The Role of Extracellular Vesicles in SARS-CoV-2-Induced Acute Kidney Injury: An Overview
Abstract
:1. Introduction
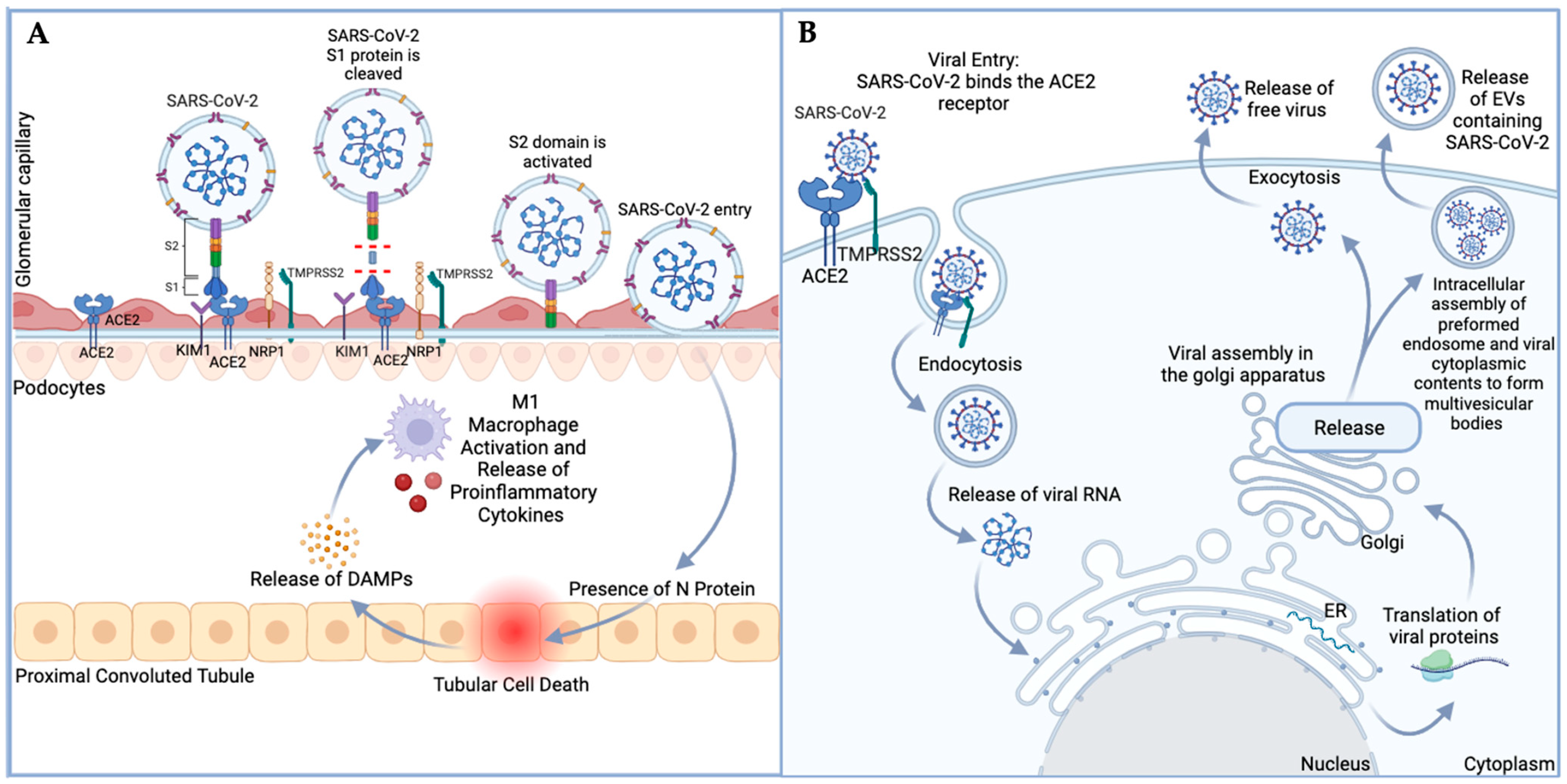
1.1. Transmission and Infection of SARS-CoV-2 into Host Cells
1.2. Extracellular Vesicles and Cell-to-Cell Communication
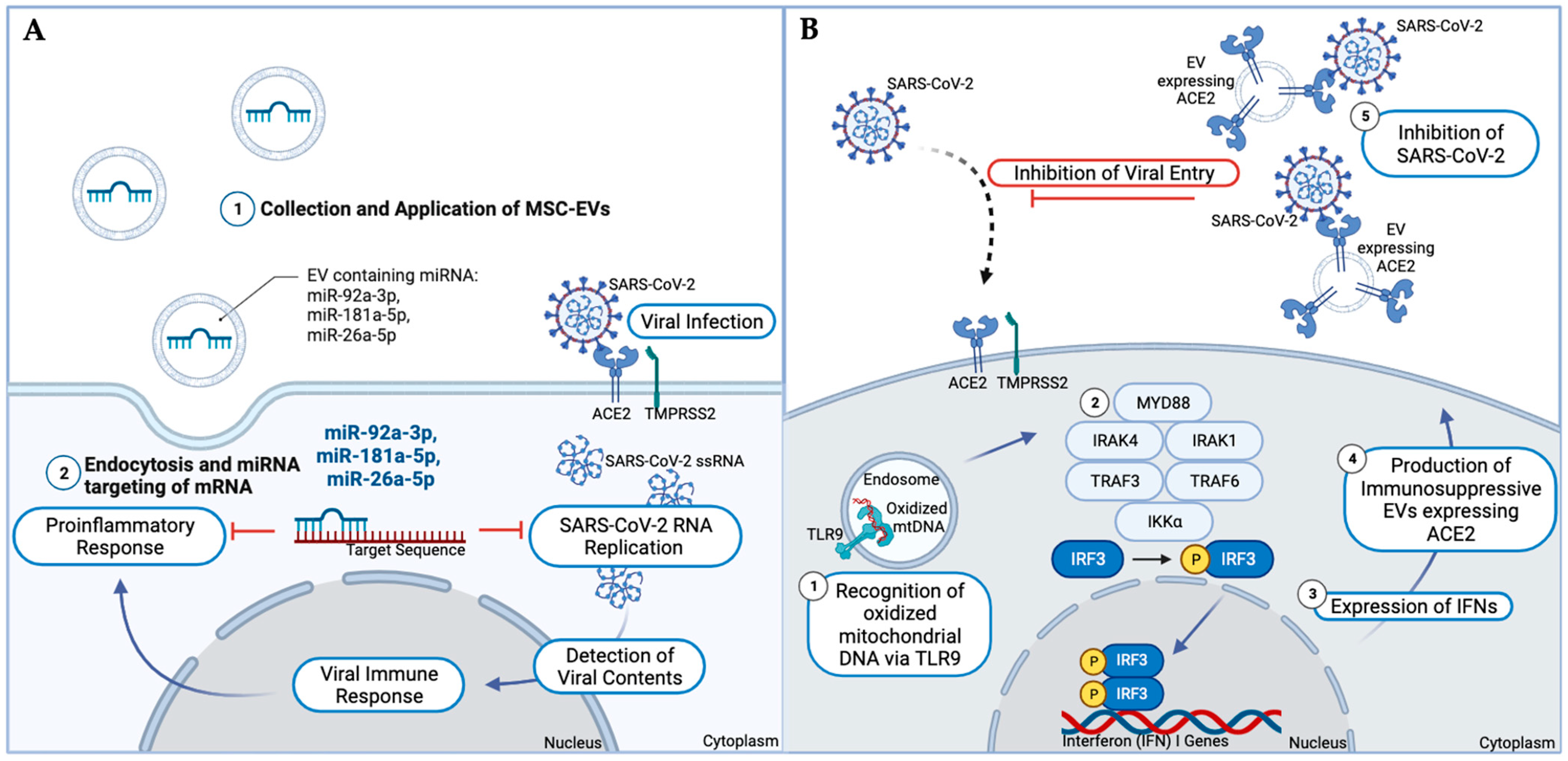
1.3. Extracellular Vesicles and Their Role in SARS-CoV-2 Pathogenesis
1.4. Extracellular Vesicles as an Indicator of Kidney Disease and COVID-19 Disease Outcome
2. Acute Kidney Injury (AKI), EVs, and COVID-19
3. Extracellular Vesicles as a Treatment Option for COVID-19
4. Limitations and Future Directions
5. Conclusions
Funding
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Civljak, R.; Markotic, A.; Kuzman, I. The third coronavirus epidemic in the third millennium: What’s next? Croat. Med. J. 2020, 61, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Eurosurveillance 2020, 25, 200131e. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Al-Awaida, W.J.; Jawabrah Al Hourani, B.; Swedan, S.; Nimer, R.; Alzoughool, F.; Al-Ameer, H.J.; Al Tamam, S.E.; Alashqar, R.; Al Bawareed, O.; Gushchina, Y.; et al. Correlates of SARS-CoV-2 Variants on Deaths, Case Incidence and Case Fatality Ratio among the Continents for the Period of 1 December 2020 to 15 March 2021. Genes 2021, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Cisewski, J.A.; Xu, J.; Anderson, R.N. Provisional Mortality Data—United States, 2022. MMWR Morb. Mortal Wkly. Rep. 2023, 72, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Khraisat, B.; Toubasi, A.; AlZoubi, L.; Al-Sayegh, T.; Mansour, A. Meta-analysis of prevalence: The psychological sequelae among COVID-19 survivors. Int. J. Psychiatry Clin. Pract. 2022, 26, 234–243. [Google Scholar] [CrossRef]
- Goudarzi, S.; Esmaeeli, S.; Valencia, J.D.; Lu, M.E.; Hales, R.R.; Fehnel, C.R.; Conley, C.M.; Quraishi, S.A.; Nozari, A. Treatment Options for COVID-19-Related Guillain-Barre Syndrome: A Systematic Review of Literature. Neurologist 2021, 26, 196–224. [Google Scholar] [CrossRef]
- Varma-Doyle, A.; Villemarette-Pittman, N.R.; Lelorier, P.; England, J. Demonstrating new-onset or worsened sudomotor function post-COVID-19 on comparative analysis of autonomic function pre-and post-SARS-CoV-2 infection. Eneurologicalsci 2023, 30, 100445. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Tsoi, K.H.; Lee, C.H.; Cheung, C.Y.Y.; Fong, C.H.Y.; Lee, A.C.H.; Tam, A.R.; Pang, P.; Ho, T.Y.; Law, C.Y.; et al. A prospective follow-up on thyroid function, thyroid autoimmunity and long COVID among 250 COVID-19 survivors. Endocrine 2023, 80, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Mart, M.F.; Ware, L.B. The long-lasting effects of the acute respiratory distress syndrome. Expert Rev. Respir. Med. 2020, 14, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Valdes, R.; Almaguer-Lopez, M.; Lopez-Marin, L.; Bacallao-Mendez, R.; Jorge, F.; Guerra-Bustillo, G. COVID-19 and the Kidneys: Risk, Damage and Sequelae. MEDICC Rev. 2020, 22, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Wilkins, K.J.; Alakwaa, F.; Liu, F.; Torre-Healy, L.A.; Krichevsky, S.; Hong, S.S.; Sakhuja, A.; Potu, C.K.; Saltz, J.H.; et al. Geographic and Temporal Trends in COVID-Associated Acute Kidney Injury in the National COVID Cohort Collaborative. Clin. J. Am. Soc. Nephrol. 2023, 18, 1006–1018. [Google Scholar] [CrossRef]
