Impact of Diaphragm-Strengthening Core Training on Postural Stability in High-Intensity Squats
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
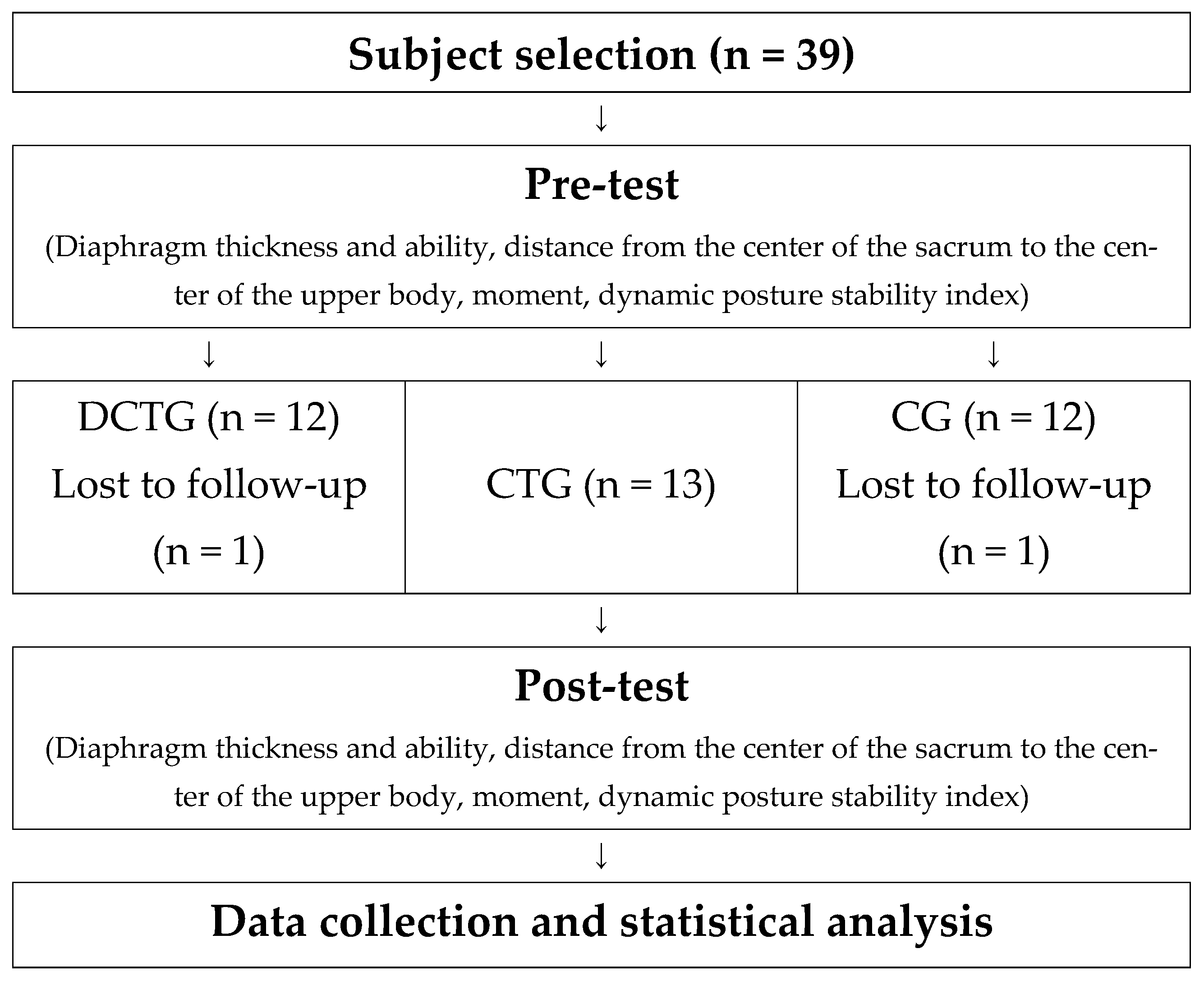
2.2. Research Design
2.3. Experimental Procedures
2.4. Measurement Methods
2.4.1. Diaphragm Thickness Measurement
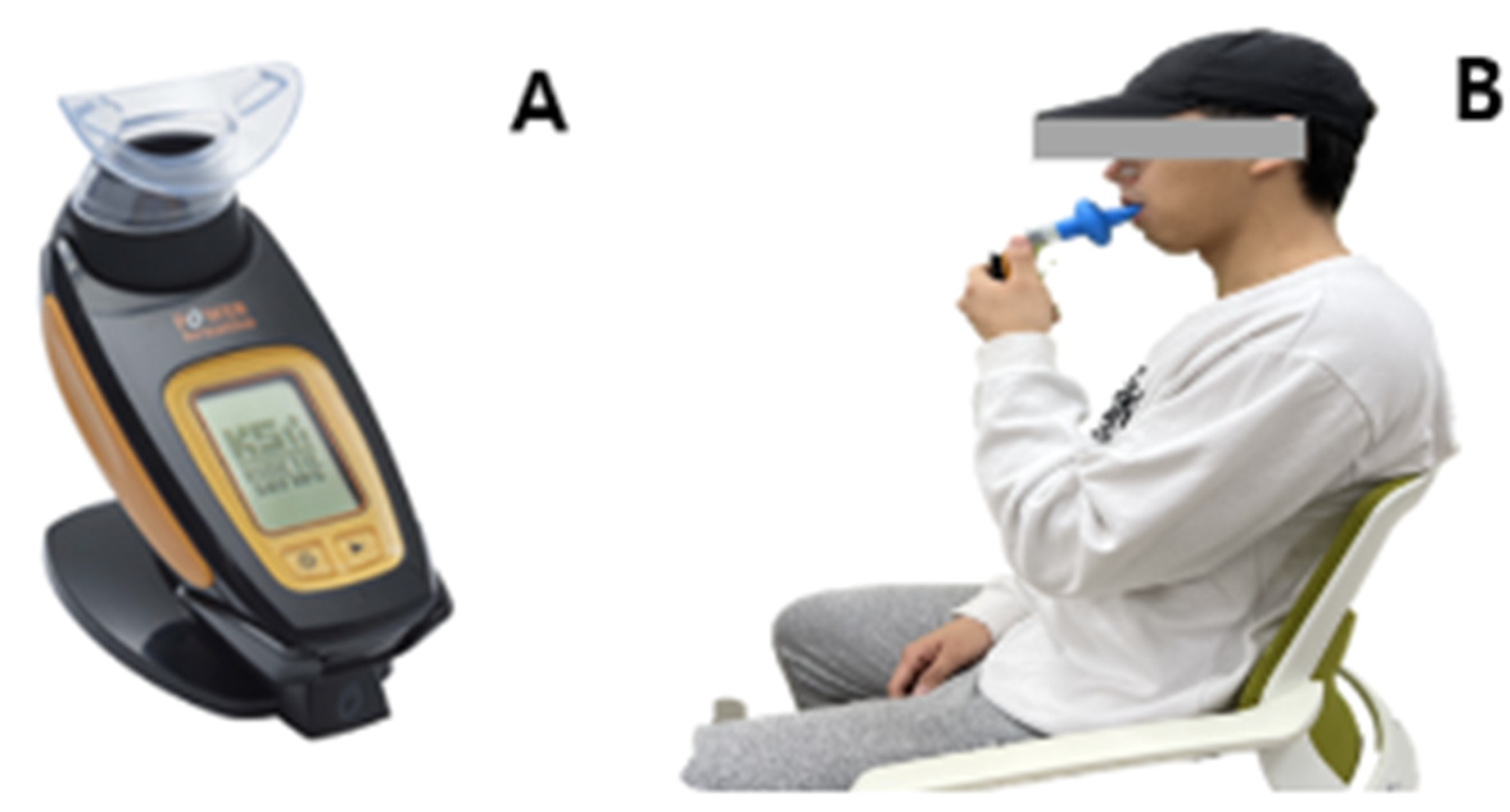
2.4.2. Diaphragm Function Assessment
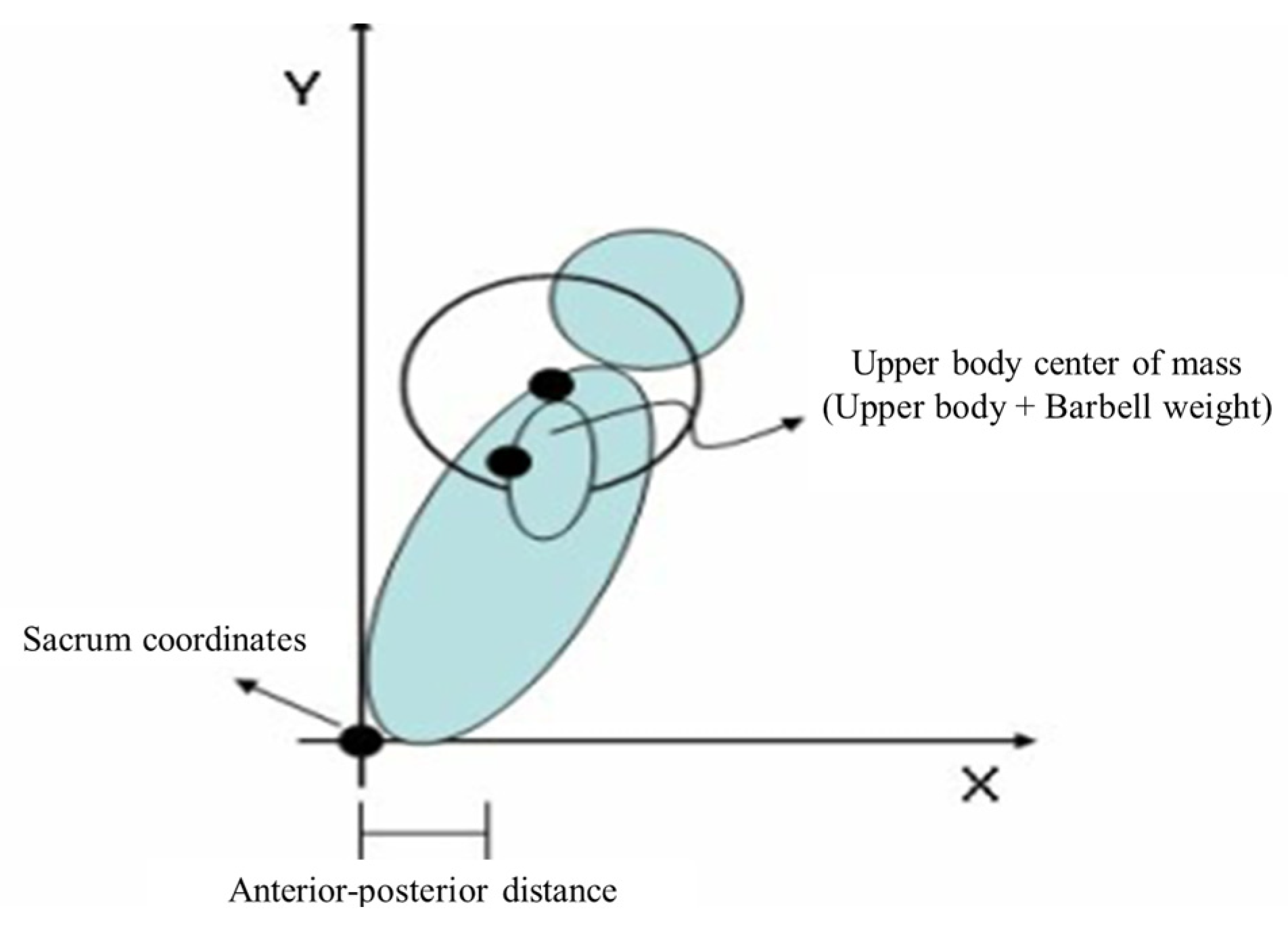
2.4.3. Squat Motion Analysis
2.4.4. Dynamic Postural Stability Assessment
2.4.5. Squat Testing Protocol
2.5. Exercise Programs
2.5.1. Diaphragmatic Training
2.5.2. Core Training
2.6. Statistical Analysis
3. Results
3.1. Effects of Diaphragm-Strengthening Core Training on Diaphragm Thickness and Function
3.2. Changes in Squat Posture Following Diaphragm-Strengthening Core Training
4. Discussion
4.1. Changes in Diaphragm Thickness and Respiratory Function
4.2. Changes in Postural Stability During High-Intensity Squats
4.3. Changes in Dynamic Stability
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sprague, E.; Reynolds, S.; Brindley, P. Patient isolation precautions: Are they worth it? Can. Respir. J. 2016, 2016, 5352625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Escamilla, R.F.; Fleisig, G.S.; Zheng, N.; Lander, J.E.; Barrentine, S.W.; Andrews, J.R.; Bergemann, B.W.; Moorman, C.T. Effects of technique variations on knee biomechanics during the squat and leg press. Med. Sci. Sports Exerc. 2001, 33, 1552–1566. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.C.; Smith, J.C.; Schilling, B.K. Effects of knee position on hip and knee torques during the barbell squat. J. Strength Cond. Res. 2003, 17, 629–633. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, K.W.; Langford, G.A.; Doscher, M.W.; Wiley, L.P.; Mallard, K.G. The effects of short-term unilateral and bilateral lower-body resistance training on measures of strength and power. J. Strength Cond. Res. 2005, 19, 9–15. [Google Scholar] [CrossRef]
- Králik, S.; Freudenfeld, D.; Gurín, D. Changes in the activity of selected muscles during deep squat correction. Slovak. J. Sports Sci. 2021, 7, 12–24. [Google Scholar] [CrossRef]
- Donnelly, D.V.; Berg, W.P.; Fiske, D.M. The effect of the direction of gaze on the kinematics of the squat exercise. J. Strength Cond. Res. 2006, 20, 145–150. [Google Scholar] [CrossRef]
- Williams, K.R. Biomechanical factors contributing to injury during trunk loading. Ergonomics 1980, 23, 135–146. [Google Scholar] [CrossRef]
- Stokes, I.A.; Gardner-Morse, M.; Henry, S.M.; Badger, G.J. Decrease in trunk muscular response to perturbation with preactivation of lumbar spinal musculature. Spine 2000, 25, 1957–1964. [Google Scholar] [CrossRef]
