Abstract
Lanolin is a fatty substance derived from sheep’s fleece. The ancient Greeks used the moisturizing and skin-protective properties of this substance. The technique of industrial production of lanolin was developed in Germany in the 19th century. Since then, this natural wax has become an extremely popular base for many different cosmetic and pharmaceutical preparations intended for the treatment and care of the skin. In addition to its medicinal and cosmetic applications, lanolin is also widely used for industrial purposes. Hypersensitivity to lanolin has raised many questions and controversies for almost 100 years. Although lanolin has significant dermoprotective properties and when applied to intact skin without inflammatory changes, it lubricates it, improves its lipid barrier, and maintains proper moisture, it can also cause contact hypersensitivity when in contact with pathologically changed or damaged skin. It can, in the same person, both protect and damage the skin, depending on the condition of the skin to which the cosmetic or medicine containing lanolin is applied. The nature of the observed reactions and the circumstances of their occurrence, as well as the lack of a clear answer to the question of whether this wax causes allergies or not, make this phenomenon one of the so-called dermatological paradoxes. Although unusual reactions to lanolin have been the subject of research for many years, they still raise many questions to which there is still no clear answer. This is mainly due to the imperfection and incompleteness of the available publications. Although many different studies have been published on hypersensitivity to lanolin, most of them are retrospective analyses of the results of routinely performed epidermal patch tests or descriptions of clinical cases. Such reports and analyses, although undoubtedly very important, are a poor tool for assessing the sensitizing potential of lanolin and/or its derivatives. It is difficult to determine the causative factors, to define lanolin allergens, to investigate immunological mechanisms, or to assess the clinical significance of this phenomenon. There is a definite lack of standardized studies on the nature of lanolin hypersensitivity involving well-selected groups of patients and healthy volunteers, which would be conducted in a reproducible manner under laboratory and/or clinical conditions. As of today, lanolin hypersensitivity seems to be both an old and new problem that still remains unresolved.
1. Introduction
Lanolin is a fatty substance, secreted by a sheep’s skin glands, which protects the animal’s fleece from moisture. It is generally believed that the ancient Greeks were the first to obtain raw wool fat from sheep’s wool and begin using it as a skincare cosmetic. The first writings presenting the process of boiling wool in water to extract the outer layer of fat come from Ancient Greece and date back to 700 BC. In 60 AD, the Greek physician Dioscorides developed an efficient method of obtaining wool fat from sheep fleece [1], and the product obtained as a result of this process, the so-called “oesypum”, is often mentioned in Greek medical writings dating from that period and in much later pharmacopoeias from various countries [2].
In 1882, Otton Braun and Oscar Liebreice developed a method of centrifuging wool fat from the water that remained after washing wool. This technique enabled the industrial production of huge amounts of lanolin. The name “lanolin” was proposed by Otto Braun. This term was created from the Latin words: “lano”—wool and “oleum”—oil, which literally meant “wool oil” and perfectly reflected the origin and nature of this substance [3]. Initially, the name “lanolin” was a patented trademark. Later, however, it began to be commonly used as the generic name of this substance.
For the next 60 years, lanolin, due to its emollient and moisturizing properties, was one of the most common ingredients in both pharmaceutical and cosmetic skincare products. It is also the most frequently used base substance for various types of medicinal ointments (Figure 1) [4].
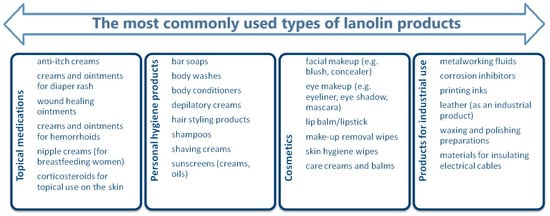
Figure 1.
Commonly used types of products containing lanolin [4].
The first description of a case of contact allergy to lanolin, which occurred in a German patient in the form of a “skin reaction” to Nivea® cream containing lanolin alcohol (eucerite), was reported in 1922 [5]. This description drew attention to a safety issue when using lanolin-based preparations in the context of the possibility of causing contact hypersensitivity. Since then, many case reports of lanolin allergy have been published and many retrospective analyses of this problem have been presented, which will be discussed later in this review. However, there is a lack of independent, well-designed studies on the allergenic properties of lanolin components. The existing studies were performed using well-characterized lanolin derivatives (free from impurities or with known impurities), conducted under standardized conditions, and involving a large group of clinically well-characterized patients and healthy volunteers. The only currently available studies of this type date back to the 1950s [6,7]. However, they were conducted with the participation of only a few patients who were randomly selected (the only condition for inclusion was a hypersensitivity reaction to lanolin products) and have never been repeated.
The problem of hypersensitivity to lanolin, although not new, still remains unresolved. The available retrospective analyses, based mainly on an evaluation of the results of routinely performed diagnostic tests, do not allow for unambiguous and objective conclusions about the nature of hypersensitivity reactions to lanolin derivatives. In addition, they encourage the creation of various assumptions or presumptions that are difficult to translate into clinical situations.
This review is an attempt to collect and summarize the currently available clinical case reports, retrospective analyses, and other publications on hypersensitivity to lanolin in order to draw attention to the simultaneously old and new, and still insufficiently investigated problem of hypersensitivity to lanolin and its derivatives.
2. Methodological Strategy of the Review
Publications reported in the PubMed and Google Scholar databases were analyzed. The search terms used and the preliminary results are presented in Table 1. The last verification of the available data was performed on 17 November 2024. A targeted search was conducted using generally available web browsers for specific publications cited in the analyzed studies, unless they were indicated in PubMed or Google Scholar. After analyzing the available data, the final compilation presents publications (case reports, retrospective analyses, and original studies) from the years 1950–2024.

Table 1.
Search terms used and preliminary results.
3. Lanolin
Lanolin (wool wax, wool fat, wool tallow) is a purified secretory product of the sebaceous glands that is deposited on the wool fibers of sheep (Ovis spp.). Wool wax is obtained from sheep wool by washing. Raw lanolin constitutes approximately 5–25% of the weight of freshly sheared wool. The wool from one sheep can yield approximately 250–300 mL of recoverable wool fat [8,9].
Lanolin is a yellow, fatty substance with a yellow color (from light to dark), quite a hard consistency (density is approx. 0.9 g/cm3), and a specific smell. The melting point of lanolin is 36–42 °C. Heating the wool wax leads to its separation and the eradication of water. Lanolin is highly soluble in fats, sparingly soluble in cold alcohol (performing better in hot alcohol), and is easily soluble in benzene, chloroform, ether, carbon disulfide, acetone, and petroleum ether. Wool tallow does not dissolve in water, but instead absorbs water, creating an emulsion. This natural emulsifier can absorb a mass of water that is equal to twice its own weight, forming stable water/oil emulsions [9,10]. Lanolin is a very chemically unstable substance. It is sensitive to oxidation, which means that it is not stable in aerobic conditions (i.e., the usual conditions for the storage of cosmetics and medicines). The stability of lanolin in cosmetic and pharmaceutical products can be increased by the addition of antioxidants [11,12].
Lanolin is a substance of natural origin, which means that the share of individual substances and their mutual proportions vary, depending on the source material. It is a complex mixture of esters and polyesters. Lanolin does not contain triglycerides, so it is not a fat. The composition of this substance is typical of waxes and varies depending on its state of matter. The main components of lanolin are cholesterol, isocholesterol, and unsaturated monohydric alcohols, both free and combined with lanoceric acid, lanopalmitic acid, carnaubic acid, and other fatty acids. Lanolin also contains the esters of oleic and myristic acids and aliphatic alcohols such as cetyl, ceryl and carnaubyl alcohols, lanosterol, and agnosterol. Using solvent fractionation or crystallization, raw lanolin can be separated into lanolin wax (solid lanolin) and lanolin oil (liquid lanolin) (Table 2) [11,12,13].

