interval
2.2.4. Corneal Staining
2.3. Statistical Analysis
3. Results
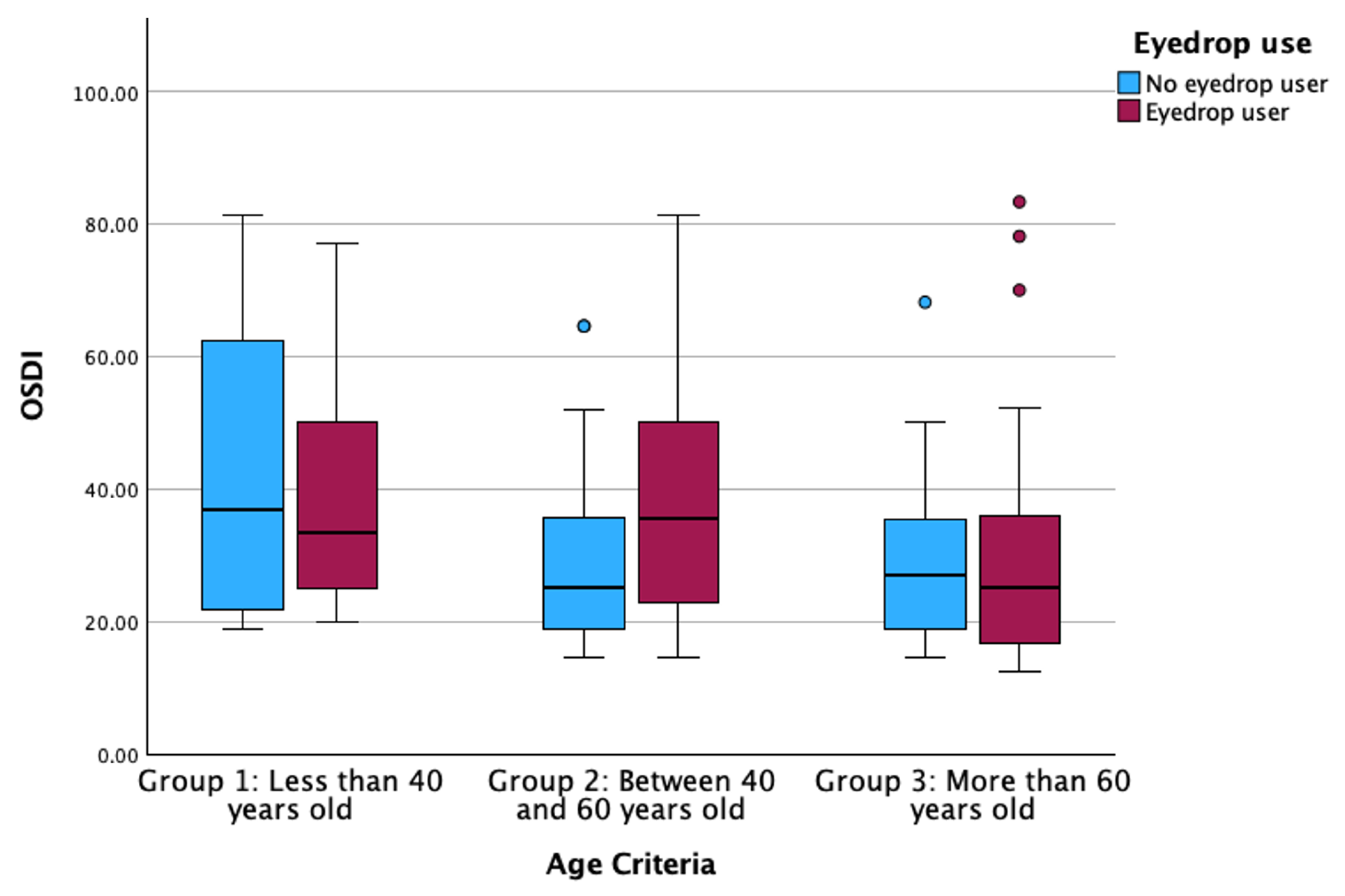
3.1. Descriptive Statistics and Differences Between Groups
3.2. Correlations Between the Studied Parameters
3.3. Multiple Linear Regression of OSDI, Osmolarity, FBUT, and MBI Regarding the Age and Lifestyle Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, S. Biochemistry of human tear film: A review. Exp. Eye Res. 2022, 220, 109101. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed]
- Ayaki, M.; Negishi, K.; Kawashima, M.; Uchino, M.; Kaido, M.; Tsubota, K. Age Is a Determining Factor of Dry Eye-Related Signs and Symptoms. Diagnostics 2020, 10, 193. [Google Scholar] [CrossRef]
- Jaiswal, S.; Asper, L.; Long, J.; Lee, A.; Harrison, K.; Golebiowski, B. Ocular and visual discomfort associated with smartphones, tablets and computers: What we do and do not know. Clin. Exp. Optom. 2019, 102, 463–477. [Google Scholar] [CrossRef]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Meibomian gland secretion quality association with ocular parameters in university students during COVID-19 restrictions. Int. Ophthalmol. 2023, 43, 2349–2362. [Google Scholar] [CrossRef]
- Craig, J.P.; Alves, M.; Wolffsohn, J.S.; Downie, L.E.; Efron, N.; Galor, A.; Gomes, J.A.P.; Jones, L.; Markoulli, M.; Stapleton, F.; et al. TFOS Lifestyle Report Introduction: A Lifestyle Epidemic—Ocular Surface Disease. Ocul. Surf. 2023, 28, 304–309. [Google Scholar] [CrossRef]
- Galor, A.; Britten-Jones, A.C.; Feng, Y.; Ferrari, G.; Goldblum, D.; Gupta, P.K.; Merayo-Lloves, J.; Na, K.S.; Naroo, S.A.; Nichols, K.K.; et al. TFOS Lifestyle: Impact of lifestyle challenges on the ocular surface. Ocul. Surf. 2023, 28, 262–303. [Google Scholar] [CrossRef]