- Zadeh, F.H.; Wilson, D.R.; Agrawal, D.K. Long COVID: Complications, Underlying Mechanisms, and Treatment Strategies. Arch. Microbiol. Immunol. 2023, 7, 36–61. [Google Scholar] [PubMed]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney Outcomes in Long COVID. J. Am. Soc. Nephrol. 2021, 32, 2851–2862. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. COVID-19: Infection increases the risk of kidney disease even in mild cases, finds study. BMJ 2021, 374, n2189. [Google Scholar] [CrossRef]
- Farkash, E.A.; Wilson, A.M.; Jentzen, J.M. Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. J. Am. Soc. Nephrol. 2020, 31, 1683–1687. [Google Scholar] [CrossRef]
- Sun, R.; Cai, Y.; Zhou, Y.; Bai, G.; Zhu, A.; Kong, P.; Sun, J.; Li, Y.; Liu, Y.; Liao, W.; et al. Proteomic profiling of single extracellular vesicles reveals colocalization of SARS-CoV-2 with a CD81/integrin-rich EV subpopulation in sputum from COVID-19 severe patients. Front. Immunol. 2023, 14, 1052141. [Google Scholar] [CrossRef]
- Bartas, M.; Volna, A.; Beaudoin, C.A.; Poulsen, E.T.; Cerven, J.; Brazda, V.; Spunda, V.; Blundell, T.L.; Pecinka, P. Unheeded SARS-CoV-2 proteins? A deep look into negative-sense RNA. Brief. Bioinform. 2022, 23, bbac045. [Google Scholar] [CrossRef]
- Kutter, J.S.; de Meulder, D.; Bestebroer, T.M.; Lexmond, P.; Mulders, A.; Richard, M.; Fouchier, R.A.M.; Herfst, S. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nat. Commun. 2021, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Mulay, A.; Konda, B.; Garcia, G., Jr.; Yao, C.; Beil, S.; Villalba, J.M.; Koziol, C.; Sen, C.; Purkayastha, A.; Kolls, J.K.; et al. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery. Cell Rep. 2021, 35, 109055. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Shum, M.H.; Leung, G.M.; Lam, T.T.; Wu, J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Eurosurveillance 2021, 26, 2002106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Pohlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784.e5. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Muller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lutgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, J.; Liu, L.; Chen, R.; Wang, M.; Xiong, M.; Li, Z.Q.; Zhao, Y.; Li, H.; Guan, C.; et al. SARS-CoV-2 Causes Acute Kidney Injury by Directly Infecting Renal Tubules. Front. Cell Dev. Biol. 2021, 9, 664868. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Nakayama, T.; Wu, C.T.; Goltsev, Y.; Jiang, S.; Gall, P.A.; Liao, C.K.; Shih, L.C.; Schurch, C.M.; McIlwain, D.R.; et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat. Commun. 2020, 11, 5453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Zhang, H. Genetic Roadmap for Kidney Involvement of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Clin. J. Am. Soc. Nephrol. 2020, 15, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Fink, C.; Ichimura, T.; Sako, K.; Mori, M.; Lee, N.N.; Aschauer, P.; Padmanabha Das, K.M.; Hong, S.; Song, M.; et al. KIM-1/TIM-1 is a Receptor for SARS-CoV-2 in Lung and Kidney. medRxiv 2022. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zeng, X.; Chen, H.; Chen, Y.; Yang, D.; Shen, Z.; Wang, X.; Liu, X.; Xiong, M.; et al. Kidney injury molecule-1 is a potential receptor for SARS-CoV-2. J. Mol. Cell Biol. 2021, 13, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Snijder, E.J.; van der Meer, Y.; Zevenhoven-Dobbe, J.; Onderwater, J.J.; van der Meulen, J.; Koerten, H.K.; Mommaas, A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006, 80, 5927–5940. [Google Scholar] [CrossRef]