Table 2.
Percentage composition of the various forms of lanolin [13].
The physical state of lanolin also affects its properties and suitability for specific applications (Table 3).

Table 3.
Main features and applications of lanolin oil and wax [14].
Lanolin, when used in cosmetic and pharmaceutical applications, is classified as an anhydrous lipophilic base. It has the ability to bind with water-soluble substances, which facilitates the absorption of active ingredients through the skin. Free sterols, their esters, and free and esterified fatty acids make lanolin highly effective for moisturizing [15,16,17].
The composition and structure of lanolin mimic the lipid matrix of the stratum corneum. Due to this similarity, lanolin components can penetrate the structures of the stratum corneum and react with corneocytes at the border of the granular and stratum corneum [18,19]. This allows for overcoming the natural barrier of the stratum corneum for hydrophilic substances and delivering various nutritional and/or therapeutic substances to the dermis [15,16].
Lanolin also has softening and occlusive properties and a natural ability to slow down the loss of moisture from the skin [20].
3.1. Forms, Fractions, and Derivatives of Lanolin
Lanolin wax and lanolin oil (Table 3) are the two basic fractions of lanolin. Other modified and unmodified forms of wool fat are also widely used. These substances have different compositions, which determine their physical and chemical properties. They can also penetrate the epidermis and affect the tissues with varying intensity and effectiveness. These individual characteristics of particular varieties and/or derivatives of lanolin predispose them to specific applications. The largest group of lanolin-derived substances are forms that are chemically modified by hydrolysis, hydrogenation, alkoxylation, acetylation, and transesterification [8,21].
3.1.1. Anhydrous Lanolin
This is a yellowish, persistent, semi-solid fat with a slight odor. It is practically insoluble in water. It is sparingly soluble in alcohol and is easily soluble in benzene, acetone, ether, and petroleum ether. Anhydrous lanolin is able to absorb about twice as much water as it weighs. The melting point of anhydrous lanolin is about 40 °C [11,21]. Anhydrous lanolin is the final product in the lanolin extraction process and is used as a raw material with a wide range of applications in the pharmaceutical and cosmetic industries [11,12].
3.1.2. Hydrated Lanolin
This form of lanolin contains 25–28% water (usually a ratio of 25% water to 75% fat). It is most often used for the production of lubricants and in wood preservation and fabric processing [11,12].
3.1.3. Refined Lanolin
This is a pale yellow solid that is insoluble in water (although soluble in ether and chloroform). It contains 25–30% of absorbed water. A significant part of this form of lanolin consists of high-molecular-weight fatty acids and alcohols. Refined lanolin melts at 40 °C [11,12,21].
3.1.4. Lanolin Alcohols
Lanolin alcohols, including modified lanolin alcohols (e.g., acetylated and oxyoxygenated alcohols and oxypropylene), have a much greater emulsifying ability than unmodified lanolin. For this reason, they are used as plasticizers and dispersants [8,11,12,21].
3.1.5. Eucerite
Eucerite is a mixture of steroidal alcohols: cholesterol and isocholesterol, lanosterol, and higher aliphatic alcohols, obtained from the hydrolysesters contained in lanolin. The history of eucerite dates back to the early 20th century, when in 1902, the German chemist Isaac Lifschütz patented an emulsifying agent that he called “eucerite”, which means “beautiful wax” in Greek [22]. Eucerite became the basis for the development of the first-ever stable oil-in-water emulsions. Mixed with Vaseline, it produces Eucerin (or eucerol), and, in this form, it is the technological base for many medicinal and cosmetic creams, including the extremely popular NIVEA® cream, which has been constantly present on the cosmetics market since 1911. Interestingly, the name of the cream “NIVEA” is derived from the Latin words “nix, nivis” (snow), which refer to the snow-white color of the cream [22].
Eucerin is highly soluble in water, so it is also used as a lubricating ingredient in syndetics, emollients, shampoos, tonics, varnishes, and nail polish removers, as well as in lipsticks and lip glosses [11,12,21,23].
3.1.6. Lanolinic Acids
Lanolinic acids are a mixture of branched fatty acids, hydroxy acids, and other compounds with a low degree of saturation. This group of lanolin derivatives is obtained during the hydrolysis process. Lanolin acids are used as starting compounds for the synthesis of further lanolin derivatives, such as isopropyl esters, glycerol esters, and oxyethylene derivatives. Lanolin acids and their derivatives are used as solubilizers, emulsifiers, dispersants, and wetting agents in cosmetics and pharmaceuticals. Lanolin fatty acids have softening, lubricating, and shiny properties. They work very well in haircare cosmetics (shampoos, conditioners, and lotions) [11,12,21].
3.1.7. Lanolin Esters
The isopropyl esters of lanolin acids are obtained by the esterification of lanolin fatty acids with isopropyl alcohol or the transesterification of raw lanolin. Lanolin esters are less viscous than lanolin and spread better on the skin [21]. Due to the presence of hydroxyl groups, they have surface-active properties, including wetting ability and dispersing pigments. The consistency of lanolin esters depends on their molecular weight and viscosity. Esters with low viscosity and low molecular weight are liquid in nature. The average molecular weight causes them to become substances with a soft and buttery consistency. High-molecular-weight esters are semi-solid [21,24].
The consistency of lanolin isopropyl esters determines their usefulness and application. Liquid ester derivatives are waterproof and are easily absorbed by human skin, strongly smoothing it. They are usually used in oil preparations for baby care. Derivatives of medium molecular weight, which have a buttery consistency, easily become liquid when applied to the skin. They are mainly used in toilet soaps, cosmetic creams, and lotions. Higher-molecular-weight semi-solid derivatives are non-greasy and have good emollient and lubricating properties. They are widely used in lipsticks to reduce drag and improve shine [21,24].
Interestingly, although lanolin isopropyl esters are the most widely used, it is also possible to esterify lanolin acids with alcohols other than isopropanol. For example, after esterification with glycerin, lanolin acids create a mixture of mono-, di-, and tri-ester glycerides, dominated by monoesters. They are typically used as emulsifying emollients in light creams and lotions. The glycerin esters of lanolin often leave a heavy, hydrophobic, non-greasy film on the skin, which can be easily washed off with water and detergents due to its poor resistance to these agents [21,24].
3.1.8. Acetylated Lanolin Derivatives
Acetylated lanolin is an almost odorless, semi-solid, yellowish gooey substance. It has a lower melting point (36 °C) and reduced emulsifying properties than the starting substance. It is also more hydrophobic than lanolin. It dissolves in mineral oils and some vegetable oils. Acetylated lanolin disperses easily in an oil-in-water emulsion. It does not form a water-in-oil emulsion and is used as an auxiliary agent in pharmaceutical preparations and cosmetics, especially the group of sunscreen preparations especially intended for children [20,21,25].
3.1.9. Amerchol L-101
Amerchol L-101 is a substance obtained from the hydrolysis of lanolin (lanolin alcohol or sheep wool alcohol). In cosmetics, Amerchol L-101 is used as a moisturizer and emollient. It is also a component of the base used in topical medicines. Amerchol L-101 is also used in industrial preparations, such as pastes for the care of furniture and leather products. It is also an ingredient of anti-corrosion preparations, paints, and inks. Amerchol L-101 is present in paper, textiles, furs, machine oils and waxes. Amerchol L-101 is also important as a substance that is commonly used in the diagnosis of a contact allergy to lanolin. The substance used for epidermal patch tests is Amerchol L-101 (10% lanolin alcohol dissolved in mineral oil) at 50% in Vaseline [4,12,26,27].