- Jones, L.; Efron, N.; Bandamwar, K.; Barnett, M.; Jacobs, D.S.; Jalbert, I.; Pult, H.; Rhee, M.K.; Sheardown, H.; Shovlin, J.P.; et al. TFOS Lifestyle: Impact of contact lenses on the ocular surface. Ocul. Surf. 2023, 29, 175–219. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Lingham, G.; Downie, L.E.; Huntjens, B.; Inomata, T.; Jivraj, S.; Kobia-Acquah, E.; Muntz, A.; Mohamed-Noriega, K.; Plainis, S.; et al. TFOS Lifestyle: Impact of the digital environment on the ocular surface. Ocul. Surf. 2023, 28, 213–252. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Willcox, M.D.; Argueso, P.; Maissa, C.; Stahl, U.; Tomlinson, A.; Wang, J.; Yokoi, N.; Stapleton, F. The TFOS International Workshop on Contact Lens Discomfort: Report of the contact lens interactions with the tear film subcommittee. Investig. Ophthalmol. Vis. Sci. 2013, 54, TFOS123–TFOS156. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. A cross-sectional study of non-modifiable and modifiable risk factors of dry eye disease states. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2023, 46, 101800. [Google Scholar] [CrossRef]
- Walston, J.D. Common clinical sequelae of aging. In Goldman-Cecil Medicine, 27th ed.; Goldman, L., Cooney, K.A., Eds.; Elsevier: Philadelphia, PA, USA, 2024; Chapter 24. [Google Scholar]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef]
- Fricke, T.R.; Tahhan, N.; Resnikoff, S.; Papas, E.; Burnett, A.; Ho, S.M.; Naduvilath, T.; Naidoo, K.S. Global Prevalence of Presbyopia and Vision Impairment from Uncorrected Presbyopia: Systematic Review, Meta-analysis, and Modelling. Ophthalmology 2018, 125, 1492–1499. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Davies, L.N. Presbyopia: Effectiveness of correction strategies. Prog. Retin. Eye Res. 2019, 68, 124–143. [Google Scholar] [CrossRef]
- Gomes, J.R.M.; Franco, S.M.B. Near Vision Tasks and Optical Quality of the Eye. J. Ophthalmic Vis. Res. 2021, 16, 620–630. [Google Scholar] [CrossRef]
- Fuentes-Paez, G.; Herreras, J.M.; Cordero, Y.; Almaraz, A.; Gonzalez, M.J.; Calonge, M. Lack of concordance between dry eye syndrome questionnaires and diagnostic tests. Arch. Soc. Esp. Oftalmol. 