Inhibitor of DNA Binding Protein 2 (ID2) Mediates the Anti-Proliferative and Pro-Differentiation Effects of Insulin-like Growth Factor-1 (IGF-1)
Abstract
1. Introduction
2. Methods
3. Results
3.1. IGF-1 Decreases ID2 Expression in Trophoblasts in a Dose-Dependent Manner
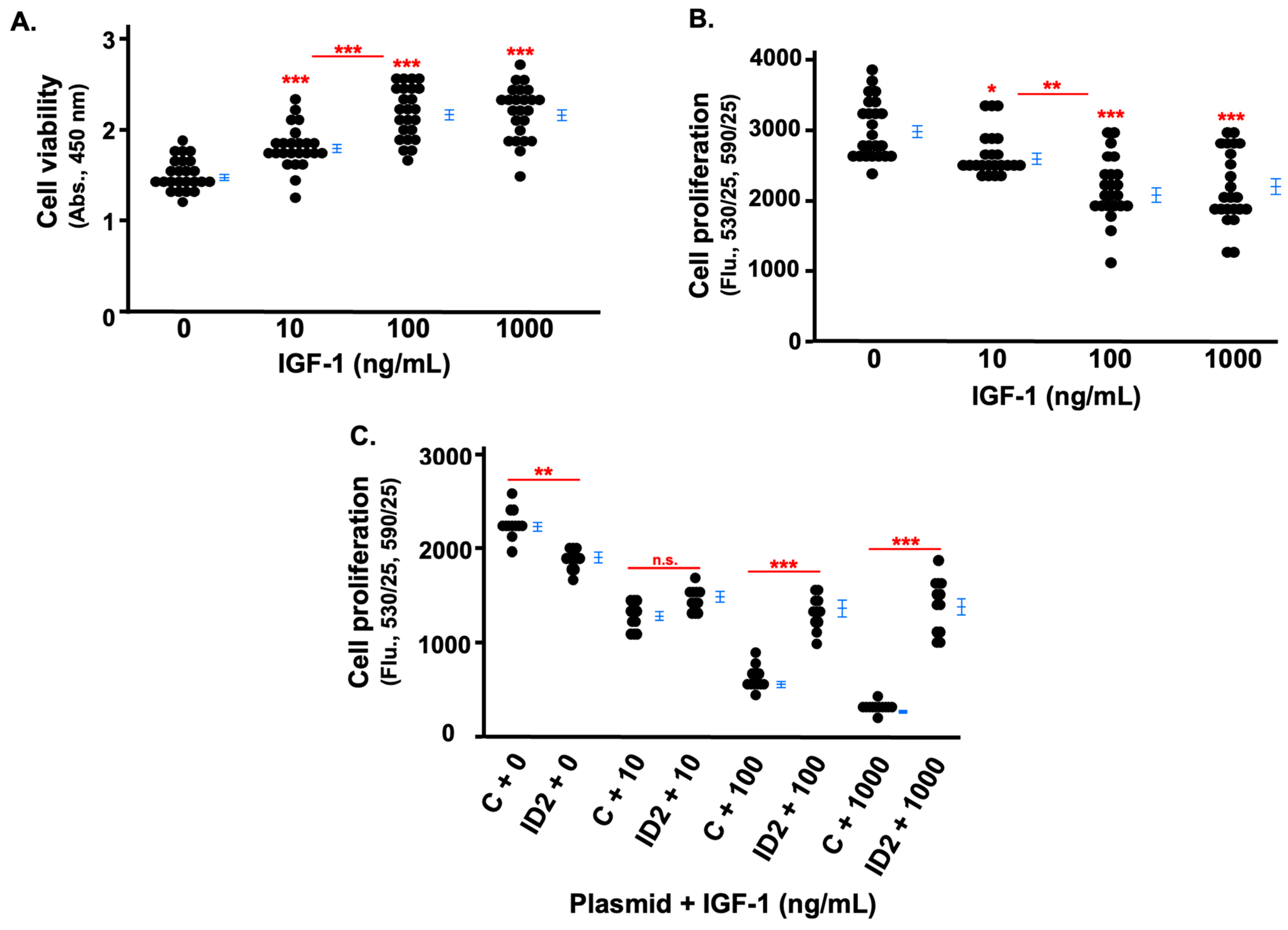
3.2. IGF-1 Inhibits the Proliferation of Trophoblasts Which Is Rescued by ID2 Overexpression
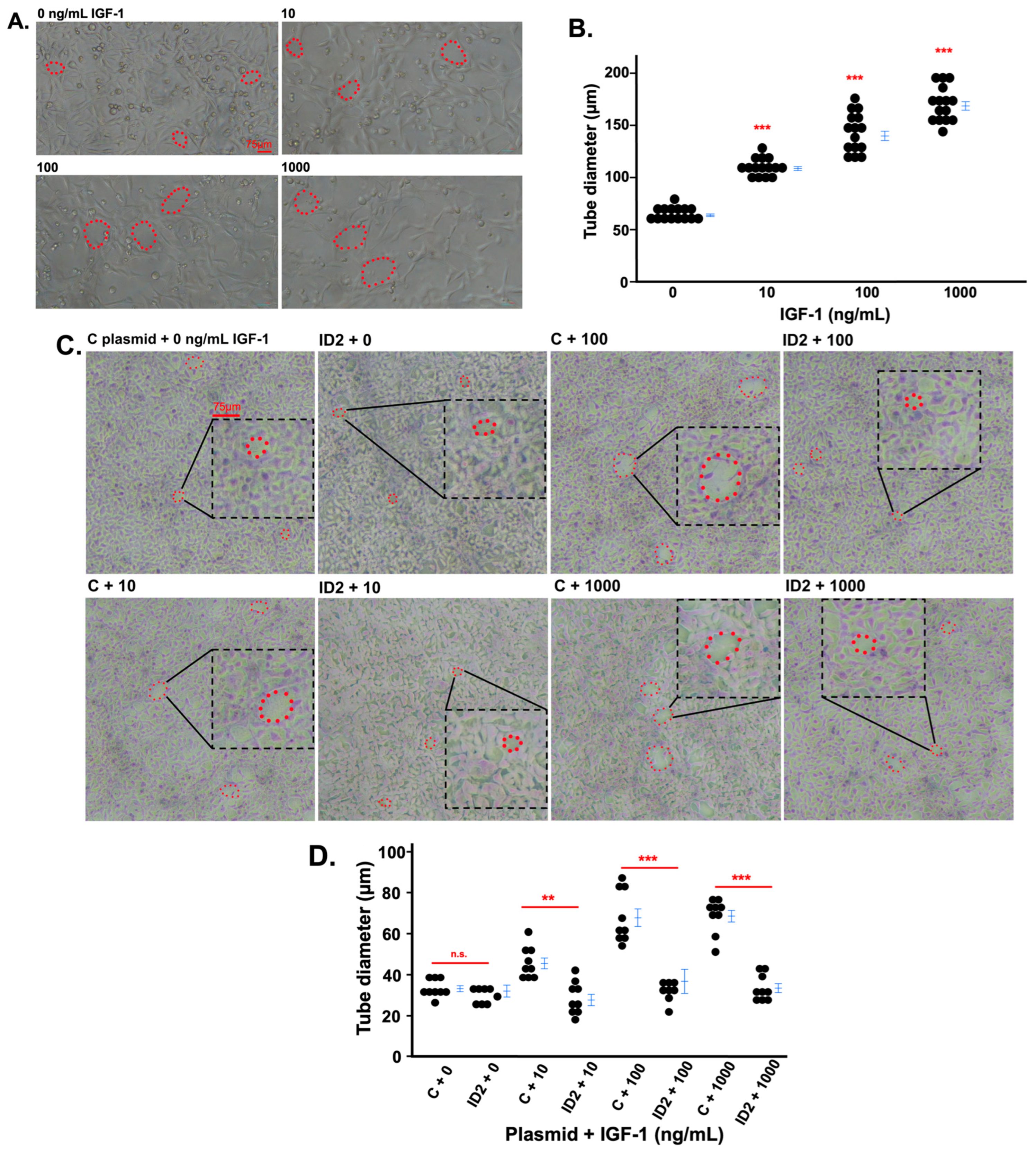
3.3. IGF-1 Promotes Tube Formation in Trophoblasts Which Is Mitigated by ID2 Overexpression
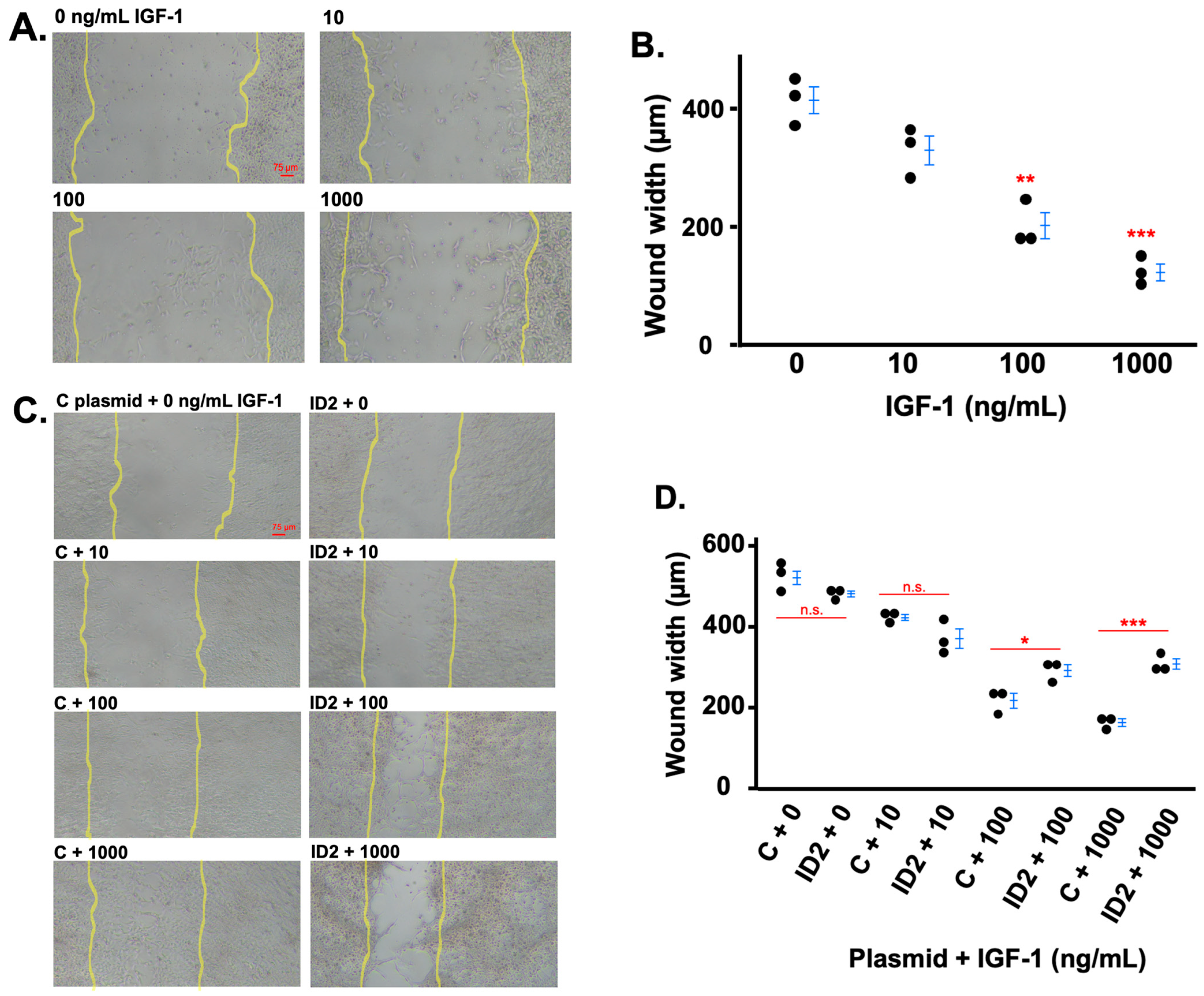
3.4. IGF-1 Promotes Trophoblast Migration Which Is Mitigated by ID2 Overexpression
3.5. IGF-1 Administration to WT Female Mice Decreases Id2 in Placentas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef]
- Surico, D.; Bordino, V.; Cantaluppi, V.; Mary, D.; Gentilli, S.; Oldani, A.; Farruggio, S.; Melluzza, C.; Raina, G.; Grossini, E. Preeclampsia and intrauterine growth restriction: Role of human umbilical cord mesenchymal stem cells-trophoblast cross-talk. PLoS ONE 2019, 14, e0218437. [Google Scholar] [CrossRef] [PubMed]
- Selesniemi, K.; Albers, R.E.; Brown, T.L. Id2 Mediates Differentiation of Labyrinthine Placental Progenitor Cell Line, SM10. Stem Cells Dev. 2016, 25, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Kang, B.; Sun, X.H. Id proteins: Small molecules, mighty regulators. Curr. Top. Dev. Biol. 2014, 110, 189–216. [Google Scholar]
- Florio, M.; Hernandez, M.-C.; Yang, H.; Shu, H.-K.; Cleveland, J.L.; Israel, M.A. Id2 Promotes Apoptosis by a Novel Mechanism Independent of Dimerization to Basic Helix-Loop-Helix Factors. Mol. Cell. Biol. 2023, 18, 5435–5444. [Google Scholar] [CrossRef] [PubMed]