4. Allergy and/or Hypersensitivity to Lanolin
Interest in the problem of possible allergy to lanolin began at the turn of the 1920s and 1930s. In 1922, A. Marcus [5] presented a case concerning a skin reaction that occurred as a result of the use of Nivea® cream containing Eucerin, which is considered the first description of hypersensitivity to lanolin. In 1931, Sulzberger and Morse [28] reported two cases of probable lanolin allergy. A description of three more cases of this hypersensitivity was provided by Sezary [29] in 1936. In turn, in 1939, Bonnevie [30], in a study of 2358 patients, identified a single case of lanolin allergy. In 1947, Ellis [31] described nine cases of contact allergic dermatitis caused by an ointment (“Aquaphor”) based on oxycholesterol, petroleum jelly, and lanolin-derived substances. The author tried to draw attention to the underestimated problem of hypersensitivity to medicinal substances applied to the skin, which may be caused by additional substances that constitute the basis for the preparation of the medicine.
Overall, until 1950, reported clinical cases of the various skin reactions associated with the use of cosmetic or medicinal preparations based on lanolin were rather few [32]. Due to the reports being so few, it was concluded that the problem of hypersensitivity to lanolin and its derivatives was of no major clinical importance, estimating the incidence of this phenomenon in a quite wide range from 0.25% to 18.6% in the general population [32]. It is worth noting that according to data from 2022, among North American patients with dermatitis, the incidence of contact allergy to lanolin ranges from 1.2% to 6.9% of these people [33]. In the European population, this allergy affects approximately 0.4% of people [34].
In the 1950s, subsequent case reports of skin reactions to lanolin again focused attention on this topic. Analysis of the published clinical data on hypersensitivity to lanolin suggests that the problem of allergy to wool wax and its derivatives particularly affects certain groups of patients whose risk of hypersensitivity to lanolin is higher than in the general population (Table 4).

Table 4.
Allergy/hypersensitivity to lanolin: a review of the reports on lanolin allergy published between 1950 and 2024, based on a search of the PubMed database with the queries “lanolin allergy” [35] and “lanolin hypersensitivity” [36].
Based on the data collected in Table 4 and the extensive analysis in the “Safety Assessment of Lanolin-Derived Ingredients as Used in Cosmetics” prepared by the Expert Panel for Cosmetic Ingredient Safety [12], it appears that hypersensitivity to lanolin particularly affects several groups of patients (Table 5).

Table 5.
Groups of patients at high risk of developing hypersensitivity to lanolin.
5. In Search of Lanolin Allergens
The beginning of research on the allergenic potential of lanolin dates back to the 1950s. In 1950, Sulzberger and Lazar [6] published the results of their research, in which they focused on an analysis of the immunogenic properties of lanolin components, both their allergenic properties and the possibility of promoting an autoimmune response. These researchers associated the possible autoimmune potential of lanolin with the similarity of the components in wool fat to components in the secretions of human sebaceous glands (sebum) [6]. During their research, as a result of their analysis of epidemiological data on hypersensitivity to lanolin, Sulzberger and Lazar [6] drew attention to an interesting phenomenon regarding the small number of reported cases of allergic reactions to wool fat in the general population, in the context of the common exposure of people to contact with lanolin and its derivatives.
In the research of Sulzberger and Lazar [6], what draws attention to the allergenic properties of lanolin and its derivatives is the authors’ well-thought-out concept and attention to detail. Unfortunately, and importantly, these studies, although conducted over 70 years ago, still remain the best-planned and most thoroughly executed research project on hypersensitivity to lanolin. For this reason, in this review, both the study project developed by Sulzberger and Lazar [6] and its results are described in detail, in the hope that it will inspire readers to undertake new, definitely needed, studies in this area.
Sulzberger and Lazar [6] enrolled four people in their study. The inclusion criteria were a clinical history of contact hypersensitivity in the skin (Table 6) and a positive reaction in a 48-hour lanolin patch test. To exclude the primary irritating effect of the tested substances, three randomly selected people (without skin contact reactions) were tested with the same substances as the patients.

Table 6.
Clinical characteristics of the patients included in the Sulzberger and Lazar study [6].
The study participants underwent a series of patch tests (48 h) using 12 selected substances that were lanolin components, lanolin from various sources, and chemical substances used in the extraction process of lanolin components. The substances used in the experiment were found in pharmaceutical and cosmetic preparations (base ingredients) (Table 7).

Table 7.
Test substances used in the Sulzberger and Lazar experiment [6].
In all patients, a clearly positive reaction was observed to each of the lanolin preparations (substances Nos. 7–9), a proprietary ointment containing lanolin alcohols (substance No. 2 and No. 6), a fraction of mixed lanolin alcohol, and a fraction of lanolin alcohol mixed with fatty acids (substance No. 4). None of the other tested substances caused skin reactions (Table 8) [6].

Table 8.
Results of the Sulzberger and Lazar experiment [6].
Based on the presented results (Table 8), Sulzberger and Lazar [6] concluded that the immunologically active factor (responsible allergen) was a specific component or components present in the mixture of wool fat alcohols that are not present in other fractions (e.g., fatty acids, cholesterol, and lanosterols). However, this substance has not been definitively identified. The authors also did not find confirmation of their hypothesis about the relationship between hypersensitivity to lanolin and the autoimmune response to sebum.
In subsequent years, Sulzberger et al. [7] extended the previous project by testing the allergenicity of various lanolin derivatives (acetylated derivatives and aliphatic alcohols) and other additional substances (olive oil and cetyl alcohol) found in cosmetics and/or medicines. Nineteen patients with contact-type hypersensitivity were recruited for the study. The results of this project confirmed previous observations indicating that aliphatic lanolin alcohols were the most likely lanolin allergen. At the same time, these researchers noticed that acetylated lanolin derivatives are probably hypoallergenic [7]. Both of these observations were confirmed in the study by Everall and Truter [97], which showed that lanolin alcohols have allergenic properties and acetylation deprives them of this property. In turn, Clark et al. [98,99] noticed that removing the free fatty alcohol fraction from lanolin to a level below 3% results in a reduction in the incidence of hypersensitivity to lanolin by 96%–99%. Independent confirmation of the allergenic potential of lanolin alcohols was provided by the results of studies on an animal model [100,101,102]. Bourrinet and Berkovic [100,101] performed a series of patch tests with lanolin (fresh, in long-term storage, and purified), lanolin alcohols, and semi-liquid lanolin on the skins of guinea pigs. They observed positive reactions only for wool alcohols.
Another candidate for the allergenic component of lanolin was the lanolin sterol fraction [103], although it raised considerable doubts [104,105]. Fregert et al. [102] performed tests with various lanolin fractions on the skins of guinea pigs (including lanolin alcohols and sterol-like fractions) and observed that lanolin alcohols caused hypersensitivity reactions in 10 to 30% of animals, but two sterol-like fractions did not cause such reactions.
It is also worth considering another theory of lanolin allergenicity, which assumes that the long-term storage of wool fat may lead to the oxidation of lanolin and its derivatives. Oxidation changes the conformation of the molecules, which may increase their immunogenicity. Attention was drawn to the connection between the aging process of lanolin and the answer to the question of why some samples of lanolin and its derivatives sometimes cause hypersensitivity reactions and sometimes do not (in the same people) [33,106]. Hjorth and Trolle-Lassen [41] studied 20 patients who were initially diagnosed as sensitive to lanolin and performed patch tests with “fresh” Eucerin and “old” Eucerin (it was stored for 7 years in a tightly sealed package). These researchers noticed that all patients reacted to “old” Eucerin but only 55% of them also had positive results with “fresh” Eucerin. These results suggest that lanolin oxidation products may take on allergenic properties that they did not have before the oxidation process. The use of Eucerin and not lanolin is the weak point of this study. Its composition may vary depending on the origin, source material, and production series. This means that it is impossible to fully predict how the aging of this product will affect its properties because this depends on the share of individual fractions in a specific series of lanolin. For example, Bourrinet and Berkovic [101], when conducting tests on guinea pigs with samples of fresh and old lanolin, did not notice any difference in their sensitizing potential. Of course, the involvement of aging processes in modulating the allergenicity of lanolin cannot be completely ruled out, but this hypothesis certainly requires further, more advanced research with a larger group of patients.