2011, 86, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the ocular surface disease index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Beltran, F.; Betancourt, N.R.; Martinez, J.; Valdes, C.S.; Babayan, A.; Ramírez-Assad, C.; Juarez, E.M.; Hernandez-Quintela, E.; Group, M.O.S.D.S. Transcultural validation of ocular surface disease index (osdi) questionnaire for mexican population. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6050. [Google Scholar]
- Tavakoli, A.; Markoulli, M.; Flanagan, J.; Papas, E. The validity of point of care tear film osmometers in the diagnosis of dry eye. Ophthalmic Physiol. Opt. 2022, 42, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Pena-Verdeal, H.; Garcia-Resua, C.; Vazquez-Sanchez, C.; Garcia-Queiruga, J.; Giraldez, M.J.; Yebra-Pimentel, E. Inter-eye osmolarity differences in patients with symptomatic and non-symptomatic dry eyes. Arq. Bras. Oftalmol. 2020, 83, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Pena-Verdeal, H.; Ramos, L.; Garcia-Queiruga, J.; Garcia-Resua, C.; Giraldez, M.J.; Yebra-Pimentel, E. Validation of a New Software Application for Tear Breakup Measurement. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2022, 99, 159–166. [Google Scholar] [CrossRef]
- Pena-Verdeal, H.; Garcia-Resua, C.; Ramos, L.; Yebra-Pimentel, E.; Giraldez, M.J. Diurnal variations in tear film break-up time determined in healthy subjects by software-assisted interpretation of tear film video recordings. Clin. Exp. Optom. 2016, 99, 142–148. [Google Scholar] [CrossRef]
- Bron, A.J.; Argueso, P.; Irkec, M.; Bright, F.V. Clinical staining of the ocular surface: Mechanisms and interpretations. Prog. Retin. Eye Res. 2015, 44, 36–61. [Google Scholar] [CrossRef] [PubMed]
- Pena-Verdeal, H.; Garcia-Queiruga, J.; Sabucedo-Villamarin, B.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Capability of the Inter-Eye Differences in Osmolarity, Break-Up Time and Corneal Staining on the Diagnostic of Dry Eye. Ocul. Immunol. Inflamm. 2023, 32, 1674–1681. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Davies, L.N.; Dunne, M.C.; Gilmartin, B. Statistical guidelines for clinical studies of human vision. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2011, 31, 123–136. [Google Scholar] [CrossRef]
