Can Aluminum Affect Social Behavior and Cortisol Plasma Profile in the Neotropical Freshwater Teleost Astyanax lacustris (Teleostei: Characidae)?
Abstract
:1. Introduction
2. Materials and Methods
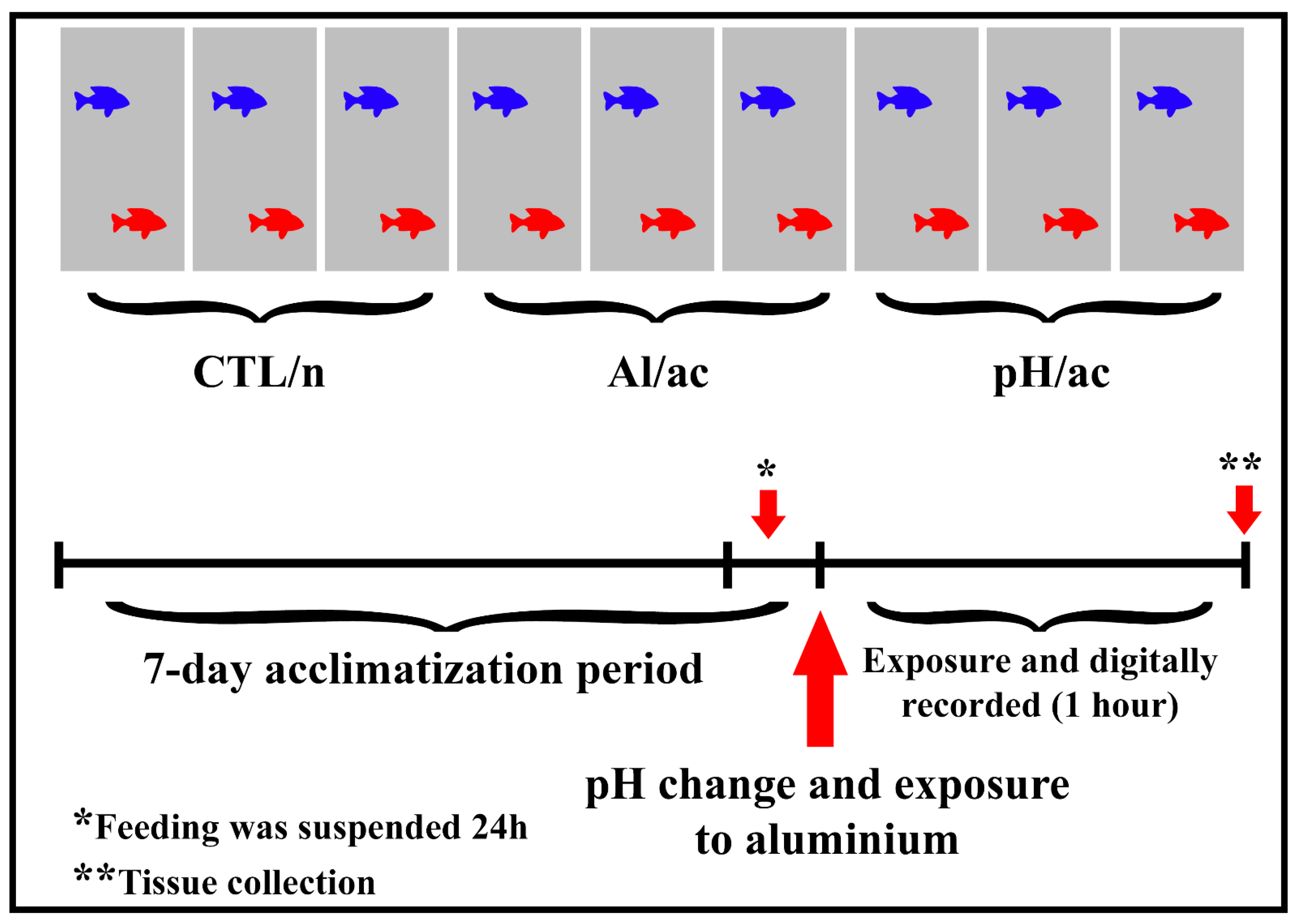
2.1. Animals and Experimental Design
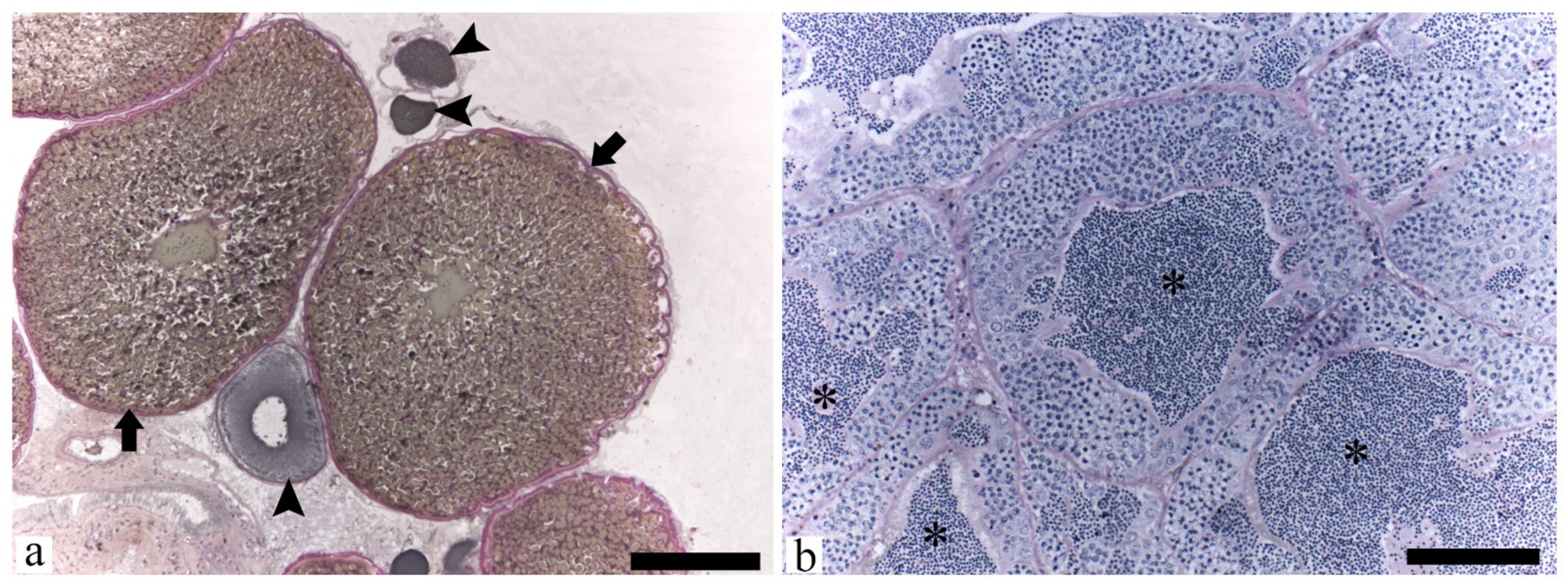
2.2. Histological and Hormone Analyses
2.3. Statistical Analysis
3. Results
3.1. Water Analysis
3.2. Animals and Biometrical Parameters
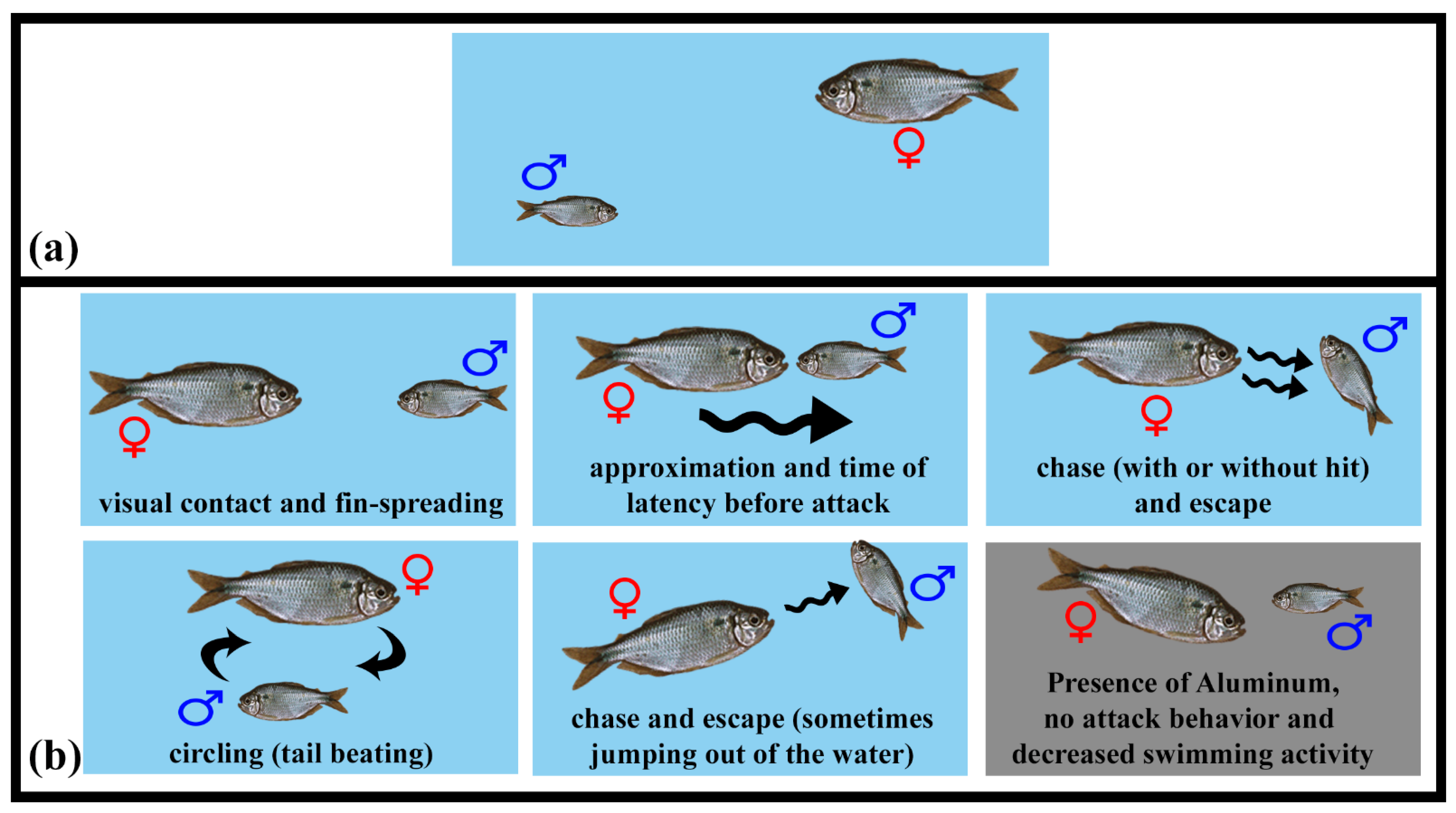
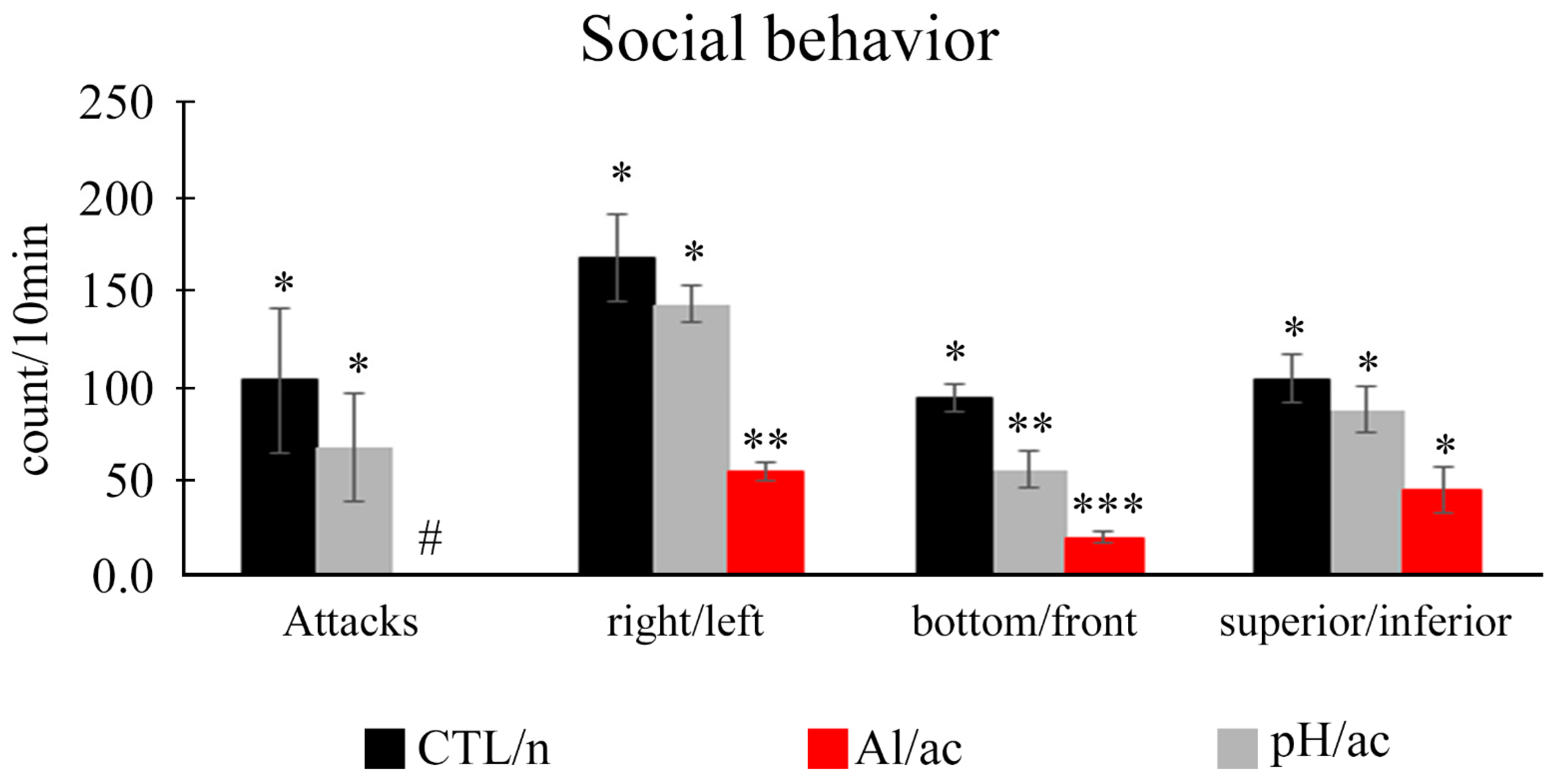
3.3. Social Behavior
3.4. Hormone Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.; Ahmed, G.; Vedika, S.; Kumar, P.; Chaturvedi, S.K.; Rai, S.N.; Vamanu, E.; Kumar, A. Toxic heavy metal ions contamination in water and their sustainable reduction by eco-friendly methods: Isotherms, thermodynamics and kinetics study. Sci. Rep. 2024, 14, 7595. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Saravanan, V.; Rajeshkannan, R.; Arnica, G.; Rajasimman, M.; Baskar, G.; Pugazhendhi, A. Comprehensive review on toxic heavy metals in the aquatic system: Sources, identification, treatment strategies, and health risk assessment. Environ. Res. 2024, 258, 119440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta. 2008, 606, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Sloman, K.A. Effects of trace metals on salmonid fish: The role of social hierarchies. Appl. Anim. Behav. Sci. 2007, 104, 326–345. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water Contamination by heavy metals and their toxic effect on aquaculture and human health through food chain. Lett. Appl. NanoBioScience 2021, 10, 2148–2166. [Google Scholar] [CrossRef]
- Capriello, T.; Félix, L.M.; Monteiro, S.M.; Santos, D.; Cofone, R.; Ferrandino, I. Exposure to aluminium causes behavioural alterations and oxidative stress in the brain of adult zebrafish. Environ. Toxicol. Pharmacol. 2021, 85, 103636. [Google Scholar] [CrossRef] [PubMed]