- Franklin, T.C.; Granata, K.P. Role of reflex gain and reflex delay in spinal stability—A dynamic simulation. J. Biomech. 2007, 40, 1762–1767. [Google Scholar] [CrossRef]
- Cholewicki, J.; Ivancic, P.C.; Radebold, A. Can increased intra-abdominal pressure in humans be decoupled from trunk muscle co-contraction during steady state isometric exertions? Eur. J. Appl. Physiol. 2002, 87, 127–133. [Google Scholar] [CrossRef]
- Lee, P.J.; Rogers, E.L.; Granata, K.P. Active trunk stiffness increases with co-contraction. J. Electromyogr. Kinesiol. 2006, 16, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.R.; Myer, G.D.; Hewett, T.E. Increased trunk motion in female athletes compared to males during single leg landing. Med. Sci. Sports Exerc. 2007, 39, S70. [Google Scholar] [CrossRef]
- Myer, G.D.; Chu, D.A.; Brent, J.L.; Hewett, T.E. Trunk and hip control neuromuscular training for the prevention of knee joint injury. Clin. Sports Med. 2008, 27, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Essendrop, M.; Andersen, T.B.; Schibye, B. Increase in spinal stability obtained at levels of intra-abdominal pressure and back muscle activity realistic to work situations. Appl. Ergon. 2002, 33, 471–476. [Google Scholar] [CrossRef]
- Baechle, T.; Earle, W. Essentials of Strength Training and Conditioning; Human Kinetics Publishers: Champaign, IL, USA, 2008. [Google Scholar]
- Barker, P.J.; Briggs, C.A.; Bogeski, G. Tensile transmission across the lumbar fasciae in unembalmed cadavers: Effects of tension to various muscular attachments. Spine 2004, 29, 129–138. [Google Scholar] [CrossRef]
- Janssens, L.; McConnell, A.K.; Pijnenburg, M.; Claeys, K.; Goossens, N.; Lysens, R.; Troosters, T.; Brumagne, S. Inspiratory muscle training affects proprioceptive use and low back pain. Med. Sci. Sports Exerc. 2015, 47, 12–19. [Google Scholar] [CrossRef]
- Hodges, P.W.; Moseley, G.L.; Gabrielsson, A.; Gandevia, S.C. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp. Brain Res. 2003, 151, 262–271. [Google Scholar] [CrossRef]
- Cholewicki, J.; Juluru, K.; McGill, S.M. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J. Biomech. 1999, 32, 13–17. [Google Scholar] [CrossRef]
- Williams, M.J.; Gibson, N.V.; Sorbie, G.G.; Ugbolue, U.C.; Brouner, J.; Easton, C. Activation of the gluteus maximus during performance of the back squat, split squat, and barbell hip thrust and the relationship with maximal sprinting. J. Strength Cond. Res. 2021, 35, 16–24. [Google Scholar] [CrossRef]