- Pucker, A.D.; Jones-Jordan, L.A.; Marx, S.; Powell, D.R.; Kwan, J.T.; Srinivasan, S.; Sickenberger, W.; Jones, L.; Contact Lens Assessment of Symptomatic Subjects Study, G. Clinical factors associated with contact lens dropout. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2019, 42, 318–324. [Google Scholar] [CrossRef]
- Guillon, M.; Maissa, C. Tear film evaporation--effect of age and gender. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2010, 33, 171–175. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Muntz, A.; Lim, J.; Kim, J.S.; Lacerda, L.; Arora, A.; Craig, J.P. Ageing and the natural history of dry eye disease: A prospective registry-based cross-sectional study. Ocul. Surf. 2020, 18, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Ryden, L. Technological Development and Lifestyle Changes. In Sustainable Development, Knowledge Society and Smart Future Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2015; pp. 113–124. [Google Scholar]
- Woessner, M.N.; Tacey, A.; Levinger-Limor, A.; Parker, A.G.; Levinger, P.; Levinger, I. The Evolution of Technology and Physical Inactivity: The Good, the Bad, and the Way Forward. Front. Public Health 2021, 9, 655491. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R. Lifestyle and mental health. Am. Psychol. 2011, 66, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Stonerock, G.L.; Blumenthal, J.A. Role of Counseling to Promote Adherence in Healthy Lifestyle Medicine: Strategies to Improve Exercise Adherence and Enhance Physical Activity. Prog. Cardiovasc. Dis. 2017, 59, 455–462. [Google Scholar] [CrossRef]
- Wong, V.W.; Ho, F.Y.; Shi, N.K.; Sarris, J.; Chung, K.F.; Yeung, W.F. Lifestyle medicine for depression: A meta-analysis of randomized controlled trials. J. Affect Disord 2021, 284, 203–216. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Dong, N.; Yang, F.; Lin, Z.; Shang, X.; Li, C. Meibomian gland dysfunction determines the severity of the dry eye conditions in visual display terminal workers. PLoS ONE 2014, 9, e105575. [Google Scholar] [CrossRef]