- Closset, M.; Cailliau, K.; Slaby, S.; Marin, M. Effects of aluminium contamination on the nervous system of freshwater aquatic vertebrates: A review. Int. J. Mol. Sci. 2021, 23, 31. [Google Scholar] [CrossRef]
- Bergmann, M.; Graça, M.A.S. Bioaccumulation and dispersion of uranium by freshwater organisms. Arch. Environ. Contam. Toxicol. 2020, 78, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Windsor, F.M.; Pereira, M.G.; Morrissey, C.A.; Tyler, C.R.; Ormerod, S.J. Environment and food web structure interact to alter the trophic magnification of persistent chemicals across river ecosystems. Sci. Total. Environ. 2020, 717, 137271. [Google Scholar] [CrossRef]
- Monneret, C. What is an endocrine disruptor? Biologies 2017, 340, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Atchison, G.J.; Henry, M.G.; Sandheinrich, M.B. Effects of metals on fish behavior: A review. Environ. Biol. Fishes. 1987, 18, 11–25. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Isaifan, R.J. Aluminum environmental pollution: The silent killer. Environ. Sci. Pollut. Res. 2021, 28, 44587–44597. [Google Scholar] [CrossRef]
- Botté, A.; Zaidi, M.; Guery, J.; Fichet, D.; Leignel, V. Aluminium in aquatic environments: Abundance and ecotoxicological impacts. Aquat. Ecol. 2022, 56, 751–773. [Google Scholar] [CrossRef]
- Nilsen, T.O.; Ebbesson, L.O.; Handeland, S.O.; Kroglund, F.; Finstad, B.; Angotzi, A.R.; Stefansson, S.O. Atlantic salmon (Salmo salar L.) smolts require more than two weeks to recover from acidic water and aluminum exposure. Aquat. Toxicol. 2013, 142–143, 33–44. [Google Scholar] [CrossRef]
- Pinheiro, J.P.S.; Assis, C.B.A.; Peñuela, M.M.; Júnior, F.B.; Correia, T.G.; Moreira, R.G. Water temperature and acid pH influence the cytotoxic and genotoxic effects of aluminum in the freshwater teleost Astyanax altiparanae (Teleostei: Characidae). Chemosphere 2019, 220, 266–274. [Google Scholar] [CrossRef]