- Vantrease, W.C.; Townsend, J.R.; Sapp, P.A.; Henry, R.N.; Johnson, K.D. Maximal strength, muscle activation, and bar velocity comparisons between squatting with a traditional or safety squat bar. J. Strength Cond. Res. 2021, 35 (Suppl. 1), S1–S5. [Google Scholar] [CrossRef]
- Coratella, G.; Tornatore, G.; Caccavale, F.; Longo, S.; Esposito, F.; Cè, E. The activation of gluteal, thigh, and lower back muscles in different squat variations performed by competitive bodybuilders: Implications for resistance training. Int. J. Environ. Res. Public Health 2021, 18, 772. [Google Scholar] [CrossRef] [PubMed]
- Warneke, K.; Keiner, M.; Schiemann, S.; Lohmann, L.; Wirth, K. Influence of maximal strength performance in front squat and deadlift on linear sprint and jump performance in male youth elite basketball players. Ger. J. Exerc. Sport Res. 2023, 53, 10–18. [Google Scholar] [CrossRef]
- Junior, G.D.B.V.; Passos, R.P.; Júnior, J.R.G.; dos Santos Carvalho, A.; Abdalla, P.P. Squat with front and back bar position in parallel and sumo feet techniques: A cross-sectional study. Rev. CPAQV 2022, 14, 2. [Google Scholar]
- Barrett, K.B.; Sievert, Z.A.; Bennett, H.J. A comparison of squat depth and sex on knee kinematics and muscle activation. J. Biomech. Eng. 2023, 145, 071010. [Google Scholar] [CrossRef]
- Chan, C.K.; Azah, H.N.; Yeow, C.H.; Goh, S.K.; Ting, H.N.; Salmah, K. Effects of squatting speed and depth on lower extremity kinematics, kinetics and energetics. J. Mech. Med. Biol. 2022, 22, 2250032. [Google Scholar] [CrossRef]
- Sinclair, J.; Taylor, P.J.; Jones, B.; Butters, B.; Bentley, I.; Edmundson, C.J. A multi-experiment investigation of the effects stance width on the biomechanics of the barbell squat. Sports 2022, 10, 136. [Google Scholar] [CrossRef]
- Boon, A.J.; Harper, C.J.; Ghahfarokhi, L.S.; Strommen, J.A.; Watson, J.C.; Sorenson, E.J. Two-dimensional ultrasound imaging of the diaphragm: Quantitative values in normal subjects. Muscle Nerve 2013, 47, 884–889. [Google Scholar] [CrossRef]
- Hellyer, N.J.; Andreas, N.M.; Bernstetter, A.S.; Cieslak, K.R.; Donahue, G.F.; Steiner, E.A.; Hollman, J.H.; Boon, A.J. Comparison of diaphragm thickness measurements among postures via ultrasound imaging. PM R 2017, 9, 21–25. [Google Scholar] [CrossRef]