- Uchino, M.; Yokoi, N.; Uchino, Y.; Dogru, M.; Kawashima, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Schaumberg, D.A.; et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka study. Am. J. Ophthalmol. 2013, 156, 759–766. [Google Scholar] [CrossRef]
- Kamoy, B.; Magno, M.; Noland, S.T.; Moe, M.C.; Petrovski, G.; Vehof, J.; Utheim, T.P. Video display terminal use and dry eye: Preventive measures and future perspectives. Acta Ophthalmol. 2022, 100, 723–739. [Google Scholar] [CrossRef]
- Uchino, M.; Kawashima, M.; Uchino, Y.; Tsubota, K.; Yokoi, N. Association between tear film break up time and blink interval in visual display terminal users. Int. J. Ophthalmol. 2018, 11, 1691–1697. [Google Scholar] [CrossRef]
- Srivastav, S.; Basu, S.; Singh, S. Tear film changes in symptomatic versus asymptomatic video display terminal users following computer challenge test. Ocul. Surf. 2023, 30, 53–56. [Google Scholar] [CrossRef]
- Dresp-Langley, B.; Hutt, A. Digital Addiction and Sleep. Int. J. Envrion. Res. Public Health 2022, 19, 6910. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.N.; Banda, J.A.; Hale, L.; Lu, A.S.; Fleming-Milici, F.; Calvert, S.L.; Wartella, E. Screen Media Exposure and Obesity in Children and Adolescents. Pediatrics 2017, 140, S97–S101. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Henderson, R.; Bradley, L.; Galloway, B.; Hunter, L. Effect of visual display unit use on blink rate and tear stability. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 1991, 68, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Talens-Estarelles, C.; Cervino, A.; Garcia-Lazaro, S.; Fogelton, A.; Sheppard, A.; Wolffsohn, J.S. The effects of breaks on digital eye strain, dry eye and binocular vision: Testing the 20-20-20 rule. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2023, 46, 101744. [Google Scholar] [CrossRef]