- Eymieux, S.; Uzbekov, R.; Rouille, Y.; Blanchard, E.; Hourioux, C.; Dubuisson, J.; Belouzard, S.; Roingeard, P. Secretory Vesicles Are the Principal Means of SARS-CoV-2 Egress. Cells 2021, 10, 2047. [Google Scholar] [CrossRef]
- Yoo, M.H.; Lee, A.R.; Moon, K.S. Characteristics of Extracellular Vesicles and Preclinical Testing Considerations Prior to Clinical Applications. Biomedicines 2022, 10, 869. [Google Scholar] [CrossRef]
- Mastronardi, M.L.; Mostefai, H.A.; Soleti, R.; Agouni, A.; Martinez, M.C.; Andriantsitohaina, R. Microparticles from apoptotic monocytes enhance nitrosative stress in human endothelial cells. Fundam. Clin. Pharmacol. 2011, 25, 653–660. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Statello, L.; Maugeri, M.; Garre, E.; Nawaz, M.; Wahlgren, J.; Papadimitriou, A.; Lundqvist, C.; Lindfors, L.; Collen, A.; Sunnerhagen, P.; et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS ONE 2018, 13, e0195969. [Google Scholar] [CrossRef]
- Ipinmoroti, A.O.; Matthews, Q.L. Extracellular Vesicles: Roles in Human Viral Infections, Immune-Diagnostic, and Therapeutic Applications. Pathogens 2020, 9, 1056. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Wurdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef]
- Meckes, D.G., Jr.; Gunawardena, H.P.; Dekroon, R.M.; Heaton, P.R.; Edwards, R.H.; Ozgur, S.; Griffith, J.D.; Damania, B.; Raab-Traub, N. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc. Natl. Acad. Sci. USA 2013, 110, E2925–E2933. [Google Scholar] [CrossRef]
- Plazolles, N.; Humbert, J.M.; Vachot, L.; Verrier, B.; Hocke, C.; Halary, F. Pivotal advance: The promotion of soluble DC-SIGN release by inflammatory signals and its enhancement of cytomegalovirus-mediated cis-infection of myeloid dendritic cells. J. Leukoc. Biol. 2011, 89, 329–342. [Google Scholar] [CrossRef]
- Tamai, K.; Shiina, M.; Tanaka, N.; Nakano, T.; Yamamoto, A.; Kondo, Y.; Kakazu, E.; Inoue, J.; Fukushima, K.; Sano, K.; et al. Regulation of hepatitis C virus secretion by the Hrs-dependent exosomal pathway. Virology 2012, 422, 377–385. [Google Scholar] [CrossRef]
- Kadiu, I.; Narayanasamy, P.; Dash, P.K.; Zhang, W.; Gendelman, H.E. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. J. Immunol. 2012, 189, 744–754. [Google Scholar] [CrossRef]
- Kongsomros, S.; Pongsakul, N.; Panachan, J.; Khowawisetsut, L.; Somkird, J.; Sangma, C.; Kanjanapruthipong, T.; Wongtrakoongate, P.; Chairoungdua, A.; Pattanapanyasat, K.; et al. Comparison of viral inactivation methods on the characteristics of extracellular vesicles from SARS-CoV-2 infected human lung epithelial cells. J. Extracell. Vesicles 2022, 11, e12291. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Bihl, J. Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell. Oxid. Med. Cell. Longev. 2020, 2020, 4213541. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Dreux, M.; Garaigorta, U.; Boyd, B.; Decembre, E.; Chung, J.; Whitten-Bauer, C.; Wieland, S.; Chisari, F.V. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 2012, 12, 558–570. [Google Scholar] [CrossRef]