- Gultice, A.D.; Selesniemi, K.L.; Brown, T.L. Hypoxia Inhibits Differentiation of Lineage-Specific Rcho-1 Trophoblast Giant Cells. Biol. Reprod. 2006, 74, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Guo, W.; Zheng, S.; Fu, C.; Ma, Y.; Pan, S.; Liu, Q.; Yang, X. Enhancement of neural stem cell survival, proliferation and differentiation by IGF-1 delivery in graphene oxide-incorporated PLGA electrospun nanofibrous mats. RSC Adv. 2019, 9, 8315–8325. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Meng, Z. Insulin growth factor-1 promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells through the Wnt/β-catenin pathway. Exp. Ther. Med. 2021, 22, 891. [Google Scholar] [CrossRef]
- Liao, S.; Vickers, M.H.; Taylor, R.S.; Jones, B.; Fraser, M.; McCowan, L.M.; Baker, P.N.; Perry, J.K. Maternal serum IGF-1, IGFBP-1 and 3, and placental growth hormone at 20 weeks’ gestation in pregnancies complicated by preeclampsia. Pregnancy Hypertens. 2017, 10, 149–154. [Google Scholar] [CrossRef]
- Balachandiran, M.; Bobby, Z.; Dorairajan, G.; Gladwin, V.; Vinayagam, V.; Packirisamy, R.M. Decreased maternal serum adiponectin and increased insulin-like growth factor-1 levels along with increased placental glucose transporter-1 expression in gestational diabetes mellitus: Possible role in fetal overgrowth. Placenta 2021, 104, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Troja, W.; Sumser, E.K.; Maupin, A.; Lampe, K.; Jones, H.N. Insulin-like growth factor 1 signaling in the placenta requires endothelial nitric oxide synthase to support trophoblast function and normal fetal growth. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R653–R662. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Yu, L. Insulin-like growth factor 1 ameliorates pre-eclampsia by inhibiting zinc finger E-box binding homeobox 1 by up-regulation of microRNA-183. J. Cell. Mol. Med. 2023, 27, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Valentinis, B.; Belletti, B.; Romano, G.; Reiss, K.; Baserga, R. Regulation of Id2 Gene Expression by the Type 1 IGF Receptor and the Insulin Receptor Substrate-1. Endocrinology 2001, 142, 5149–5157. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.H.; Hawley, T.S.; Hawley, R.C.