The allergenic properties of lanolin may also be related to the natural origin of this substance. Products from natural sources may contain admixtures of various foreign substances that were present in the original sources. These foreign additives may cause hypersensitivity, regardless of the main substance (in this case, lanolin). It has been proven that sheep wool may contain pesticides from grass spraying, insecticides used to combat the external parasites of sheep, and residues of the detergents or other chemicals used for the washing and technological processing of wool to produce lanolin [99,107,108,109,110,111]. Even if their concentrations are not toxic [109], it cannot be ruled out that each of these “foreign substances” may cause allergies unconnected to lanolin.
6. The Unusual Face of Hypersensitivity to Lanolin—The ”Lanolin Paradox”
In the literature on hypersensitivity to lanolin, non-standard terms have also been used in an attempt to illustrate the unusual features of this allergy and the associated risk, such as the “lanolin paradox” [112], “lanolin myth” [105] or “lanolin—the wolf in the sheep’s clothing” [4]. All these terms are justified by the unusual phenomena and facts related to hypersensitivity to lanolin. If we take into account the general population’s exposure to the lanolin present in medicines, cosmetics, and many other industrial products and household chemicals, the percentage of people allergic to this substance is disproportionately low. For this reason, lanolin is considered a compound with relatively low allergenic potential. However, if we analyze the published reports and clinical cases devoted to lanolin allergy (Table 4), it is easy to see that the percentage of people allergic to this substance in certain groups of patients (such as those with atopic dermatitis or leg ulcers, small children, or the elderly) is higher than in the general population.
The term “lanolin paradox”, used to describe the unusual features of hypersensitivity to lanolin, was proposed in 1996 by R. Wolf [112]. It refers to the term “paraben paradox”, illustrating the surprising face of paraben allergy, which was proposed over 20 years earlier by A.A. Fisher [113].
R. Wolf [112] included four atypical features of lanolin allergy in the “lanolin paradoxes” (Table 9).

Table 9.
“Lanolin paradoxes” according to R. Wolf [112].
According to Wolf [112], the first and second paradoxes are caused by the fact that lanolin is probably a weak sensitizing agent. Therefore, healthy skin with a normal tissue structure is a sufficient barrier for this hapten. In the case of mechanically or inflammatorily damaged skin, lanolin penetrates deeply enough to trigger an immune system response, manifesting itself in a contact hypersensitivity reaction. Several studies have also noted that the use of skin-irritating substances (such as sodium lauryl sulfate or salicylic acid), together with lanolin and/or its derivatives, in patch tests increases the percentage of positive results [32,114,115]. It is also likely that the greater susceptibility of atopic skin to irritation, resulting from damage to the epidermal barrier and inflammation, may affect the results of patch tests with various substances. This causes slight differences in patch-test reaction patterns compared to non-atopic skin [116].
The third lanolin paradox is a direct result of the second lanolin paradox and addresses the fact that patch testing is performed on lesion-free skin. Moreover, according to Wolf [112], a lack of or low reactivity to lanolin in patch tests in patients with lanolin hypersensitivity may be the result of a low concentration of the sensitizing fraction of wool fat, which is most likely lanolin alcohols [6], found in the pure lanolin used in standard skin tests.
Wolf’s fourth lanolin paradox [112] results from the third paradox and was also observed by numerous researchers dealing with hypersensitivity to lanolin. Analysis of the data in Table 4 provides many such observations. A similar diagnostic problem was previously observed by Fisher [113] concerning paraben allergy, and he included these findings in the “paraben paradoxes”. He also suggested that using only a mixture of parabens in the diagnosis of hypersensitivity to these substances does not provide reliable results [113].
It is worth noting that Wolf’s “lanolin paradoxes” theory [112] also has opponents. An interesting example is the position reported by Kligman [105], according to which lanolin allergy is a myth that was created, mainly for commercial purposes, to increase demand for products marked as “lanolin-free”. According to this author, lanolin is, at best, a weak contact allergen and only affects a specific population. Kligman [105] believed that the risk of allergy to products containing lanolin is the result of erroneous scientific knowledge and a misunderstanding of the limitations of patch tests. Moreover, according to Kligman [105], no one has managed to sensitize animals or humans to lanolin or wool wax alcohols.
This statement does not seem to be entirely true, as Bourrinet and Berkovic [100,101] proved in their experiments, which induced hypersensitivity to lanolin and its derivatives in guinea pigs. It is also very interesting that earlier, Kligman [104] had been a strong supporter of the idea of lanolin allergy and pointed out the great importance of this problem.
Lanolin hypersensitivity has also been called a “wolf in sheep’s clothing” [4]. Johnson et al. [4] wanted to point out that despite the many advantages and benefits of using lanolin, we should not forget that wool fat can also be harmful. This is particularly important in those cases where its properties could be most useful (e.g., inflammation and/or skin damage). These authors also drew attention to the interesting fact that allergic contact dermatitis to wool fat is not related to an allergy to wool. Moreover, it is believed that wool allergy probably does not exist [117]. Wool causes non-immunological (non-allergic) irritating contact dermatitis, especially in atopic people [4].
7. Patch Tests for Lanolin Hypersensitivity—A Diagnostic Problem
The unusual nature of hypersensitivity reactions to lanolin (“lanolin paradoxes”) seems to result in difficulties in the diagnosis and diagnosis of contact allergy to wool fat and derivatives.
Diagnostic patch tests are performed on skin that is free from lesions. According to Wolf’s second “lanolin paradox” [112], in patients whose hypersensitivity to lanolin usually occurs at the site of application of medicinal and/or cosmetic products with lanolin, the results of patch tests on skin that is free from lesions may be falsely negative. These patients may tolerate the use of lanolin on healthy skin and develop hypersensitivity on inflamed skin [34,118]. In such a situation, it is possible that hypersensitivity to lanolin will not be confirmed in the diagnostic process.
The fourth “lanolin paradox”, according to Wolf [112], draws attention to the fact that the use of a single substance in epidermal patch tests for the diagnosis of contact hypersensitivity to lanolin may not be effective. Moreover, such a strategy may lead to both false negative and false positive results, and this is largely dependent on the condition of the patient’s skin at the time of testing. An analysis of the literature data presented in Table 4 of this review also allows us to notice that the use of additional patch tests using Amerchol L-101 significantly improves the quality of diagnostics for lanolin allergy. Matthieu and Dockx [61] analyzed routine patch test results in 393 patients diagnosed with contact dermatitis to investigate this issue (Table 10). Substances used in the patch tests were a standard mixture of lanolin alcohols (30%), Amerchol L-101 (100%), and (in 223 patients) Amerchol L-101 (50%). Based on the collected results, the authors noted that the observation that many lanolin allergy diagnoses could be missed in the case of contact allergy when using only a standard test with a mixture of lanolin alcohols (30%) seems to be accurate [61].

Table 10.
Combinations of positive results in the retrospective study of Matthieu and Dockx [61].
Although the validity of such a diagnostic strategy seems to be confirmed by the results of studies from other research teams [27,49,82], there may also be opinions questioning the advisability of testing with many lanolin derivatives. For example, according to Uldahl et al. [118], such a strategy may result in the overdiagnosis of lanolin allergy.