- Barberis, E.; Vanella, V.V.; Falasca, M.; Caneapero, V.; Cappellano, G.; Raineri, D.; Ghirimoldi, M.; De Giorgis, V.; Puricelli, C.; Vaschetto, R.; et al. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Front. Mol. Biosci. 2021, 8, 632290. [Google Scholar] [CrossRef]
- Jacobs, J.L.; Naqvi, A.; Shah, F.A.; Boltz, V.F.; Kearney, M.F.; McVerry, B.J.; Ray, P.; Schaefer, C.; Fitzpatrick, M.; Methe, B.; et al. Plasma SARS-CoV-2 RNA levels as a biomarker of lower respiratory tract SARS-CoV-2 infection in critically ill patients with COVID-19. medRxiv 2022. [Google Scholar] [CrossRef]
- Andersson, M.I.; Arancibia-Carcamo, C.V.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beneke, T.; Bibi, S.; Brooks, T.; Carroll, M.; Crook, D.; et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. 2020, 5, 181. [Google Scholar] [CrossRef]
- Amati, F.; Vancheri, C.; Latini, A.; Colona, V.L.; Grelli, S.; D’Apice, M.R.; Balestrieri, E.; Passarelli, C.; Minutolo, A.; Loddo, S.; et al. Expression profiles of the SARS-CoV-2 host invasion genes in nasopharyngeal and oropharyngeal swabs of COVID-19 patients. Heliyon 2020, 6, e05143. [Google Scholar] [CrossRef]
- Latini, A.; Vancheri, C.; Amati, F.; Morini, E.; Grelli, S.; Matteucci, C.; Petrone, V.; Colona, V.L.; Murdocca, M.; Andreoni, M.; et al. Expression analysis of miRNA hsa-let7b-5p in naso-oropharyngeal swabs of COVID-19 patients supports its role in regulating ACE2 and DPP4 receptors. J. Cell. Mol. Med. 2022, 26, 4940–4948. [Google Scholar] [CrossRef]
- Miller, D.; Eagle-Hemming, B.; Sheikh, S.; Joel-David, L.; Adebayo, A.; Lai, F.Y.; Roman, M.; Kumar, T.; Aujla, H.; Murphy, G.J.; et al. Urinary extracellular vesicles and micro-RNA as markers of acute kidney injury after cardiac surgery. Sci. Rep. 2022, 12, 10402. [Google Scholar] [CrossRef]
- Kanno, K.; Sasaki, S.; Hirata, Y.; Ishikawa, S.; Fushimi, K.; Nakanishi, S.; Bichet, D.G.; Marumo, F. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N. Engl. J. Med. 1995, 332, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.E.; Schrier, R.W. Pathophysiological roles of arginine vasopressin and aquaporin-2 in impaired water excretion. Clin. Endocrinol. 2003, 58, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, T.; Alves, L.S.; Cabral-Castro, M.J.; Silva, A.R.O.; Xavier, A.R.; Burger, D.; Almeida, J.R.; Silva, A.A. Exploring Urinary Extracellular Vesicles and Immune Mediators as Biomarkers of Kidney Injury in COVID-19 Hospitalized Patients. Diagnostics 2022, 12, 2600. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Birkhoff, W.; Menzies, R.I.; Webb, D.J.; Bailey, M.A.; Dear, J.W. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J. Physiol. 2011, 589 Pt 24, 6119–6127. [Google Scholar] [CrossRef] [PubMed]
- Gildea, J.J.; Seaton, J.E.; Victor, K.G.; Reyes, C.M.; Bigler Wang, D.; Pettigrew, A.C.; Courtner, C.E.; Shah, N.; Tran, H.T.; Van Sciver, R.E.; et al. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin. Biochem. 2014, 47, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Lee, B.R.; Park, K.H.; Nihalani, D.; Yoon, J.H.; Ikeda, M.; Kwon, S.H. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci. Rep. 2019, 9, 4692. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Hoshina, T.; Matsuzaki, J.; Yoshioka, Y.; Kadota, T.; Hosaka, Y.; Fujimoto, S.; Kawamoto, H.; Watanabe, N.; Sawaki, K.; et al. Early prediction of COVID-19 severity using extracellular vesicle COPB2. J. Extracell. Vesicles 2021, 10, e12092. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Rubin, S.; Orieux, A.; Prevel, R.; Garric, A.; Bats, M.L.; Dabernat, S.; Camou, F.; Guisset, O.; Issa, N.; Mourissoux, G.; et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin. Kidney J. 2020, 13, 354–361. [Google Scholar] [CrossRef]