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and Characterization of First Trimester Human Trophoblast Cells with Extended Lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.J.; Takao, T.; Asanoma, K.; Kato, K.; Fukushima, K.; Tsunematsu, R.; Hirakawa, T.; Matsumura, S.; Seki, H.; Takeda, S.; et al. Isolation and Characterization of Human Trophoblast Side-Population (SP) Cells in Primary Villous Cytotrophoblasts and HTR-8/SVneo Cell Line. PLoS ONE 2011, 6, e21990. [Google Scholar] [CrossRef]
- Nandi, P.; Lim, H.; Torres-Garcia, E.J.; Lala, P.K. Human trophoblast stem cell self-renewal and differentiation: Role of decorin. Sci. Rep. 2018, 8, 8977. [Google Scholar] [CrossRef]
- Li, F.; Kakoki, M.; Smid, M.; Boggess, K.; Wilder, J.; Hiller, S.; Bounajim, C.; Parnell, S.E.; Sulik, K.K.; Smithies, O.; et al. Causative Effects of Genetically Determined High Maternal/Fetal Endothelin-1 on Preeclampsia-Like Conditions in Mice. Hypertension 2018, 71, 894–903. [Google Scholar] [CrossRef]
- Vatten, L.J.; Nilsen, T.I.L.; Juul, A.; Jeansson, S.; Jenum, P.A.; Eskild, A. Changes in circulating level of IGF-I and IGF-binding protein-1 from the first to second trimester as predictors of preeclampsia. Eur. J. Endocrinol. 2008, 158, 101–105. [Google Scholar] [CrossRef]
- Sun, K.; Visser, A.; Beijer, M.; Oudejans, C.B.M.; van Dijk, M. The effect of maternal NODAL on STOX1 expression in extravillous trophoblasts is mediated by IGF1. PLoS ONE 2018, 13, e0202190. [Google Scholar] [CrossRef]
- Ayesha, A.; Bahnson, E.M.; Kayashima, Y.; Wilder, J.; Huynh, P.K.; Hiller, S.; Maeda-Smithies, N.; Li, F. Vitamin B12 does not increase cell viability after hydrogen peroxide induced damage in mouse kidney proximal tubular cells and brain endothelial cells. Adv. Redox Res. 2022, 4, 100029. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, L.; Chen, X.; Pang, Y.; Jia, B.; Sun, J.; Quan, X. BMP9 maintains the phenotype of HTR-8/Svneo trophoblast cells by activating the SDF1/CXCR4 pathway. BMC Mol. Cell Biol. 2023, 24, 24. [Google Scholar] [CrossRef]