- Ozdemir, M.; Temizdemir, H. Age- and gender-related tear function changes in normal population. Eye 2010, 24, 79–83. [Google Scholar] [CrossRef]
- Mann, A.; Campbell, D.; Mirza, Z.; Hunt, O.; Wolffsohn, J.S.; Tighe, B.J. Clinical and biochemical analysis of the ageing tear film. Br. J. Ophthalmol. 2020, 104, 1028–1032. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef]
- Hirosawa, K.; Inomata, T.; Sung, J.; Nakamura, M.; Okumura, Y.; Midorikawa-Inomata, A.; Miura, M.; Fujio, K.; Akasaki, Y.; Fujimoto, K.; et al. Diagnostic ability of maximum blink interval together with Japanese version of Ocular Surface Disease Index score for dry eye disease. Sci. Rep. 2020, 10, 18106. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, K.; Caffery, B.; Dogru, M.; Hickson-Curran, S.; Kern, J.; Kojima, T.; Morgan, P.B.; Purslow, C.; Robertson, D.M.; Nelson, J.D.; et al. The TFOS International Workshop on Contact Lens Discomfort: Report of the subcommittee on epidemiology. Investig. Ophthalmol. Vis. Sci. 2013, 54, TFOS20–TFOS36. [Google Scholar] [CrossRef]
- Giannaccare, G.; Blalock, W.; Fresina, M.; Vagge, A.; Versura, P. Intolerant contact lens wearers exhibit ocular surface impairment despite 3 months wear discontinuation. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht Von Graefes Arch. Fur. Klin. Und Exp. Ophthalmol. 2016, 254, 1825–1831. [Google Scholar] [CrossRef]
- Cho, P.; Boost, M. Daily disposable lenses: The better alternative. Contact Lens Anterior Eye 2013, 36, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Misu, N.; Mimura, T.; Noma, H.; Shinbo, K. Patient Satisfaction and Tear Film Break-Up Time After First-Time Wearing of Silicone Hydrogel Contact Lenses. Cureus 2024, 16, e52516. [Google Scholar] [CrossRef] [PubMed]
- Siddireddy, J.S.; Vijay, A.K.; Tan, J.; Willcox, M. The eyelids and tear film in contact lens discomfort. Contact Lens Anterior Eye 2018, 41, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Wong, K.; Jia, T.; Siddireddy, J.S.; Briggs, N.; Tan, J. The effect of hydroxypropyl-guar nanoemulsion on signs and symptoms of dry eye. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2022, 45, 101736. [Google Scholar] [CrossRef]