Among the diagnostic difficulties associated with diagnosing hypersensitivity to lanolin, many authors also point out that the reproducibility and repeatability of patch tests for this contact allergy is low. This problem is clearly illustrated by the research of the Bourke team [119] and the Ale team [120]. When Bourke et al. [119] analyzed the available literature data, they noticed that various inconsistencies in the results of this type of test were recorded in as many as 44% of cases. To verify these data, they conducted their own study, in which 383 patients underwent simultaneous, dual-patch tests on opposite sides of the upper back with 10 allergens from the European standard test series. Completely discordant patch test results, i.e., a negative test on one side and a positive test on the opposite side of the back, were recorded in 30 patients (i.e., 8%), including 28 for a single allergen and 2 for more than one allergen. For lanolin, non-compliance concerned 0.8% of the results. A similar study was conducted by Ale et al. [120], who performed simultaneous, dual-patch testing on opposite sides of the upper backs of 500 patients. They used 2 panels, each containing 12 standard allergens. A total of 435 positive patch test reactions were observed in 289 patients (i.e., 58.8%), on either one or both sides of the back. There were 22 (i.e., 5%) pairs of results that were discordant, i.e., they were interpreted as a positive result on one side and a negative or questionable result on the other side of the back. For lanolin alcohol, such an incompatibility was found in 1% of the results [120].
8. Summary and Conclusions
Hypersensitivity to lanolin and/or its derivatives, although not the only unusual phenomenon in dermatology [121,122], has been the subject of observations and studies for almost 100 years. Nevertheless, it still raises many questions and controversies. The significance and importance of this problem seems to be confirmed by the fact that the American Contact Dermatitis Society (ACDS) designated lanolin as the “contact allergen of 2023” [4]. The unusual nature of this allergy has been reflected in various attempts to understand and systematize this phenomenon. Hypersensitivity to lanolin has been called the “lanolin paradox”. There is also the theory of the “lanolin myth”, created for commercial purposes. Lanolin itself has been called “a wolf in sheep’s clothing”. These paradoxical properties of lanolin mean that it is not recommended for use on damaged skin, while, at the same time, it is considered useful for preventive skin care, helping to keep it in good condition.
The fact that lanolin is a substance that both causes and does not cause hypersensitivity in the same patient, depending on the skin’s condition, is a problem that researchers have been studying for decades. Despite many studies and analyses, this problem remains unsolved. Analysis of the available literature data allows us to assume that there is a lack of independent, well-developed programs in lanolin hypersensitivity studies. There is a lack of studies with well-selected, uniform groups of patients, conducted in standardized conditions. There is also a lack of studies concerning various, well-characterized lanolin preparations. Most of the available studies on the problem are studies based on retrospective analyses. In this type of study, neither the patient groups nor the preparations used are unified. This does not allow for the formulation of clear conclusions. The causative factor cannot be clearly determined. It is also impossible to use the results of these analyses to improve diagnostics and therapeutic procedures. Considering the continuing interest in the problem of lanolin hypersensitivity, the unusual nature of this reaction, and the diagnostic and therapeutic problems resulting from it, there seems to be a need to thoroughly investigate and understand all the mechanisms of this reaction, based on well-designed, extensive, standardized research projects.
9. Weaknesses, Limitations, and Possible Perspectives
This review is paradoxically limited by the seemingly large number of studies available for analysis in the field of lanolin hypersensitivity. Available publications are primarily retrospective analyses of test results performed during routine diagnostics or reports from clinical cases. In the context of an attempt to determine the sensitizing potential of lanolin and/or its derivatives, and the pathogenic mechanisms of hypersensitivity to these substances, the value and usefulness of this type of data are significantly limited. These data were collected from a mixed population, during a routine diagnostic process, by different doctors and in different medical facilities. The manner of conducting the clinical interview, the physical examination, the applied diagnostic strategies, test materials, and the manner of documenting medical data may differ. The collected clinical data are often incomplete in such cases (there is no standardized method of data collection), which may hinder coherent analysis. There is also a lack of independent studies involving healthy individuals. There is a lack of studies on cell models and possibly animal models. It seems that carefully planned, standardized, and reproducible studies, conducted under laboratory conditions, will be absolutely necessary to finally explain and understand the paradox of lanolin hypersensitivity.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Lee, B.; Warshaw, E. Lanolin allergy: History, epidemiology, responsible allergens, and management. Dermatitis 2008, 19, 63–72. [Google Scholar] [CrossRef]
- Liebreich, O. Note on the Employment of Impure Lanolin (Oesypus) as an External Application in Classical Times. Glasg. Med. J. 1890, 34, 97–99. [Google Scholar]
- Available online: https://patentimages.storage.googleapis.com/76/06/df/f797626d43e356/US271192.pdf (accessed on 21 June 2024).
- Johnson, H.; Norman, T.; Adler, B.L.; Yu, J. Lanolin: The 2023 American Contact Dermatitis Society Allergen of the Year. Cutis 2023, 112, 78–81. [Google Scholar] [CrossRef]
- Marcus, A. Zum Kapital der Hautkrankheiten auf “nervöser” Basis. Munch. Med. Wochenschr. 1922, 69, 1510. [Google Scholar]
- Sulzberger, M.B.; Lazar, M.P. A study of the allergenic constituents of lanolin (wool fat). J. Investig. Dermatol. 1950, 15, 453–458. [Google Scholar] [CrossRef]
- Sulzberger, M.B.; Warshaw, T.; Herrmann, F. Studies of skin-hypersensitivity to lanolin. J. Investig. Dermatol. 1953, 20, 33–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlossman, M.L.; McCarthy, J.P. Lanolin and its derivatives. J. Am. Oil. Chem. Soc. 1978, 55, 447–450. [Google Scholar] [CrossRef]
- Tinto, W.F.; Elufioye, T.O.; Roach, J. Chapter 22—Waxes. In Pharmacognosy, 1st ed.; Badal, S., Delgoda, R., Eds.; Academic Press: London, UK, 2017; pp. 443–455. [Google Scholar]
- Chapter: Pharmacognosy and Phytochemistry: Drugs Containing Lipids. Available online: https://www.pharmacy180.com/article/lanolin-306/ (accessed on 28 June 2024).
- Sengupta, A.; Behera, J. Comprehensive view on chemistry, manufacturing and applications of lanolin extracted from wool pretreatment. Am. J. Eng. Res. 2014, 3, 33–43. [Google Scholar]
- Safety Assessment of Lanolin-Derived Ingredients as Used in Cosmetics. Available online: https://www.cir-safety.org/sites/default/files/Lanolin.pdf (accessed on 2 July 2024).
- Chemtob, C.; Fawaz, F.; Puiseux, F. Analysis of ointments, oils and waxes. XVIII. Study of the chemical composition of liquid lanolin. II. Study of alcohols. Ann. Pharm. Fr. 1974, 32, 433–441. [Google Scholar]
- Available online: https://www.cir-safety.org/sites/default/files/TAR_Lanolin_032024.pdf (accessed on 24 June 2024).