- Pinheiro, J.P.S.; Windsor, F.M.; Wilson, R.W.; Tyler, C.R. Global variation in freshwater physico-chemistry and its influence on chemical toxicity in aquatic wildlife. Biol. Rev. 2021, 96, 1528–1546. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Chappell, J.S.; Birchall, J.D. A mechanism for acute aluminum toxicity in fish. J. Theor. Biol. 1991, 151, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Teien, H.C.; Salbu, B.; Kroglund, F.; Heier, L.S.; Rosseland, B.O. The influence of colloidal material on aluminum speciation and estimated acid neutralizing capacity (ANC). Appl. Geochem. 2007, 22, 1202–1208. [Google Scholar] [CrossRef]
- Correia, T.G.; Vieira, V.A.R.O.; Narcizo, A.M.; Zampieri, R.A.; Floeter-Winter, L.M.; Moreira, R.G. Endocrine disruption caused by the aquatic exposure to aluminum and manganese in Astyanax altiparanae (Teleostei: Characidae) females during the final ovarian maturation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 249, 109132. [Google Scholar] [CrossRef] [PubMed]
- Narcizo, A.M.; Correia, T.G.; Bianchini, A.; Mayer, M.G.; Zampieri, R.; Floeter-Winter, L.M.; Moreira, R.G. Aluminum bioconcentration in female Nile tilapia Oreochromis niloticus (Perciformes: Cichlidae) and the effects on pituitary gonadotropins. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 243, 108965. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.G.; Narcizo, A.M.; Bianchini, A.; Moreira, R.G. Aluminum as an endocrine disruptor in female Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 461–466. [Google Scholar] [CrossRef]