- Morales-Garza, L.A.; Puche, J.E.; Aguirre, G.A.; Muñoz, Ú.; García-Magariño, M.; De la Garza, R.G.; Castilla-Cortazar, I. Experimental approach to IGF-1 therapy in CCl4-induced acute liver damage in healthy controls and mice with partial IGF-1 deficiency. J. Transl. Med. 2017, 15, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Townley-Tilson, W.H.; Wu, Y.; Ferguson, J.E., 3rd; Patterson, C. The ubiquitin ligase ASB4 promotes trophoblast differentiation through the degradation of ID2. PLoS ONE 2014, 9, e89451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, S.-M.; Sharma, G.; Verma, R.; Byun, S.; Rudra, D.; Im, S.-H. Inflammation-induced Id2 promotes plasticity in regulatory T cells. Nat. Commun. 2018, 9, 4736. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Haugen, M.J.; Woods, D.C. Role for inhibitor of differentiation/deoxyribonucleic acid-binding (Id) proteins in granulosa cell differentiation. Endocrinology 2008, 149, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Jakubison, B.L.; Sarkar, T.; Gudmundsson, K.O.; Singh, S.; Sun, L.; Morris, H.M.; Klarmann, K.D.; Keller, J.R. ID2 and HIF-1α collaborate to protect quiescent hematopoietic stem cells from activation, differentiation, and exhaustion. J. Clin. Investig. 2022, 132, e152599. [Google Scholar] [CrossRef]
- Liu, F.; Chen, S.; Yu, Y.; Huang, C.; Chen, H.; Wang, L.; Zhang, W.; Wu, J.; Ye, Y. Inhibitor of DNA binding 2 knockdown inhibits the growth and liver metastasis of colorectal cancer. Gene 2022, 819, 146240. [Google Scholar] [CrossRef] [PubMed]
- Zinina, V.V.; Ruehle, F.; Winkler, P.; Rebmann, L.; Lukas, H.; Möckel, S.; Diefenbach, A.; Mendez-Lago, M.; Soshnikova, N. ID2 controls differentiation of enteroendocrine cells in mouse small intestine. Acta Physiol. 2022, 234, e13773. [Google Scholar] [CrossRef] [PubMed]
- Forbes, K.; Westwood, M.; Baker, P.N.; Aplin, J.D. Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. Am. J. Physiol. Cell Physiol. 2008, 294, C1313–C1322. [Google Scholar] [CrossRef] [PubMed]