- Sheppard, J.; Shen Lee, B.; Periman, L.M. Dry eye disease: Identification and therapeutic strategies for primary care clinicians and clinical specialists. Ann. Med. 2023, 55, 241–252. [Google Scholar] [CrossRef]
- Turner, A.W.; Layton, C.J.; Bron, A.J. Survey of eye practitioners’ attitudes towards diagnostic tests and therapies for dry eye disease. Clin. Exp. Ophthalmol. 2005, 33, 351–355. [Google Scholar] [CrossRef]
- Cardona, G.; Serés, C.; Quevedo, L.; Augé, M. Knowledge and use of tear film evaluation tests by spanish practitioners. Optom. Vis. Sci. 2011, 88, 1106–1111. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Rocha, E.M.; Aragona, P.; Clayton, J.A.; Ding, J.; Golebiowski, B.; Hampel, U.; McDermott, A.M.; Schaumberg, D.A.; Srinivasan, S.; et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul. Surf. 2017, 15, 284–333. [Google Scholar] [CrossRef]

| N | Group 1 (<40 Years Old) | Group 2 (40–60 Years Old) | Group 3 (>60 Years Old) | p-Value | |
|---|---|---|---|---|---|
| 38 | 101 | 46 | |||
| Sex (% women) | 185 | 76.3% | 83.2% | 82.6% | 0.666 †† |
| Age (Mean ± SD) | 185 | 28.2 ± 1 | 51.7 ± 0.4 | 66.8 ± 0.7 | - |
| OSDI values (Median [IQR]) | 185 | 33.3 [22.9–50] | 27.5 [20.6–38.5] | 25 [17.7–35.9] | 0.029 * |
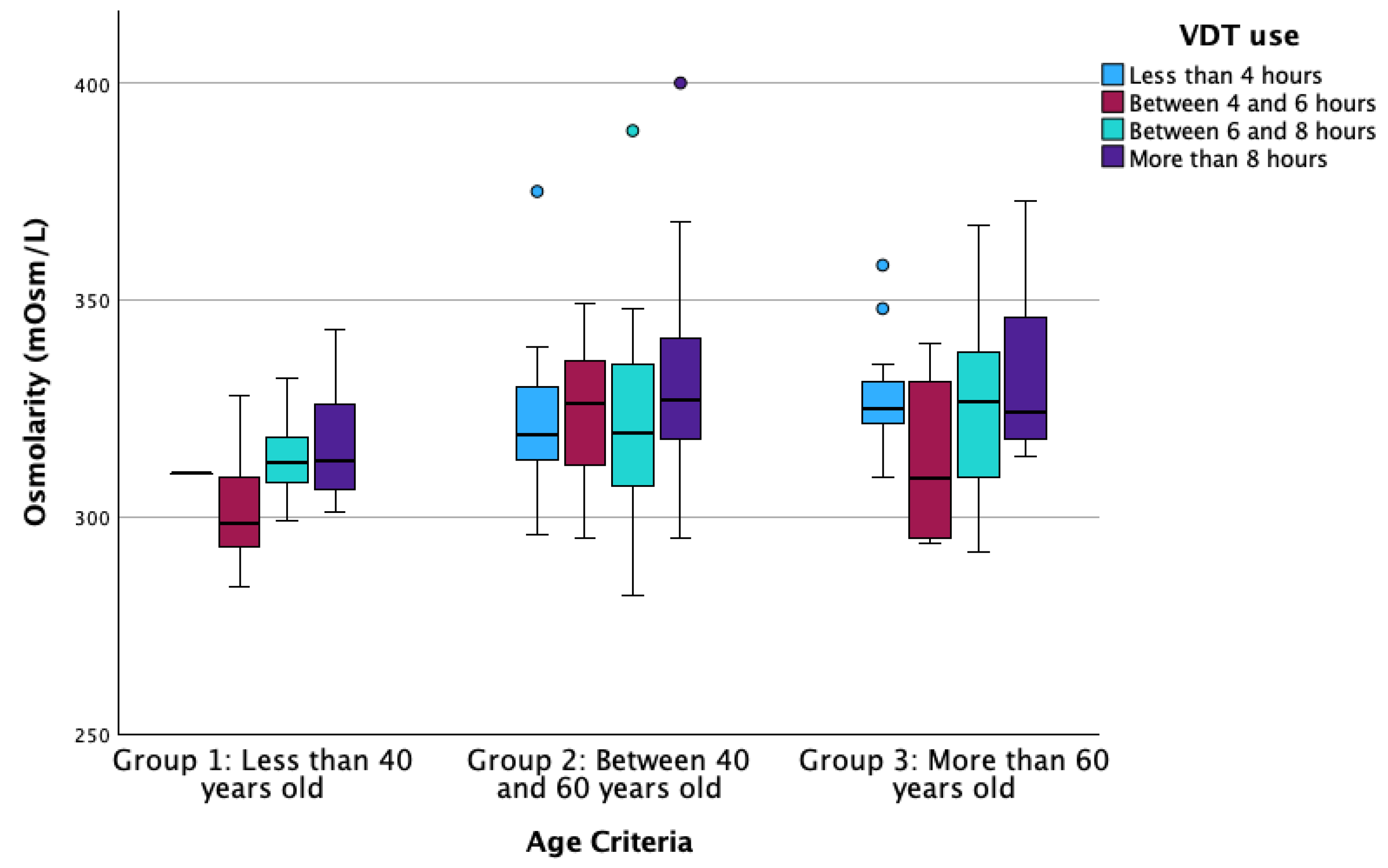
| Osmolarity (mOsm/L) (Median [IQR]) | 185 | 310 [303–318.5] | 323 [314–337] | 324 [314–338] | <0.001 * |
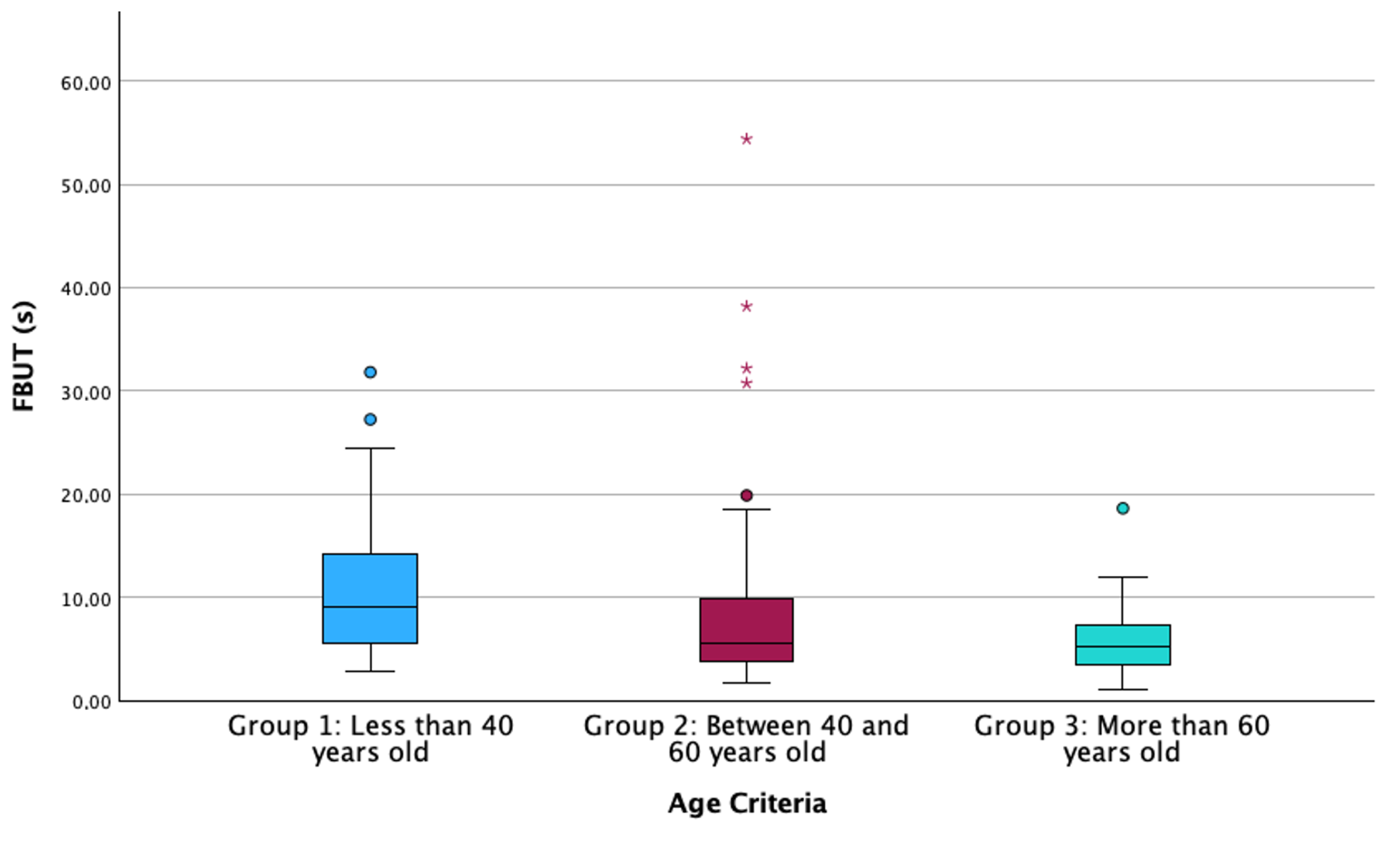
| FBUT (s) (Median [IQR]) | 185 | 9.4 [5.5–14.2] | 4.9 [3.8–8.9] | 5.1 [3.3–7.2] | <0.001 * |
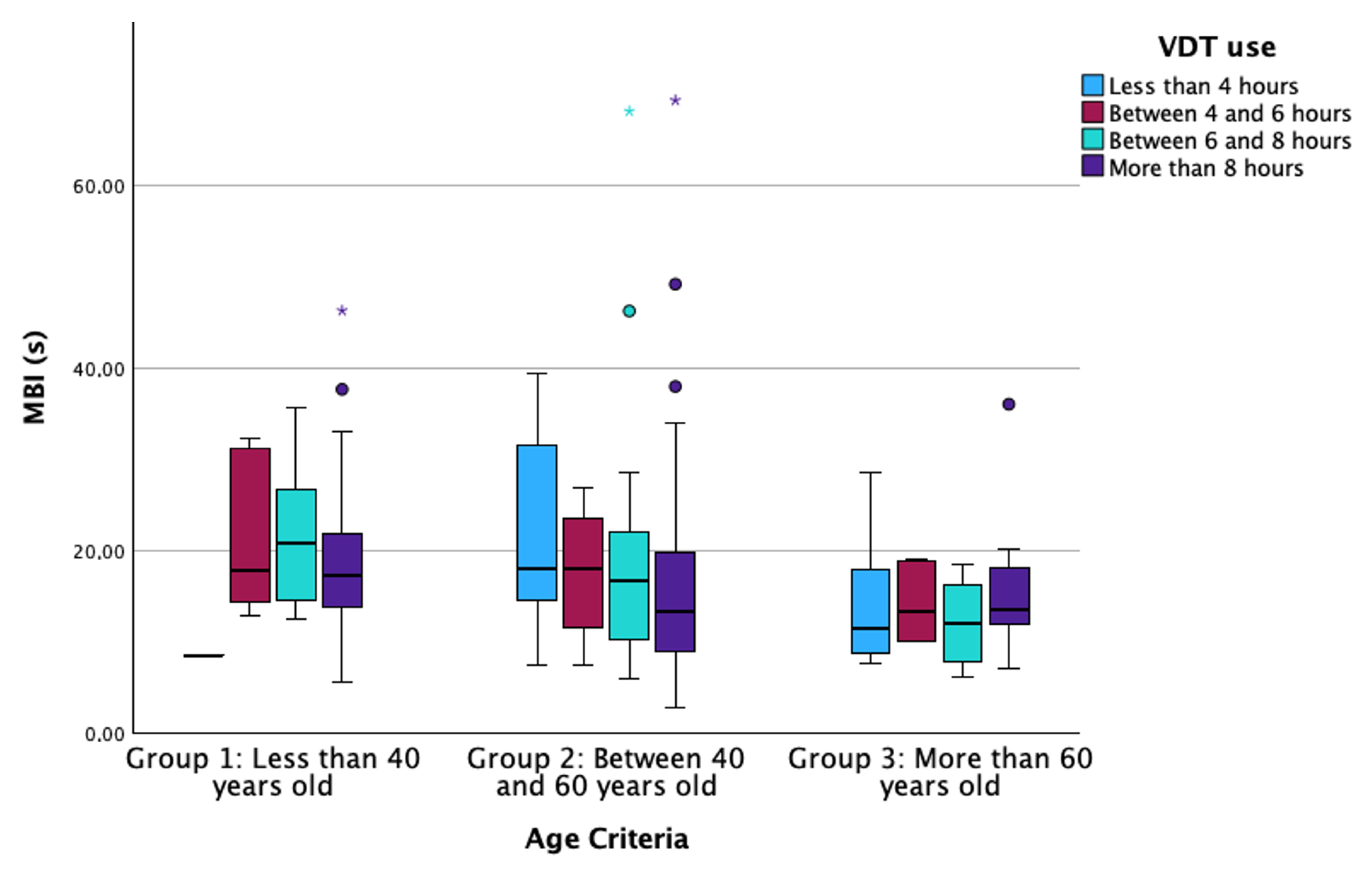
| MBI (s) (Median [IQR]) | 185 | 20.3 [14.9–24.6] | 15.44 [9–20] | 12.6 [8.8–17.5] | 0.006 * |
| Corneal staining (Oxford scheme) (Median [IQR]) | 185 | 0 [0–1] | 1 [0–2] | 1 [0–1.5] | 0.425 † |
| VDT use (Median [IQR]) | 185 | 3 [3–4] | 3 [2–4] | 3 [1–3] | <0.001 †† |
| CL wear (Median [IQR]) | 172 | 1 [0–1] | 0 [0–0] | 0 [0–0] | <0.001 †† |