- Alonso, C.; Collini, I.; Martí, M.; Barba, C.; Coderch, L. Lanolin-Based Synthetic Membranes for Transdermal Permeation and Penetration Drug Delivery Assays. Membranes 2021, 11, 444. [Google Scholar] [CrossRef]
- Carrer, V.; Guzmán, B.; Martí, M.; Alonso, C.; Coderch, L. Lanolin-Based Synthetic Membranes as Percutaneous Absorption Models for Transdermal Drug Delivery. Pharmaceuticals 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Chrzastek, L.; Dondela, B.; Deska, M. Safe ingredients of cosmetics—Lipids and their derivatives. Int. J. Eng. Saf. Sci. 2015, 3, 9–27. [Google Scholar]
- Clark, E.W. Short-term penetration of lanolin into human stratum corneum. J. Soc. Cosmet. Chem. 1992, 43, 219–222. [Google Scholar]
- Clark, E.W.; Steel, I. Investigations into biomechanisms of the moisturizing function of lanolin. J. Soc. Cosmet. Chem. 1993, 44, 181–195. [Google Scholar]
- Pavlou, P.; Siamidi, A.; Varvaresou, A.; Vlachou, M. Skin Care Formulations and Lipid Carriers as Skin Moisturizing Agents. Cosmetics 2021, 8, 89. [Google Scholar] [CrossRef]
- Dixit, S. Lanolin for Silky, Soft, Smooth, Skin. Chem. Weekly 2001, 30, 153–154. [Google Scholar]
- Available online: https://www.nivea.co.uk/about-us/nivea-history (accessed on 24 June 2024).
- Jacob, S. The lanolion-woll wax alcohol update. Consultant 2014, 54, 303–304. [Google Scholar]
- Yao, L.; Hammond, E.G. Isolation and melting properties of branched-chain esters from lanolin. J. Am. Oil. Chem. Soc. 2006, 83, 547–552. [Google Scholar] [CrossRef]
- Płocica, J.; Tal-Figiel, B.; Figiel, W. Correlation between rheological studies and organoleptic cosmetic emulsion with lanolin—Natural emulsifier. Tech. Trans.—Chem. 2013, 1, 61–68. [Google Scholar]
- Uter, W.; Schnuch, A.; Geier, J. Contact sensitization to lanolin alcohols and Amerchol®L101—Analysis of IVDK data. Contact Dermat. 2018, 78, 367–369. [Google Scholar] [CrossRef]
- Knijp, J.; Bruynzeel, D.P.; Rustemeyer, T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermat. 2019, 80, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Sulzberger, M.B.; Morse, J.L. Hypersensitivity to Wool Fat-Report of 2 Cases. JAMA 1931, 96, 2099. [Google Scholar] [CrossRef]
- Sezary, A. Intolerance cutanée a la lanolin. Presse Med. 1936, 93, 1880. [Google Scholar]
- Bonnevie, P. Aetiologie und Pathogenese der Ekzemkrankheiten: Klinische Studien über die Ursachen der Ekzeme unter besonderer Berücksichtigung des diagnostischen Wertes der Ekzemproben. Arch. Derm. Syphilol. 1939, 40, 861–862. [Google Scholar]
- Ellis, F.A. Allergic contact dermatitis due to wool fat and cholesterol. Arch. Derm. Syphilol. 1947, 56, 801–806. [Google Scholar] [CrossRef]
- Thune, P. Allergy to wool fat. The addition of salicylic acid for patch test purposes. Acta Derm. Venereol. 1969, 49, 282–287. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Patel, N.; Warshaw, E.M.; DeKoven, J.G.; Atwater, A.R.; Belsito, D.V.; Dunnick, C.A.; Houle, M.C.; Reeder, M.J.; Maibach, H.I.; et al. Lanolin Allergic Reactions: North American Contact Dermatitis Group Experience, 2001 to 2018. Dermatitis 2022, 33, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.A.; Belsito, D.V. Lanolin. Dermatitis 2023, 34, 4–12. [Google Scholar] [CrossRef]
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=lanolin+allergy&sort=pubdate&size=200 (accessed on 24 June 2024).
- Available online: https://pubmed.ncbi.nlm.nih.gov/?term=lanolin+hypersensitivity&sort=pubdate&size=200 (accessed on 24 June 2024).
- Warshaw, T.G. On the Incidcnce of Allergic Skin Reactions to Lanolin, to its Components and Certain Lanolin Modification. J. Soc. Cosmet. Chem. 1953, 4, 290. [Google Scholar]
- Baer, R.L.; Serri, F.; Weissenbachvial, C. Studies on allergic sensitization to certain topical therapeutic agents. AMA Arch. Derm. 1955, 71, 19–23. [Google Scholar] [CrossRef]
- Hjorth, N. Cosmetic allergy. J. Soc. Cosmet. Chem. 1959, 10, 96–101. [Google Scholar]
- Bandmann, H.J.; Reichenberger, M. Beobachtungen und Untersuchungen zur Frage der durch Eucerin bedingten seltenen Allergie. Der Hautarzt. 1957, 8, 11–13. [Google Scholar] [PubMed]
- Hjorth, N.; Trolle-Lassen, C. Skin reactions to ointment bases. Trans. St. Johns. Hosp. Dermatol. Soc. 1963, 49, 127–140. [Google Scholar]
- Wereide, K. Contact allergy to wool-fat (“lanolin”). Its incidence in a dermatological in-patient department. Acta Derm. Venereol. 1965, 45, 15–18. [Google Scholar]
- Reichenberger, M. Zur epicutanen Sensibilisierung bei Ulcus cruris-Kranken. Über gehäufte Eucerin allergie. Arch. Klin. Exp. Derm. 1965, 223, 56–63. [Google Scholar] [CrossRef]
- Vollum, D.I. Sensitivity to hydrogenated lanolin. Arch. Dermatol. 1969, 100, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. The detection of lanolin allergy. Arch. Dermatol. 1972, 106, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Rantuccio, F.; Meneghini, C.L. Contact dermatitis in patients with leg ulcers. Contact Dermat. 1975, 1, 81–87. [Google Scholar] [CrossRef]
- Sugai, T.; Higashi, J. Hypersensitivity to hydrogenated lanolin. Contact Dermat. 1975, 1, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Hannuksela, M.; Kousa, M.; Pirilä, V. Allergy to ingredients of vehicles. Contact Dermat. 1976, 2, 105–110. [Google Scholar] [CrossRef]
- Mortensen, T. Allergy to lanolin. Contact Dermat. 1979, 5, 137–139. [Google Scholar] [CrossRef] [PubMed]
- von Liebe, V.; Karge, H.J.; Burg, G. Contac turticaria. Hautarzt 1979, 30, 544–546. [Google Scholar] [PubMed]
- Jenni, C.; Zala, L. Eczema of the lower leg—Clinical, allergological and differential diagnostic aspects. Schweiz. Med. Wochenschr. 1980, 110, 124–128. [Google Scholar] [PubMed]
- Förg, T.; Burg, G.; Zirbs, S. Frequency of contact allergy in housewives. Derm. Beruf. Umwelt. 1982, 30, 48–50. [Google Scholar] [PubMed]
- Frenzel, U.; Gutekunst, A. Allergic phenomena in the treatment of leg ulcer. Phlebologie 1985, 38, 389–394. [Google Scholar]
- Edman, B. Sites of contact dermatitis in relationship to particular allergens. Contact Dermat. 1985, 13, 129–135. [Google Scholar] [CrossRef]
- Wilson, C.L.; Cameron, J.; Powell, S.M.; Cherry, G.; Ryan, T.J. High incidence of contact dermatitis in leg-ulcer patients--implications for management. Clin. Exp. Dermatol. 1991, 16, 250–253. [Google Scholar] [CrossRef]
- Lever, R.; Forsyth, A. Allergic contact dermatitis in atopic dermatitis. Acta Derm. Venereol. Suppl. 1992, 176, 95–98. [Google Scholar]