- Minahan, C.; Sheehan, B.; Doutreband, R.; Kirkwood, T.; Reeves, D.; Cross, T. Repeated-sprint cycling does not induce respiratory muscle fatigue in active adults: Measurements from the powerbreathe® inspiratory muscle trainer. J. Sports Sci. Med. 2015, 14, 233–238. [Google Scholar]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Tillman, M.D.; Schenker, S.M.; Borsa, P.A. Jump-landing direction influences dynamic postural stability scores. J. Sci. Med. Sport 2008, 11, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, L.A.; McConnell, A.K. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur. J. Appl. Physiol. 2007, 99, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Romer, L.M.; McConnell, A.K.; Jones, D.A. Inspiratory muscle fatigue in trained cyclists: Effects of inspiratory muscle training. Med. Sci. Sports Exerc. 2002, 34, 785–792. [Google Scholar] [CrossRef]
- Klusiewicz, A.; Borkowski, L.; Zdanowicz, R.; Boros, P.; Wesołowski, S. The inspiratory muscle training in elite rowers. J. Sports Med. Phys. Fitness 2008, 48, 279–284. [Google Scholar]
- Cosio-Lima, L.M.; Reynolds, K.L.; Winter, C.; Paolone, V.; Jones, M.T. Effects of physioball and conventional floor exercises on early phase adaptations in back and abdominal core stability and balance in women. J. Strength Cond. Res. 2003, 17, 721–725. [Google Scholar] [CrossRef]
- Mills, J.D.; Taunton, J.E.; Mills, W.A. The effect of a 10-week training regimen on lumbo-pelvic stability and athletic performance in female athletes: A randomized-controlled trial. Phys. Ther. Sport 2005, 6, 60–66. [Google Scholar] [CrossRef]
- American College of Sports Medicine (ACSM). ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Clark, M.A.; Lucett, S.; Corn, R.J. NASM Essentials of Personal Fitness Training; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Levitzky, M.G. Pulmonary Physiology, 7th ed.; McGraw-Hill Medical: New York, NY, USA, 2007. [Google Scholar]
- Kraemer, W.J.; Fleck, S.J.; Evans, W.J. Strength and power training: Physiological mechanisms of adaptation. Exerc. Sport Sci. Rev. 1996, 24, 363–397. [Google Scholar] [CrossRef]
- Enright, S.J.; Unnithan, V.B.; Heward, C.; Withnall, L.; Davies, D.H. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys. Ther. 2006, 86, 345–354. [Google Scholar] [CrossRef]
- Helms, E.R.; Byrnes, R.K.; Cooke, D.M.; Haischer, M.H.; Carzoli, J.P.; Johnson, T.K.; Cross, M.R.; Cronin, J.B.; Storey, A.G.; Zourdos, M.C. RPE vs. percentage 1RM loading in periodized programs matched for sets and repetitions. Front. Physiol. 2018, 9, 247. [Google Scholar] [CrossRef]
- Keens, T.G.; Bryan, A.C.; Levison, H.; Ianuzzo, C.D. Developmental pattern of muscle fiber types in human ventilatory muscles. J. Appl. Physiol. 1978, 44, 909–913. [Google Scholar] [CrossRef]
- Leith, D.E.; Bradley, M. Ventilatory muscle strength and endurance training. J. Appl. Physiol. 1976, 41, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Brannon, F.J.; Foley, M.W.; Starr, J.A.; Saul, L.M. Cardiopulmonary Rehabilitation: Basic Theory & Application, 3rd ed.; F.A. Davis Company: Philadelphia, PA, USA, 1998. [Google Scholar]