- Hung, A.M.; Shah, S.C.; Bick, A.G.; Yu, Z.; Chen, H.C.; Hunt, C.M.; Wendt, F.; Wilson, O.; Greevy, R.A.; Chung, C.P.; et al. APOL1 Risk Variants, Acute Kidney Injury, and Death in Participants with African Ancestry Hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern. Med. 2022, 182, 386–395. [Google Scholar] [CrossRef]
- Chen, K.; Lei, Y.; He, Y.; Xiao, F.; Yu, Y.; Lai, X.; Liu, Y.; Wang, J.; Dai, H. Clinical outcomes of hospitalized COVID-19 patients with renal injury: A multi-hospital observational study from Wuhan. Sci. Rep. 2021, 11, 15205. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.S.; James, B.D.; Al-Chalabi, S.; Sykes, L.; Kalra, P.A.; Green, D. Community-versus hospital-acquired acute kidney injury in hospitalised COVID-19 patients. BMC Nephrol. 2021, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, C.; Prasad, P.; Elumalai, R.; Matcha, J. Clinical profile and outcomes of COVID-19 patients with acute kidney injury: A tertiary centre experience from South India. Clin. Exp. Nephrol. 2022, 26, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, H.; Kazemian, S.; Rahbar, M.; Farrokhpour, H.; Montazeri, M.; Kafan, S.; Salimzadeh, A.; Talebpour, M.; Majidi, F.; Jannatalipour, A.; et al. The Risk Factors and Clinical Outcomes Associated with Acute Kidney Injury in Patients with COVID-19: Data from a Large Cohort in Iran. Kidney Blood Press Res. 2021, 46, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Scarpioni, R.; Valsania, T.; Albertazzi, V.; Blanco, V.; DeAmicis, S.; Manini, A.; Melfa, L.; Ricardi, M.; Rocca, C.; Gandolfi, S. Acute kidney injury, a common and severe complication in hospitalized patients during the COVID-19 pandemic. J. Nephrol. 2021, 34, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Kilis-Pstrusinska, K.; Akutko, K.; Braksator, J.; Dancewicz, A.; Grosman-Dziewiszek, P.; Jamer, T.; Juszczynska, K.; Konikowska, K.; Koruba, M.; Pupek, M.; et al. Kidney Dysfunction and Its Progression in Patients Hospitalized Duo to COVID-19: Contribution to the Clinical Course and Outcomes. J. Clin. Med. 2021, 10, 5522. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Gameiro, J.; Oliveira, J.; Fonseca, J.A.; Duarte, I.; Bernardo, J.; Branco, C.; Costa, C.; Carreiro, C.; Braz, S.; et al. Acute Kidney Disease and Mortality in Acute Kidney Injury Patients with COVID-19. J. Clin. Med. 2021, 10, 4599. [Google Scholar] [CrossRef]
- Procaccini, F.L.; Alcazar Arroyo, R.; Albalate Ramon, M.; Torres Aguilera, E.; Martin Navarro, J.; Ryan Murua, P.; Cintra Cabrera, M.; Ortega Diaz, M.; Puerta Carretero, M.; de Sequera Ortiz, P. Acute kidney injury in 3182 patients admitted with COVID-19: A single-center, retrospective, case-control study. Clin. Kidney J. 2021, 14, 1557–1569. [Google Scholar] [CrossRef]