- Vieira, V.A.R.O. Avaliação da Toxicidade de Metais No Metabolismo de Fêmeas Vitelogênicas de Astyanax bimaculatus (Teleostei: Characidae). Tese (Doutorado). Ph.D. Thesis, Departamento de Fisiologia. Instituto de Biociências da Universidade de São Paulo, São Paulo, Brazil, 2012; p. 143. Available online: https://teses.usp.br/teses/disponiveis/41/41135/tde-23012013-084758/pt-br.php (accessed on 25 September 2024).
- Abdalla, R.P.; Kida, B.M.; Pinheiro, J.P.S.; Oliveira, L.F.; Martinez, C.B.F.; Moreira, R.G. Exposure to aluminum, aluminum + manganese and acid pH triggers different antioxidant responses in gills and liver of Astyanax altiparanae (Teleostei: Characiformes: Characidae) males. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 33–40. [Google Scholar] [CrossRef]
- Kida, B.M.S.; Abdalla, R.P.; Moreira, R.G. Effects of acidic water, aluminum, and manganese on testicular steroidogenesis in Astyanax altiparanae. Fish Physiol. Biochem. 2016, 42, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.P.S.; Assis, C.B.; Sanches, E.A.; Moreira, R.G. Aluminum, at an environmental concentration, associated with acidic pH and high water temperature, causes impairment of sperm quality in the freshwater teleost Astyanax altiparanae (Teleostei: Characidae). Environ. Pollut. 2020, 262, 114252. [Google Scholar] [CrossRef]
- Pinheiro, J.P.S.; Lima, J.; Assis, C.B.; Branco, G.S.; Gomes, A.D.O.; Moreira, R.G. Paternal exposure to aluminum, acidity, and temperature affect fatty acid seminal profile, embryonic and larval development of Astyanax altiparanae. Chemosphere 2021, 266, 128935. [Google Scholar] [CrossRef]
- Sloman, K.A.; McNeil, P.L. Using physiology and behaviour to understand the responses of fish early life stages to toxicants. J. Fish Biol. 2012, 81, 2175–2198. [Google Scholar] [CrossRef]