- Gething, A.D.; Williams, M.; Davies, B. Inspiratory resistive loading improves cycling capacity: A placebo controlled trial. Br. J. Sports Med. 2004, 38, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.; Stanton, W.; Wilson, S.; Freke, M.; McMahon, S.; Sims, K. Retraining motor control of abdominal muscles among elite cricketers with low back pain. Scand. J. Med. Sci. Sports 2012, 22, 517–525. [Google Scholar] [CrossRef]
- Mohan, V.; Paungmali, A.; Sitilertpisan, P.; Henry, L.J.; Omar, F.A.; Azhar, F.Z. The effect of core stability training with ball and balloon exercise on respiratory variables in chronic non-specific low back pain: An experimental study. J. Bodyw. Mov. Ther. 2020, 24, 196–202. [Google Scholar] [CrossRef]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Reid, W.D. Effects of respiratory muscle training on performance in athletes: A systematic review with meta-analyses. J. Strength Cond. Res. 2013, 27, 1643–1663. [Google Scholar] [CrossRef]
- Seixas, M.B.; Almeida, L.B.; Trevizan, P.F.; Martinez, D.G.; Laterza, M.C.; Vanderlei, L.C.M.; Silva, L.P. Effects of Inspiratory Muscle Training in Older Adults. Respir. Care 2020, 65, 535–544. [Google Scholar] [CrossRef]
- Downey, A.E.; Chenoweth, L.M.; Townsend, D.K.; Ranum, J.D.; Ferguson, C.S.; Harms, C.A. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir. Physiol. Neurobiol. 2007, 156, 137–146. [Google Scholar] [CrossRef]
- Condessa, R.L.; Brauner, J.S.; Saul, A.L.; Baptista, M.; Silva, A.C.T.; Vieira, S.R.R. Inspiratory muscle training did not accelerate weaning from mechanical ventilation but did improve tidal volume and maximal respiratory pressures: A randomised trial. J. Physiother. 2013, 59, 101–107. [Google Scholar] [CrossRef]
- Neves, L.; Chiappa, A.; da Silva, V.; Vieira, P.; Cipriano, G.; Arena, R.; Chiappa, G. Comparative effects of inspiratory muscle training and resistance training on respiratory and skeletal muscle strength in COPD: Responses of the pulmonary rehabilitation program. Eur. Respir. J. 2014, 44, 598. [Google Scholar]
- Kraemer, W.J.; Adams, K.; Cafarelli, E.; Dudley, G.A.; Dooly, C.; Feigenbaum, M.S.; Fleck, S.J.; Franklin, B.; Fry, A.C.; Hoffman, J.R.; et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2002, 34, 364–380. [Google Scholar] [CrossRef]