- Sullivan, M.K.; Lees, J.S.; Drake, T.M.; Docherty, A.B.; Oates, G.; Hardwick, H.E.; Russell, C.D.; Merson, L.; Dunning, J.; Nguyen-Van-Tam, J.S.; et al. Acute kidney injury in patients hospitalized with COVID-19 from the ISARIC WHO CCP-UK Study: A prospective, multicentre cohort study. Nephrol. Dial. Transplant. 2022, 37, 271–284. [Google Scholar] [CrossRef]
- Jewell, P.D.; Bramham, K.; Galloway, J.; Post, F.; Norton, S.; Teo, J.; Fisher, R.; Saha, R.; Hutchings, S.; Hopkins, P.; et al. COVID-19-related acute kidney injury; incidence, risk factors and outcomes in a large UK cohort. BMC Nephrol. 2021, 22, 359. [Google Scholar] [CrossRef]
- Wan, Y.I.; Bien, Z.; Apea, V.J.; Orkin, C.M.; Dhairyawan, R.; Kirwan, C.J.; Pearse, R.M.; Puthucheary, Z.A.; Prowle, J.R. Acute kidney injury in COVID-19: Multicentre prospective analysis of registry data. Clin. Kidney J. 2021, 14, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Strohbehn, I.A.; Zhao, S.; Seethapathy, H.; Lee, M.; Rusibamayila, N.; Allegretti, A.S.; Parada, X.V.; Sise, M.E. Acute Kidney Injury Incidence, Recovery, and Long-term Kidney Outcomes among Hospitalized Patients with COVID-19 and Influenza. Kidney Int. Rep. 2021, 6, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Gupta, S.; Tighiouart, H.; Goyal, N.; Faugno, A.J.; Tariq, A.; Raichoudhury, R.; Sharma, J.H.; Meyer, L.; Kshirsagar, R.K.; et al. Kidney Recovery and Death in Critically Ill Patients with COVID-19-Associated Acute Kidney Injury Treated with Dialysis: The STOP-COVID Cohort Study. Am. J. Kidney Dis. 2022, 79, 404–416.e1. [Google Scholar] [CrossRef]
- Sharma, P.; Uppal, N.N.; Wanchoo, R.; Shah, H.H.; Yang, Y.; Parikh, R.; Khanin, Y.; Madireddy, V.; Larsen, C.P.; Jhaveri, K.D.; et al. COVID-19-Associated Kidney Injury: A Case Series of Kidney Biopsy Findings. J. Am. Soc. Nephrol. 2020, 31, 1948–1958. [Google Scholar] [CrossRef]
- Remmelink, M.; De Mendonca, R.; D’Haene, N.; De Clercq, S.; Verocq, C.; Lebrun, L.; Lavis, P.; Racu, M.L.; Trepant, A.L.; Maris, C.; et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit. Care 2020, 24, 495. [Google Scholar] [CrossRef]
- Santoriello, D.; Khairallah, P.; Bomback, A.S.; Xu, K.; Kudose, S.; Batal, I.; Barasch, J.; Radhakrishnan, J.; D’Agati, V.; Markowitz, G. Postmortem Kidney Pathology Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 2158–2167. [Google Scholar] [CrossRef]
- Golmai, P.; Larsen, C.P.; DeVita, M.V.; Wahl, S.J.; Weins, A.; Rennke, H.G.; Bijol, V.; Rosenstock, J.L. Histopathologic and Ultrastructural Findings in Postmortem Kidney Biopsy Material in 12 Patients with AKI and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1944–1947. [Google Scholar] [CrossRef]
- Miller, S.E.; Brealey, J.K. Visualization of putative coronavirus in kidney. Kidney Int. 2020, 98, 231–232. [Google Scholar] [CrossRef]
- Braun, F.; Lutgehetmann, M.; Pfefferle, S.; Wong, M.N.; Carsten, A.; Lindenmeyer, M.T.; Norz, D.; Heinrich, F.; Meissner, K.; Wichmann, D.; et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 2020, 396, 597–598. [Google Scholar] [CrossRef]
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.S.; Willemsen, B.; et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 2022, 29, 217–231.e8. [Google Scholar] [CrossRef] [PubMed]