- Øverli, Ø.; Sørensen, C.; Nilsson, G.E. Behavioral indicators of stress coping style in rainbow trout: Do males and females react differently to novelty? Physiol. Behav. 2006, 87, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, C.; Øverli, Ø.; Summers, C.H.; Nilsson, G.E. Social regulation of neurogenesis in teleosts. Brain Behav. Evol. 2007, 70, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Milla, S.; Wang, N.; Mandiki, S.; Kestemont, P. Corticosteroids: Friends of foes of teleost fish reproduction? Comp. Biochem. Physiol. Part A Mol. Int. Physiol. 2009, 153, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef]
- Hontela, A.; Lacroix, A. Heavy metals. Biological basis for health effects in wildlife and humans. In Endocrine Disruptors; Carr, J., Norris, D.O., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 356–374. [Google Scholar]
- Urbinati, E.C.; Zanuzzo, F.S.; Biller, J.D. Stress and immune system in fish. In Biology and Physiology of Freshwater Neotropical Fish; Baldisserotto, B., Urbinati, E.C., Cyrino, J.E.P., Eds.; Academic Press: San Diego, CA, USA, 2020; pp. 93–114. [Google Scholar] [CrossRef]
- Grassie, C.; Braithwaite, V.A.; Nilsson, J.; Nilsen, T.O.; Teien, H.-C.; Handeland, S.O.; Stefansson, S.O.; Tronci, V.; Gorissen, M.; Flik, G.; et al. Aluminum exposure impacts brain plasticity and behavior in Atlantic salmon (Salmo salar). J. Exp. Biol. 2013, 216, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Korzan, W.; Åtland, A.; Haraldstad, T.; Høgberget, R.; Mayer, I.; Øverli, Ø. Neuroendocrine indicators of allostatic load reveal the impact of environmental acidification in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 229, 108679. [Google Scholar] [CrossRef] [PubMed]
- de Lucena, C.A.S.; Soares, H.G. Review of species of the Astyanax bimaculatus “caudal peduncle spot” subgroup sensu Garutti & Langeani (Characiformes, Characidae) from the rio La Plata and rio São Francisco drainages and coastal systems of southern Brazil and Uruguay. Zootaxa 2016, 4072, 101–125. [Google Scholar] [CrossRef]