- Morton, R.W.; Oikawa, S.Y.; Wavell, C.G.; Mazara, N.; McGlory, C.; Quadrilatero, J.; Baechler, B.L.; Baker, S.K.; Phillips, S.M. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J. Appl. Physiol. 2016, 121, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and hypertrophy adaptations between low-vs. high-load resistance training: A systematic review and meta-analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Schoenfeld, B.J. Are the hypertrophic adaptations to high and low-load resistance training muscle fiber type specific? Front. Physiol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Cresswell, A.G.; Daggfeldt, K.; Thorstensson, A. Three dimensional preparatory trunk motion precedes asymmetrical upper limb movement. Gait Posture 2000, 11, 92–101. [Google Scholar] [CrossRef]
- Russell, P.J.; Phillips, S.J. A preliminary comparison of front and back squat exercises. Res. Q. Exerc. Sport 1989, 60, 201–208. [Google Scholar] [CrossRef]
- Raymakers, J.A.; Samson, M.M.; Verhaar, H.J.J. The assessment of body sway and the choice of stability parameter(s). Gait Posture 2005, 21, 48–58. [Google Scholar] [CrossRef]
- Bojairami, I.E.; Driscoll, M. Coordination between trunk muscles, thoracolumbar fascia, and intra-abdominal pressure toward static spine stability. Spine 2022, 47, E423–E431. [Google Scholar] [CrossRef]
- Fan, C.; Fede, C.; Gaudreault, N.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Anatomical and functional relationships between external abdominal oblique muscle and posterior layer of thoracolumbar fascia. Clin. Anat. 2018, 31, 1092–1098. [Google Scholar] [CrossRef]
- Vleeming, A.; Schuenke, M. Form and force closure of the sacroiliac joints. PM R 2019, 11, S24–S31. [Google Scholar] [CrossRef]
- Kitamura, T.; Kido, A.; Ishida, Y.; Kobayashi, Y.; Tsukamoto, S.; Tanaka, Y. Muscle activity pattern with a shifted center of pressure during the squat exercise. J. Sports Sci. Med. 2019, 18, 248–252. [Google Scholar] [PubMed Central]
- Braidot, A.A.; Brusa, M.H.; Lestussi, F.E.; Parera, G.P. Biomechanics of front and back squat exercises. J. Phys. Conf. Ser. 2007, 90, 012009. [Google Scholar] [CrossRef]
- Zink, A.J.; Whiting, W.C.; Vincent, W.J.; McLaine, A.J. The effects of a weight belt on trunk and leg muscle activity and joint kinematics during the squat exercise. J. Strength Cond. Res. 2001, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Lander, J.E.; Simonton, R.L.; Giacobbe, J.K. The effectiveness of weight-belts during the squat exercise. Med. Sci. Sports Exerc. 1990, 22, 117–126. [Google Scholar] [CrossRef]
- Gholami-Borujeni, B.; Yalfani, A.; Ahmadnezhad, L. Eight-week inspiratory muscle training alters electromyography activity of the ankle muscles during overhead and single-leg squats: A randomized controlled trial. J. Appl. Biomech. 2020, 37, 13–20. [Google Scholar] [CrossRef]