- Radovic, S.; Meng, W.; Chen, L.; Paniz Mondolfi, A.E.; Bryce, C.; Grimes, Z.; Sordillo, E.M.; Cordon-Cardo, C.; Guo, H.; Huang, Y.; et al. SARS-CoV-2 infection of kidney tissues from severe COVID-19 patients. J. Med. Virol. 2023, 95, e28566. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.E.; Dhar, N.; Timar, R.; Wills, M.; Dhar, S.; Chancellor, M.B. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC). Med. Hypotheses 2020, 145, 110375. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, K.; Okeke, T.; Hajj, J.; Siddiq, K.; Rader, D.J.; Wu, J.; Susztak, K. Longitudinal urinary biomarkers of immunological activation in COVID-19 patients without clinically apparent kidney disease versus acute and chronic failure. Sci. Rep. 2021, 11, 19675. [Google Scholar] [CrossRef]
- Medeiros, T.; Guimaraes, G.M.C.; Carvalho, F.R.; Alves, L.S.; Faustino, R.; Campi-Azevedo, A.C.; Peruhype-Magalhaes, V.; Teixeira-Carvalho, A.; de Souza Gomes, M.; Rodrigues do Amaral, L.; et al. Acute kidney injury associated to COVID-19 leads to a strong unbalance of circulant immune mediators. Cytokine 2022, 157, 155974. [Google Scholar] [CrossRef]
- Gradin, A.; Andersson, H.; Luther, T.; Anderberg, S.B.; Rubertsson, S.; Lipcsey, M.; Aberg, M.; Larsson, A.; Frithiof, R.; Hultstrom, M. Urinary cytokines correlate with acute kidney injury in critically ill COVID-19 patients. Cytokine 2021, 146, 155589. [Google Scholar] [CrossRef]
- Kellum, J.A.; Nadim, M.K.; Forni, L.G. Sepsis-associated acute kidney injury: Is COVID-19 different? Kidney Int. 2020, 98, 1370–1372. [Google Scholar] [CrossRef]
- Alexander, M.P.; Mangalaparthi, K.K.; Madugundu, A.K.; Moyer, A.M.; Adam, B.A.; Mengel, M.; Singh, S.; Herrmann, S.M.; Rule, A.D.; Cheek, E.H.; et al. Acute Kidney Injury in Severe COVID-19 Has Similarities to Sepsis-Associated Kidney Injury: A Multi-Omics Study. Mayo Clin. Proc. 2021, 96, 2561–2575. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Sonmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Cao, Y.; Zhong, M. No causal association between COVID-19 and sepsis: A bidirectional two-sample Mendelian randomization study. Front. Immunol. 2023, 14, 1183489. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lyon, C.J.; Fletcher, J.K.; Tang, W.; Wan, M.; Hu, T.Y. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm. Sin. B 2021, 11, 1493–1512. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.L.; Feng, Y.; Wu, M.; Wang, B.; Li, Z.L.; Zhong, X.; Wu, W.J.; Chen, J.; Ni, H.F.; Tang, T.T.; et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020, 27, 210–226. [Google Scholar] [CrossRef]
- Li, Z.L.; Lv, L.L.; Tang, T.T.; Wang, B.; Feng, Y.; Zhou, L.T.; Cao, J.Y.; Tang, R.N.; Wu, M.; Liu, H.; et al. HIF-1alpha inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019, 95, 388–404. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Liang, L.; Chen, J.; Sun, S.; Wei, B.; Zhong, Y.; Huang, X.R.; Liu, J.; Wang, X.; et al. SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation. Front. Immunol. 2023, 14, 1264447. [Google Scholar] [CrossRef]
- Schurink, B.; Roos, E.; Radonic, T.; Barbe, E.; Bouman, C.S.C.; de Boer, H.H.; de Bree, G.J.; Bulle, E.B.; Aronica, E.M.; Florquin, S.; et al. Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe 2020, 1, e290–e299. [Google Scholar] [CrossRef]