- Porto-Foresti, F.; Castilho-Almeida, R.B.; Senhorini, J.A.; Foresti, F. Biologia e criação de Lambari do rabo amarelo (Astyanax altiparanae). In Espécies Nativas Para Piscicultura no Brasil (Gráfica da Univerdidade Federal de Santa Maria), 2nd ed.; Baldisserotto, B., Gomes, L.C., Eds.; Universidade Federal de Santa Maria–UFSM: Santa Maria, CA, USA, 2010; Volume 1, pp. 101–115. [Google Scholar]
- Nascimento, N.F.D.; Pereira-Santos, M.; Piva, L.H.; Manzini, B.; Fujimoto, T.; Senhorini, J.A.; Yasui, G.S.; Nakaghi, L.S.O. Growth, fatty acid composition, and reproductive parameters of diploid and triploid yellowtail tetra Astyanax altiparanae. Aquaculture 2017, 471, 163–171. [Google Scholar] [CrossRef]
- Conama. Resolução Número 357, de 17 de Março de 2005, Ministério do Meio Ambiente. 2005. Available online: https://www.siam.mg.gov.br/sla/download.pdf?idNorma=2747 (accessed on 25 September 2024).
- CETESB. CETESB (Companhia Ambiental do Estado de São Paulo). Relatório de Qualidade das Águas Interiores do Estado de São Paulo, Governo do Estado de São Paulo, Secretaria do Meio Ambiente. 2022. Available online: https://cetesb.sp.gov.br/ (accessed on 25 September 2024).
- Beghelli, F.G.d.S.; Lopez-Dovál, J.C.; Rosa, A.H.; Pompêo, M.; Carlos, V.M. Lethal and sublethal effects of metal-polluted sediments on Chironomus sancticaroli Strixino and Strixino, 1981. Ecotoxicology 2018, 27, 286–299. [Google Scholar] [CrossRef]
- Hinaux, H.; Rétaux, S.; Elipot, Y. Social behavior and aggressiveness in Astyanax. In Biology and Evolution of the Mexican Cavefish; Keene, A.C., Yoshizawa, M., McGaugh, S.E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 335–359. [Google Scholar] [CrossRef]
- NCR. Resolução Normativa nº44 do Conselho Nacional de Controle de Experimentação Animal; Ministério da Ciência, Tecnologia e Inovações: Federal, Brazil, 2019; p. 73.