- Pasche-Koo, F.; Piletta, P.A.; Hunziker, N.; Hauser, C. High sensitization rate to emulsifiers in patients with chronic leg ulcers. Contact Dermat. 1994, 31, 226–228. [Google Scholar] [CrossRef]
- Dotterud, L.K.; Falk, E.S. Contact allergy in relation to hand eczema and atopic diseases in north Norwegian schoolchildren. Acta Paediatr. 1995, 84, 402–406. [Google Scholar] [CrossRef]
- Van Ginkel, C.J.; Bruintjes, T.D.; Huizing, E.H. Allergy due to topical medications in chronic otitis externa and chronic otitis media. Clin. Otolaryngol. Allied Sci. 1995, 20, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Schauder, S.; Ippen, H. Contact and photocontact sensitivity to sunscreens. Review of a 15-year experience and of the literature. Contact Dermat. 1997, 37, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Matthieu, L.; Dockx, P. Discrepancy in patch test results with wool wax alcohols and Amerchol L-101. Contact Dermat. 1997, 36, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Le Coz, C.J.; Scrivener, Y.; Santinelli, F.; Heid, E. Sensibilisation de contact au cours des ulcères de jambe (Contact sensitization in leg ulcers). Ann. Dermatol. Venereol. 1998, 125, 694–699. [Google Scholar]
- Reichert-Pénétrat, S.; Barbaud, A.; Weber, M.; Schmutz, J.L. Leg ulcers. Allergologicstudies of 359 cases. Ann. Dermatol. Venereol. 1999, 126, 131–135. [Google Scholar]
- Giordano-Labadie, F.; Rancé, F.; Pellegrin, F.; Bazex, J.; Dutau, G.; Schwarze, H.P. Frequency of contact allergy in children with atopic dermatitis: Results of a prospective study of 137 cases. Contact Dermat. 1999, 40, 192–195. [Google Scholar] [CrossRef]
- Wakelin, S.H.; Smith, H.; White, I.R.; Rycroft, R.J.; McFadden, J.P. A retrospective analysis of contact allergy to lanolin. Br. J. Dermatol. 2001, 145, 28–31. [Google Scholar] [CrossRef]
- Machet, L.; Couhé, C.; Perrinaud, A.; Hoarau, C.; Lorette, G.; Vaillant, L. A high prevalence of sensitization still persists in leg ulcer patients: A retrospective series of 106 patients tested between 2001 and 2002 and a meta-analysis of 1975–2003 data. Br. J. Dermatol. 2004, 150, 929–935. [Google Scholar] [CrossRef]
- Deleo, V.A.; Taylor, S.C.; Belsito, D.V.; Fowler, J.F., Jr.; Fransway, A.F.; Maibach, H.I.; Marks, J.G., Jr.; Mathias, C.G.; Nethercott, J.R.; Pratt, M.D.; et al. The effect of race and ethnicity on patch test results. J. Am. Acad. Dermatol. 2002, 46 (Suppl. S2), S107–S112. [Google Scholar] [CrossRef]
- Kieć-Świerczyńska, M.; Kręcisz, B.; Świerczyńska-Machura, D. Most frequent causes of allergic contact dermatitis in farmers: Based on material in the Nofer Institute of Occupational Medicine, Lodz. Med. Pr. 2003, 54, 237–243. [Google Scholar]
- Machovcova, A.; Dastychova, E.; Kostalova, D.; Vojtechovska, A.; Reslova, J.; Smejkalova, D.; Vaneckova, J.; Vocilkova, A. Common contact sensitizers in the Czech Republic. Patch test results in 12,058 patients with suspected contact dermatitis. Contact Dermat. 2005, 53, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Goon, A.T.; Goh, C.L. Patch testing of Singapore children and adolescents: Our experience over 18 years. Pediatr. Dermatol. 2006, 23, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Oppel, T.; Schnuch, A. The most frequent allergens in allergic contact dermatitis. Dtsch. Med. Wochenschr. 2006, 131, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Tomljanović-Veselski, M.; Lipozencić, J.; Lugović, L. Contact allergy to special and standard allergens in patients with venous ulcers. Coll. Antropol. 2007, 31, 751–756. [Google Scholar] [PubMed]
- Beattie, P.E.; Green, C.; Lowe, G.; Lewis-Jones, M.S. Which children should we patch test? Clin. Exp. Dermatol. 2007, 32, 6–11. [Google Scholar] [CrossRef]
- Smart, V.; Alavi, A.; Coutts, P.; Fierheller, M.; Coelho, S.; Linn Holness, D.; Sibbald, R.G. Contact allergens in persons with leg ulcers: A Canadian study in contact sensitization. Int. J. Low. Extrem. Wounds 2008, 7, 120–125. [Google Scholar] [CrossRef]
- Hogeling, M.; Pratt, M. Allergic contact dermatitis in children: The Ottawa hospital patch-testing clinic experience, 1996 to 2006. Dermatitis 2008, 19, 86–89. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Nelsen, D.D.; Maibach, H.I.; Marks, J.G.; Zug, K.A.; Taylor, J.S.; Rietschel, R.L.; Fowler, J.F.; Mathias, C.G.; Pratt, M.D.; et al. Positive patch test reactions to lanolin: Cross-sectional data from the north american contact dermatitis group, 1994 to 2006. Dermatitis 2009, 20, 79–88. [Google Scholar] [CrossRef]
- Schnuch, A.; Szliska, C.; Uter, W. Facial allergic contact dermatitis. Data from the IVDK and review of literature. Hautarzt 2009, 60, 13–21. [Google Scholar] [CrossRef]
- Nguyen, J.C.; Chesnut, G.; James, W.D.; Saruk, M. Allergic contact dermatitis caused by lanolin (wool) alcohol contained in an emollient in three postsurgical patients. J. Am. Acad. Dermatol. 2010, 62, 1064–1065. [Google Scholar] [CrossRef]
- Minamoto, K. Skin sensitizers in cosmetics and skin care products. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2010, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Beliauskienė, A.; Valiukevičienė, S.; Sitkauskienė, B.; Schnuch, A.; Uter, W. Contact sensitization to the allergens of European baseline series in patients with chronic leg ulcers. Medicina 2011, 47, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Fellinger, C.; Hemmer, W.; Wantke, F.; Wöhrl, S.; Jarisch, R. Severe allergic dermatitis caused by lanolin alcohol as part of an ointment base in propolis cream. Contact Dermat. 2013, 68, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Miest, R.Y.; Yiannias, J.A.; Chang, Y.H.; Singh, N. Diagnosis and prevalence of lanolin allergy. Dermatitis 2013, 24, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.; Pratt, M. Polysensitization in recurrent lip dermatitis. J. Cutan. Med. Surg. 2015, 19, 77–80. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Maibach, H.I.; Taylor, J.S.; Sasseville, D.; DeKoven, J.G.; Zirwas, M.J.; Fransway, A.F.; Mathias, C.G.; Zug, K.A.; DeLeo, V.A.; et al. North American contact dermatitis group patch test results: 2011–2012. Dermatitis 2015, 26, 49–59. [Google Scholar] [CrossRef]
- Belloni Fortina, A.; Cooper, S.M.; Śpiewak, R.; Fontana, E.; Schnuch, A.; Uter, W. Patch test results in children and adolescents across Europe. Analysis of the ESSCA Network 2002–2010. Pediatr. Allergy Immunol. 2015, 26, 446–455. [Google Scholar] [CrossRef]
- Mahler, V. Contact allergies in the elderly. Hautarzt 2015, 66, 665–673. [Google Scholar] [CrossRef]