- Bazrgari, B.; Shirazi-Adl, A.; Arjmand, N. Analysis of squat and stoop dynamic liftings: Muscle forces and internal spinal loads. Eur. Spine J. 2007, 16, 687–699. [Google Scholar] [CrossRef]

| Group | Age (yrs) | Height (cm) | Weight (kg) |
|---|---|---|---|
| DCGT (n = 12) | 21.75 ± 3.04 | 176.97 ± 7.48 | 75.13 ± 8.43 |
| CTG (n = 13) | 21.23 ± 1.58 | 176.36 ± 7.27 | 74.55 ± 7.33 |
| CG (n = 12) | 22.08 ± 1.67 | 176.33 ± 5.84 | 76.68 ± 8.87 |
| Sig | 0.622 | 0.967 | 0.803 |
| Workout Types | Set | Rest | Intensity | |
|---|---|---|---|---|
| Warm up (5 min) | Stretching | RPE 9 | ||
| Main exercise (40 min) | Hip bridge, Plank Side Plank, crunches oblique crunches Dead bug (statics, dynamics) Bird dog (statics, dynamics) | 4 set | 60 sec | RPE 12–15 |
| Side Plank Rotation, Plank single-leg tuck Plank shoulder taps, Crawling Dead bug (one arm & one leg down) Bird dog (one arm & one leg down) | ||||
| Cool down (5 min) | Stretching | RPE 9 | ||
| Group | Pre (M ± SD) | Post (M ± SD) | Correction (M ± SD) | ∆% | p-Value | Post-Hoc | ANCOVA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | DF | MS | F-Value | p | |||||||
| The change of diaphragm thickness (Unit: mm) | |||||||||||
| DCTG (n = 12) | 2.34 ± 0.33 | 3.15 ± 0.41 | 3.06 ± 0.06 | 34.62 | 0.000 *** | a > b, c | 2.145 | 1 | 2.145 | 40.767 | 0.000 *** |
| CTG (n = 13) | 2.20 ± 0.26 | 2.23 ± 0.25 | 2.27 ± 0.06 | 1.36 | 0.281 | 4.424 | 2 | 2.212 | 42.032 | 0.000 *** | |
| CG (n = 12) | 2.21 ± 0.21 | 2.29 ± 0.32 | 2.33 ± 0.06 | 3.62 | 0.209 | 1.737 | 33 | 0.053 | |||
| The change of diaphragm average respiratory pressure (Unit: cmH2O) | |||||||||||
| DCTG (n = 12) | 94.71 ± 22.01 | 112.59 ± 19.01 | 111.79 ± 2.20 | 18.88 | 0.000 *** | a > b, c | 6997 | 1 | 6997 | 119.871 | 0.000 *** |
| CTG (n = 13) | 87.95 ± 16.66 | 89.10 ± 13.48 | 93.50 ± 2.15 | 1.31 | 0.618 | 2523.114 | 2 | 1261.557 | 21.613 | 0.000 *** | |
| CG (n = 12) | 98.81 ± 18.68 | 98.83 ± 15.85 | 94.87 ± 2.23 | 0.02 | 0.987 | 1926.239 | 33 | 58.371 | |||
| The change of diaphragm maximum respiratory pressure (Unit: cmH2O) | |||||||||||
| DCTG (n = 12) | 107.77 ± 24.29 | 127.84 ± 19.96 | 133.91 ± 2.16 | 18.62 | 0.000 *** | a > b, c | 7265.84 | 1 | 7265.84 | 137.538 | 0.000 *** |
| CTG (n = 13) | 117.96 ± 11.43 | 118.81 ± 11.51 | 116.39 ± 2.02 | 0.72 | 0.597 | 2104.511 | 2 | 1052.256 | 19.919 | 0.000 *** | |
| CG (n = 12) | 119.20 ± 14.77 | 121.47 ± 16.60 | 118.02 ± 2.11 | 1.9 | 0.132 | 1743.315 | 33 | 52.828 | |||
| Group | Pre (M ± SD) | Post (M ± SD) | Correction (M ± SD) | ∆% | p-Value | Post-Hoc | ANCOVA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | DF | MS | F | p | |||||||