- Quintero-Hunter, L.; Grier, H.; Muscato, M. Enhancement of histological detail using metanil yellow as counterstain in Periodic acid Schiff’s hematoxylin staining of glycol methacrylate tissue sections. Biotech. Histochem. 1991, 66, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.E.; White, S.A.; Kao, M.H.F.; Fernald, R.D. Stress and dominance in a social fish. J. Neurosci. 1997, 17, 6463–6469. [Google Scholar] [CrossRef]
- Sink, T.D.; Lochmann, R.T.; Fecteau, K.A. Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu, and golden shiners. Fish Physiol. Biochem. 2008, 34, 95–101. [Google Scholar] [CrossRef]
- Rambo, C.L.; Mocelin, R.; Marcon, M.; Villanova, D.; Koakoski, G.; Abreu, M.S.; Oliveira, T.A.; Barcellos, L.J.G.; Piato, A.L.; Bonan, C.D. Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiol. Behav. 2017, 171, 50–54. [Google Scholar] [CrossRef]
- Magurran, A.E.; Garcia, C.M. Sex differences in behavior as an indirect consequence of mating system. J. Fish Biol. 2000, 57, 839–857. [Google Scholar] [CrossRef]
- Pandolfi, M.; Scaia, M.F.; Fernandez, M.P. Sexual dimorphism in aggression: Sex-specific fighting strategies across species. Front. Behav. Neuros. 2021, 15, 659615. [Google Scholar] [CrossRef] [PubMed]
- Maruska, K.P.; Fernald, R.D. Astatotilapia burtoni: A model system for analyzing the neurobiology of behavior. ACS Chem. Neurosci. 2018, 9, 1951–1962. [Google Scholar] [CrossRef]
- Elcoro, M.; da Silva, S.P.; Lattal, K.A. Visual reinforcement in the female Siamese fighting fish, Betta splendens. J. Exp. Anal. Behav. 2008, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Gonçalves, D. Artificial selection for male winners in the Siamese fighting fish Betta splendens correlates with high female aggression. Front. Zool. 2019, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Brinchmann, M.F. Immune relevant molecules identified in the skin mucus of fish using-omics technologies. Mol. BioSyst. 2016, 12, 2056–2063. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Biological and ecological roles of external fish mucus: A review. Fishes. 2018, 3, 41. [Google Scholar] [CrossRef]
- Allin, C.J.; Wilson, R.W. Behavioural and metabolic effects of chronic exposure to sublethal aluminum in acidic soft water in juvenile rainbow trout (Oncorhynchus mykiss). Can. J. Fish Aquat. Sci. 1999, 56, 670–678. [Google Scholar] [CrossRef]
- Scott, G.R.; Sloman, K.A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.G.; Atchison, G.J. Metal effects on fish behavior–advances in determining the ecological significance of responses. In Metal Ecotoxicology Concepts and Applications; Newman, M.C., Mclntosh, A.W., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 131–143. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef]
- Cuña, R.H.; Pandolfi, M.; Genovese, G.; Piazza, Y.; Ansaldo, M.; Lo Nostro, F.L. Endocrine disruptive potential of endosulfan on the reproductive axis of Cichlasoma dimerus (Perciformes, Cichlidae). Aquat. Toxicol. 2013, 126, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.G. Evidências de Desregulação Endócrina Causada Pela Exposição Aquática ao Alumínio e ao Manganês em Astyanax bimaculatus (Linnaeus, 1758). Tese (Doutorado em Ciências)–PPG em Fisiologia. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2012. Available online: https://www.teses.usp.br/teses/disponiveis/41/41135/tde-11012013-115509/en.php (accessed on 25 September 2024).