- Frystyk, J.; Skjærbæk, C.; Dinesen, B.; Ørskov, H. Free insulin-like growth factors (IGF-I and IGF-II) in human serum. FEBS Lett. 2001, 348, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Bruchim, I. The insulin-like growth factor-I receptor as an oncogene. Arch. Physiol. Biochem. 2009, 115, 58–71. [Google Scholar] [CrossRef]
- Kadakia, R.; Arraztoa, J.A.; Bondy, C.; Zhou, J. Granulosa cell proliferation is impaired in the Igf1 null ovary. Growth Horm. IGF Res. 2001, 11, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ingec, M.; Gursoy, H.G.; Yildiz, L.; Kumtepe, Y.; Kadanali, S. Serum levels of insulin, IGF-1, and IGFBP-1 in pre-eclampsia and eclampsia. Int. J. Gynecol. Obstet. 2017, 84, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Williams, M.A.; Vadachkoria, S.; Muy-Rivera, M.; Frederick, I.O.; Luthy, D.A. Maternal plasma concentrations of insulinlike growth factor-1 and insulinlike growth factor-binding protein-1 in early pregnancy and subsequent risk of preeclampsia. Clin. Biochem. 2004, 37, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, A.; Salmon, A.B.; Lerner, C.; Torres, C.; Ikeno, Y.; Motch, S.; McCarter, R.; Sell, C. Mice Producing Reduced Levels of Insulin-Like Growth Factor Type 1 Display an Increase in Maximum, but not Mean, Life Span. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 69, 410–419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Irani, M.; Nasioudis, D.; Witkin, S.S.; Gunnala, V.; Spandorfer, S.D. High serum IGF-1 levels are associated with pregnancy loss following frozen-thawed euploid embryo transfer cycles. J. Reprod. Immunol. 2018, 127, 7–10. [Google Scholar] [CrossRef]
- Malik, A.; Pal, R.; Gupta, S.K. Interdependence of JAK-STAT and MAPK signaling pathways during EGF-mediated HTR-8/SVneo cell invasion. PLoS ONE 2017, 12, e0178269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, D.; Luo, D.; Ge, H.; Zhang, C.; Wei, S.; Liang, D.; Tang, D.; Li, J.; Lin, Y. Exposure to higher concentrations of exogenous ELABELA causes HTR-8/SVneo trophoblast cell dysfunction: A possible pathogenesis of pre-eclampsia. Pregnancy Hypertens. 2022, 30, 181–188. [Google Scholar] [CrossRef] [PubMed]