| The change of distance between the center of the sacrum and the center of the upper body (unit: cm) | |||||||||||
| DCTG (n = 12) | 26.32 ± 2.28 | 24.69 ± 2.71 | 24.26 ± 0.42 | −6.19 | 0.004 ** | a > c | 52.289 | 1 | 52.289 | 24.695 | 0.000 *** |
| CTG (n = 13) | 26.11 ± 2.21 | 25.26 ± 1.47 | 24.95 ± 0.40 | −3.26 | 0.153 | 16.892 | 2 | 8.446 | 3.989 | 0.028 ** | |
| CG (n = 12) | 24.20 ± 2.05 | 25.30 ± 1.16 | 26.07 ± 0.44 | 4.55 | 0.029 * | 69.873 | 33 | 2.117 | |||
| The change of maximum trunk extension moment (unit: N·m) | |||||||||||
| DCTG (n = 12) | 1.84 ± 0.10 | 1.56 ± 0.14 | 1.51 ± 0.06 | −15.22 | 0.000 *** | a > c, b > c | 0.127 | 1 | 0.127 | 2.853 | 0.000 *** |
| CTG (n = 13) | 1.70 ± 0.24 | 1.61 ± 0.15 | 1.60 ± 0.59 | −5.29 | 0.258 | 0.313 | 2 | 0.157 | 3.516 | 0.041 * | |
| CG (n = 12) | 1.45 ± 0.21 | 1.73 ± 0.31 | 1.80 ± 0.72 | 19.31 | 0.005 ** | 1.469 | 33 | 0.045 | |||
| The change of maximum knee flexion moment (unit: N·m) | |||||||||||
| DCTG (n = 12) | −1.39 ± 0.27 | −1.41 ± 0.24 | −1.35 ± 0.04 | 1.44 | 0.764 | n/a | 1.079 | 1 | 1.079 | 41.829 | 0.000 *** |
| CTG (n = 13) | −1.30 ± 0.25 | −1.38 ± 0.28 | −1.40 ± 0.04 | 6.15 | 0.189 | 0.055 | 2 | 0.027 | 1.066 | 0.346 | |
| CG (n = 12) | −1.26 ± 0.17 | −1.26 ± 0.16 | −1.30 ± 0.04 | 0 | 0.891 | 0.851 | 33 | 0.026 | |||
| The change of dynamic postural stability index | |||||||||||
| DCTG (n = 12) | 0.32 ± 0.63 | 0.23 ± 0.05 | 0.21 ± 0.01 | −28.13 | 0.000 *** | a > b > c | 0.057 | 1 | 0.057 | 15.208 | 0.000 *** |
| CTG (n = 13) | 0.28 ± 0.72 | 0.22 ± 0.08 | 0.23 ± 0.01 | −21.43 | 0.054 | 0.026 | 2 | 0.013 | 3.401 | 0.045 * | |
| CG (n = 12) | 0.26 ± 0.76 | 0.26 ± 0.08 | 0.28 ± 0.01 | 0 | 0.71 | 0.124 | 33 | 0.004 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Jeong, G.; Chun, B. Impact of Diaphragm-Strengthening Core Training on Postural Stability in High-Intensity Squats. Life 2024, 14, 1612. https://doi.org/10.3390/life14121612
Seo H, Jeong G, Chun B. Impact of Diaphragm-Strengthening Core Training on Postural Stability in High-Intensity Squats. Life. 2024; 14(12):1612. https://doi.org/10.3390/life14121612
Chicago/Turabian StyleSeo, Hyun, Guyeol Jeong, and Buongo Chun. 2024. "Impact of Diaphragm-Strengthening Core Training on Postural Stability in High-Intensity Squats" Life 14, no. 12: 1612. https://doi.org/10.3390/life14121612
APA StyleSeo, H., Jeong, G., & Chun, B. (2024). Impact of Diaphragm-Strengthening Core Training on Postural Stability in High-Intensity Squats. Life, 14(12), 1612. https://doi.org/10.3390/life14121612