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs Then and Now: The Need for Clarity in Definition and Purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120–132. [Google Scholar] [PubMed]
- Russell Wms, B.; Burch, R.L. The principles of humane experimental technique. In Health and Environmental Research Online; Universities Federation for Animal Welfare: Wheathampstead, UK, 1959. [Google Scholar]
- Sindhe, V.R.; Kulkarni, R.S. Gonadosomatic and hepatosomatic indices of the freshwater fish Notopterus notopterus (Pallas) in response to some heavy metal exposure. J. Fish. Biol. 2004, 25, 365–368. [Google Scholar]
- Rocha, M.J.; Rocha, E. The diversity of reproductive styles exhibited by fish. In Encyclopedia of Fish Physiology; Alderman, S.L., Gillis, T.E., Eds.; Academic Press: San Diego, CA, USA, 2024; pp. 567–590. [Google Scholar] [CrossRef]
- Rizzo, E.; Bazzoli, N. Reproduction and embryogenesis. In Biology and Physiology of Freshwater Neotropical Fish, 1st ed.; Baldisserotto, B., Urbinati, E.C., Cyrino, J.E.P., Eds.; Academic Press: San Diego, CA, USA, 2020; pp. 287–313. [Google Scholar] [CrossRef]
- Grempel, R.G.; Visconti, M.A. Color and physiology of pigmentation. In Biology and Physiology of Freshwater Neotropical Fish; Baldisserotto, B., Urbinati, E.C., Cyrino, J.E.P., Eds.; Academic Press: San Diego, CA, USA, 2020; pp. 147–162. [Google Scholar] [CrossRef]


| CTL/n | Al/ac | pH/ac | |||||
|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | ||
| Biometric Parameters | TL (cm) | 12.16 ± 0.44 Aa | 10.50 ± 0.50 Ab | 12.17 ± 0.17 Aa | 10.00 ± 0.29 Ab | 12.83 ± 0.17 Aa | 10.50 ± 0.29 Ab |
| TW (g) | 27.35 ± 2.95 Aa | 15.36 ± 3.16 Ab | 25.13 ± 2.01 Aa | 11.48 ± 0.97 Ab | 29.15 ± 0.90 Aa | 13.61 ± 1.15 Ab | |
| GSI (%) | 8.70 ± 0.71 Aa | 2.61 ± 0.16 Ab | 11.72 ± 2.87 Aa | 2.46 ± 0.32 Ab | 13.30 ± 2.48 Aa | 2.21 ± 0.88 Ab | |
| HSI (%) | 0.78 ± 0.51 A | 0.71 ± 0.44 A | 0.81 ± 0.17 A | 0.66 ± 0.92 A | 0.69 ± 0.78 A | 0.67 ± 0.78 A | |
| Dissolved oxygen (mg/L−1) | 6.18 ± 0.20 | 6.35 ± 0.40 | 6.16 ± 0.20 | ||||
| pH | 7.27 ± 0.01 | 5.33 ± 0.10 | 5.37 ± 0.01 | ||||
| Temperature (°C) | 24.30 ± 0.20 | 24.97 ± 0.10 | 24.93 ± 0.50 | ||||
| Astyanax lacustris female | ||
| Social interactions | Types | Behavior |
| Aggressiveness display | Physical contact | Bites with the mouth (1,2) |
| Tail hit (1,2) | ||
| Chases with attack (1,2) | ||
| Threats | Chases without attack (1,2) | |
| Approaches (1,2) | ||
| Passive coping | No threat | No sudden moves (3) |
| Astyanax lacustris male | ||
| Social interactions | Types | Behavior |
| Submission display | Activity | Escape (fish swim away) (1,2) |
| Passive coping | Did not escape or moved (3) | |
| Astyanax lacustris couple | ||
| Social interactions | Types | Behavior |
| Swimming activity | Active/Inactive | Swimming: aquarium (left/right) (1,2) |
| Swimming: aquarium (superior/inferior) (1,2) | ||
| Swimming: aquarium (front/bottom) (1,2) | ||
| Sudden rise in the water surface (3) | ||
| Water quality | Clear/Opaque | Clear (1,2) |
| Opaque (cloudy): mucus production (3) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, N.P.V.M.d.; Araújo, B.C.; Kida, B.M.S.; Abdalla, R.P.; Brito, D.d.S.; Moreira, R.G.; Honji, R.M. Can Aluminum Affect Social Behavior and Cortisol Plasma Profile in the Neotropical Freshwater Teleost Astyanax lacustris (Teleostei: Characidae)? Life 2024, 14, 1697. https://doi.org/10.3390/life14121697
Faria NPVMd, Araújo BC, Kida BMS, Abdalla RP, Brito DdS, Moreira RG, Honji RM. Can Aluminum Affect Social Behavior and Cortisol Plasma Profile in the Neotropical Freshwater Teleost Astyanax lacustris (Teleostei: Characidae)? Life. 2024; 14(12):1697. https://doi.org/10.3390/life14121697
Chicago/Turabian StyleFaria, Natália Pires Vieira Morais de, Bruno Cavalheiro Araújo, Bianca Mayumi Silva Kida, Raisa Pereira Abdalla, Diego dos Santos Brito, Renata Guimarães Moreira, and Renato Massaaki Honji. 2024. "Can Aluminum Affect Social Behavior and Cortisol Plasma Profile in the Neotropical Freshwater Teleost Astyanax lacustris (Teleostei: Characidae)?" Life 14, no. 12: 1697. https://doi.org/10.3390/life14121697
APA StyleFaria, N. P. V. M. d., Araújo, B. C., Kida, B. M. S., Abdalla, R. P., Brito, D. d. S., Moreira, R. G., & Honji, R. M. (2024). Can Aluminum Affect Social Behavior and Cortisol Plasma Profile in the Neotropical Freshwater Teleost Astyanax lacustris (Teleostei: Characidae)? Life, 14(12), 1697. https://doi.org/10.3390/life14121697