| Gene | Type | Sequence (5′–3′) |
|---|---|---|
| h-ID2 | Hs04187239_m1 (Applied Biosystems) | |
| m-Id2 | Mm00711781-m1 (Applied Biosystems) | |
| h-GAPDH | Forward | GAA GGT GAA GGT CGG AGT C |
| Reverse | GAA GAT GGT GAT GGG ATT TC | |
| Probe | FAM-CA AGC TTC CCG TTC TCA GCC-TAMRA | |
| m-Actb | Forward | AAG AGC TAT GAG CTG CCT GA |
| Reverse | TGA TGG AAT TGA ATG TAG TTT CA | |
| Probe | TET-CAC TAT TGG CAA CGA GCG GTT CCG-TAMRA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ssengonzi, R.; Wang, Y.; Zhou, J.; Kayashima, Y.; Townley-Tilson, W.H.D.; Rao, B.; Ma, Q.; Maeda-Smithies, N.; Li, F. Inhibitor of DNA Binding Protein 2 (ID2) Mediates the Anti-Proliferative and Pro-Differentiation Effects of Insulin-like Growth Factor-1 (IGF-1). Life 2024, 14, 1663. https://doi.org/10.3390/life14121663
Ssengonzi R, Wang Y, Zhou J, Kayashima Y, Townley-Tilson WHD, Rao B, Ma Q, Maeda-Smithies N, Li F. Inhibitor of DNA Binding Protein 2 (ID2) Mediates the Anti-Proliferative and Pro-Differentiation Effects of Insulin-like Growth Factor-1 (IGF-1). Life. 2024; 14(12):1663. https://doi.org/10.3390/life14121663
Chicago/Turabian StyleSsengonzi, Rebecca, Yuye Wang, Jiayi Zhou, Yukako Kayashima, W. H. Davin Townley-Tilson, Balaji Rao, Qing Ma, Nobuyo Maeda-Smithies, and Feng Li. 2024. "Inhibitor of DNA Binding Protein 2 (ID2) Mediates the Anti-Proliferative and Pro-Differentiation Effects of Insulin-like Growth Factor-1 (IGF-1)" Life 14, no. 12: 1663. https://doi.org/10.3390/life14121663
APA StyleSsengonzi, R., Wang, Y., Zhou, J., Kayashima, Y., Townley-Tilson, W. H. D., Rao, B., Ma, Q., Maeda-Smithies, N., & Li, F. (2024). Inhibitor of DNA Binding Protein 2 (ID2) Mediates the Anti-Proliferative and Pro-Differentiation Effects of Insulin-like Growth Factor-1 (IGF-1). Life, 14(12), 1663. https://doi.org/10.3390/life14121663