| Eyedrop use (Median [IQR]) | 172 | 1 [0.5–1] | 0 [0–0] | 1 [0–1] | <0.001 †† |
| Osmolarity | FBUT | MBI | Corneal Staining | VDT Use | CL Wear | Eyedrop Use | ||
|---|---|---|---|---|---|---|---|---|
| OSDI | r | −0.018 * | −0.005 * | −0.006 * | 0.017 † | 0.047 † | 0.086 † | 0.143 † |
| p | 0.811 | 0.947 | 0.932 | 0.814 | 0.523 | 0.262 | 0.061 | |
| Osmolarity | r | −0.145 * | −0.150 * | 0.149 † | 0.072 † | −0.155 † | 0.008 † | |
| p | 0.050 | 0.042 | 0.042 | 0.328 | 0.043 | 0.921 | ||
| FBUT | r | 0.719 * | −0.268 † | 0.011 † | 0.139 † | 0.077 † | ||
| p | <0.001 | 0.001 | 0.881 | 0.069 | 0.313 | |||
| MBI | r | −0.154 † | −0.037 † | 0.150 † | 0.066 † | |||
| p | 0.036 | 0.618 | 0.049 | 0.386 | ||||
| Corneal Staining | r | −0.056 † | −0.047 † | −0.154 † | ||||
| p | 0.450 | 0.537 | 0.044 | |||||
| VDT use | r | 0.123 † | −0.028 † | |||||
| p | 0.107 | 0.716 | ||||||
| CL wear | r | 0.090 † | ||||||
| p | 0.240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Noya-Padin, V.; Giraldez, M.J.; Yebra-Pimentel, E. Influence of Lifestyle Factors on Ocular Surface Parameters in Relation to Age. Life 2024, 14, 1460. https://doi.org/10.3390/life14111460
Garcia-Queiruga J, Pena-Verdeal H, Sabucedo-Villamarin B, Noya-Padin V, Giraldez MJ, Yebra-Pimentel E. Influence of Lifestyle Factors on Ocular Surface Parameters in Relation to Age. Life. 2024; 14(11):1460. https://doi.org/10.3390/life14111460
Chicago/Turabian StyleGarcia-Queiruga, Jacobo, Hugo Pena-Verdeal, Belen Sabucedo-Villamarin, Veronica Noya-Padin, Maria J. Giraldez, and Eva Yebra-Pimentel. 2024. "Influence of Lifestyle Factors on Ocular Surface Parameters in Relation to Age" Life 14, no. 11: 1460. https://doi.org/10.3390/life14111460
APA StyleGarcia-Queiruga, J., Pena-Verdeal, H., Sabucedo-Villamarin, B., Noya-Padin, V., Giraldez, M. J., & Yebra-Pimentel, E. (2024). Influence of Lifestyle Factors on Ocular Surface Parameters in Relation to Age. Life, 14(11), 1460. https://doi.org/10.3390/life14111460