- Higgins, C.L.; Nixon, R.L. Periorbital Allergic Contact Dermatitis Caused by Lanolin in a Lubricating Eye Ointment. Australas. J. Dermatol. 2016, 57, 68–69. [Google Scholar] [CrossRef]
- Uter, W.; Śpiewak, R.; Cooper, S.M.; Wilkinson, M.; Sánchez Pérez, J.; Schnuch, A.; Schuttelaar, M.L. Contact allergy to ingredients of topical medications: Results of the European Surveillance System on Contact Allergies (ESSCA), 2009–2012. Pharmacoepidemiol. Drug. Saf. 2016, 25, 1305–1312. [Google Scholar] [CrossRef]
- Erfurt-Berge, C.; Geier, J.; Mahler, V. The current spectrum of contact sensitization in patients with chronic leg ulcers or stasis dermatitis - new data from the Information Network of Departments of Dermatology (IVDK). Contact Dermat. 2017, 77, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lubbes, S.; Rustemeyer, T.; Sillevis Smitt, J.H.; Schuttelaar, M.L.; Middelkamp-Hup, M.A. Contact sensitization in Dutch children and adolescents with and without atopic dermatitis - a retrospective analysis. Contact Dermat. 2017, 76, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.E.; McGowan, M.; Silverberg, N.B.; Pelletier, J.L.; Fonacier, L.; Mousdicas, N.; Powell, D.; Scheman, A.; Goldenberg, A. Pediatric Contact Dermatitis Registry Data on Contact Allergy in Children with Atopic Dermatitis. J. Am. Acad. Dermatol. 2017, 153, 765–770. [Google Scholar] [CrossRef]
- Fransen, M.; Overgaard, L.E.K.; Johansen, J.D.; Thyssen, J.P. Contact allergy to lanolin: Temporal changes in prevalence and association with atopic dermatitis. Contact Dermat. 2018, 78, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Pap, E.B.; Temesvári, E.; Németh, I.; Sárdy, M.; Pónyai, G. Contact hypersensitivity in adolescents. Pediatr. Dermatol. 2018, 35, 769–773. [Google Scholar] [CrossRef]
- Rastogi, S.; Patel, K.R.; Singam, V.; Silverberg, J.I. Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis. J. Am. Acad. Dermatol. 2018, 79, 1028–1033. [Google Scholar] [CrossRef]
- Németh, D.; Pónyai, G. Contact Allergy in the Elderly: A Study of 600 Patients. Life 2022, 12, 1228. [Google Scholar] [CrossRef]
- Németh, D.; Temesvári, E.; Holló, P.; Pónyai, G. Preservative Contact Hypersensitivity among Adult Atopic Dermatitis Patients. Life 2022, 12, 715. [Google Scholar] [CrossRef]
- Everall, J.; Truter, E.V. Cutaneous hypersensitivity to lanolin; investigation of one case. J. Investig. Dermatol. 1954, 22, 493–496. [Google Scholar] [CrossRef]
- Clark, E.W.; Cronin, E.; Wilkinson, D.S. Lanolin with reduced sensitizing potential. A preliminary note. Contact Dermat. 1977, 3, 69–74. [Google Scholar] [CrossRef]
- Clark, E.W.; Blondeel, A.; Cronin, E.; Oleffe, J.A.; Wilkinson, D.S. Lanolin of reduced sensitizing potential. Contact Dermat. 1981, 7, 80–83. [Google Scholar] [CrossRef]
- Bourrinet, P.; Berkovic, A. Cutaneous hypersensitivity tests in guinea pig of lanolin and derivatives. Ann. Pharm. Fr. 1980, 38, 483–492. [Google Scholar]
- Bourrinet, P.; Berkovic, A. Tests of skin hypersensitivity of guinea pigs to lanolin and derivatives used in cosmetology. Int. J. Cosmet. Sci. 1981, 3, 115–123. [Google Scholar] [CrossRef]
- Fregert, S.; Dahlquist, I.; Trulsson, L. An attempt to isolate and identify allergens in lanolin. Contact Dermat. 1984, 10, 16–19. [Google Scholar] [CrossRef]
- Oleffe, L.A.; Blondeel, A.; Boschmans, S. Patchtesting with lanolin. Contact Dermat. 1978, 4, 233–247. [Google Scholar] [CrossRef]
- Kligman, A.M. Lanolin allergy: Crisis or comedy. Contact Dermat. 1983, 9, 99–107. [Google Scholar] [CrossRef]
- Kligman, A.M. The myth of lanolin allergy. Contact Dermat. 1998, 39, 103–107. [Google Scholar] [CrossRef]
- Schwarzfeld, H.K. Sensitivity to ointments containing wool fat. U.S. Armed Forces Med. J. 1952, 3, 1371–1375. [Google Scholar]
- Pérez, A.; González, G.; González, J.; Heinzen, H. Multiresidue Determination of Pesticides in Lanolin Using Matrix Solid-Phase Dispersion. J. AOAC Int. 2010, 93, 712–719. [Google Scholar] [CrossRef]
- Nurse, D.S. Dangers of the application of lanolin. Med. J. Aust. 1987, 146, 560. [Google Scholar] [CrossRef]
- Morse, J. The hazards of lanolin. MCN Am. J. Matern. Child. Nurs. 1989, 14, 204. [Google Scholar] [CrossRef]
- Paton, M.W.; Petterson, D.S. Absorption by sheep of dieldrin from contaminated soil. Aust. Vet. J. 1997, 75, 441–445. [Google Scholar] [CrossRef]
- Rosanove, R. Dangers of the application of lanolin. Med. J. Aust. 1987, 146, 232. [Google Scholar] [CrossRef]
- Wolf, R. The lanolin paradox. Dermatology 1996, 192, 198–202. [Google Scholar] [CrossRef]
- Fisher, A.A. The paraben paradox. Cutis 1973, 12, 177–181. [Google Scholar]
- Geier, J.; Uter, W.; Pirker, C.; Frosch, P.J. Patch testing with the irritant sodium lauryl sulfate (SLS) is useful in interpreting weak reactions to contact allergens as allergic or irritant. Contact Dermat. 2003, 48, 99–107. [Google Scholar] [CrossRef]
- Uter, W.; Hegewald, J.; Pfahlberg, A.; Pirker, C.; Frosch, P.J.; Gefeller, O. The association between ambient air conditions (temperature and absolute humidity), irritant sodium lauryl sulfate patch test reactions and patch test reactivity to standard allergens. Contact Dermat. 2003, 49, 97–102. [Google Scholar] [CrossRef]
- Brasch, J.; Schnuch, A.; Uter, W. Patch-test reaction patterns in patients with a predisposition to atopic dermatitis. Contact Dermat. 2003, 49, 197–201. [Google Scholar] [CrossRef]
- Zallmann, M.; Smith, P.K.; Tang, M.L.K.; Spelman, L.J.; Cahill, J.L.; Wortmann, G.; Katelaris, C.H.; Allen, K.J.; Su, J.C. Debunking the Myth of Wool Allergy: Reviewing the Evidence for Immune and Non-immune Cutaneous Reactions. Acta Derm. Venereol. 2017, 97, 906–915. [Google Scholar] [CrossRef]
- Uldahl, A.; Engfeldt, M.; Svedman, C. Clinical relevance of positive patch test reactions to lanolin: A ROAT study. Contact Dermat. 2021, 84, 41–49. [Google Scholar] [CrossRef]
- Bourke, J.F.; Batta, K.; Prais, L.; Abdullah, A.; Foulds, I.S. The reproducibility of patch tests. Br. J. Dermatol. 1999, 140, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Ale, S.I.; Maibach, H.I. Reproducibility of patch test results: A concurrent right-versus-left study using TRUE Test. Contact Dermat. 2004, 50, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Adya, K.A.; Inamadar, A.C.; Palit, A. Paradoxes in dermatology. Indian Dermatol. Online J. 2013, 4, 133–142. [Google Scholar]
- Türsen, Ü. Paradoxes in Aesthetic Dermatology. J. Turk. Acad. Dermatol. 2019, 13. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).