- Boss, K.; Konik, M.; Brasen, J.H.; Schmitz, J.; Jurgens, C.; Kribben, A.; Witzke, O.; Dolff, S.; Gackler, A. COVID-19 associated acute transplant failure after AB0-incompatible living donor kidney transplantation—A case report. BMC Nephrol. 2023, 24, 19. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Grootemaat, A.E.; Wiersma, N.; van der Niet, S.; Schimmel, I.M.; Florquin, S.; Reits, E.A.; Miller, S.E.; van der Wel, N.N. Nucleocapsid protein accumulates in renal tubular epithelium of a post-COVID-19 patient. Microbiol. Spectr. 2023, 11, e0302923. [Google Scholar] [CrossRef] [PubMed]
- Tampe, D.; Hakroush, S.; Bosherz, M.S.; Franz, J.; Hofmann-Winkler, H.; Pohlmann, S.; Kluge, S.; Moerer, O.; Stadelmann, C.; Strobel, P.; et al. Urinary Levels of SARS-CoV-2 Nucleocapsid Protein Associate with Risk of AKI and COVID-19 Severity: A Single-Center Observational Study. Front. Med. 2021, 8, 644715. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, Y.; Lim, C.W.; Park, J.M.; Yu, S.H.; Kim, Y.; Han, H.J.; Kim, C.H.; Song, Y.S.; Kim, C.; et al. Potential Therapeutic Effect of Micrornas in Extracellular Vesicles from Mesenchymal Stem Cells against SARS-CoV-2. Cells 2021, 10, 2393. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, M.; Shahrbaf, M.A.; Nouri, M.; Shekari, F.; Hosseini, S.E.; Hashemian, S.R.; Aliannejad, R.; Jamaati, H.; Khavandgar, N.; Alemi, H.; et al. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: A randomized controlled trial. Stem Cell Res. Ther. 2023, 14, 169. [Google Scholar] [CrossRef]
- Ching, K.L.; de Vries, M.; Gago, J.; Dancel-Manning, K.; Sall, J.; Rice, W.J.; Barnett, C.; Khodadadi-Jamayran, A.; Tsirigos, A.; Liang, F.X.; et al. ACE2-containing defensosomes serve as decoys to inhibit SARS-CoV-2 infection. PLoS Biol. 2022, 20, e3001754. [Google Scholar] [CrossRef]
- Duong, A.; Parmar, G.; Kirkham, A.M.; Burger, D.; Allan, D.S. Registered clinical trials investigating treatment with cell-derived extracellular vesicles: A scoping review. Cytotherapy 2023, 25, 939–945. [Google Scholar] [CrossRef]
| Stage | Description |
|---|---|
| Stage 1 | sCR 1.5–1.9 times baseline or ≥0.3 mg/dL (≥26.5 μmol/L) increase and/or urine output < 0.5 mL/kg/h for 6–12 h |
| Stage 2 | sCR 2.0–2.9 times baseline and/or urine output < 0.5 mL/kg/h for ≥12 h |
| Stage 3 | sCR 3.0 times baseline or increase in sCR to ≥4.0 mg/dL (≥353.6 μmol/L) or initiation of renal replacement therapy |
| Stage 3 for patients <18 years old | Decrease in eGFR to <35 mL/min per 1.73 m2 and/or urine output <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h |
| Author (Year) | Country | Sample Size | Male Sex (%) | Mean/Median Age (Years) | AKI (%) | Exclusion Criteria of the Cohort Study | Method of SARS-CoV-2 Diagnostic Test |
|---|---|---|---|---|---|---|---|
| Hung et al. (2022) [71] | Africa | 990 | 92.10% | 68 | 392 (39.6%) | History of ESKD, baseline eGFR < 15 mL/min/1.73 m2, no sCr levels recorded | PCR testing of nasopharyngeal specimen |
| Chen et al. (2021) [72] | China | 1851 | 48.00% | 62 | 115 (6.7%) | Lack of renal function tests | PCR testing of nasal and pharyngeal specimens |
| Bell et al. (2021) [73] | England | 448 | 54.80% | 69.4 | 118 (26.3%) | ESKD, dialysis, kidney transplant, or no sCr levels recorded | Positive COVID-19 swab |
| Sindhu et al. (2022) [74] | India | 2650 | 81.60% | 62.6 | 190 (7.20%) | Stage 5 CKD on dialysis | PCR testing of nasopharyngeal specimen |
| Rahimzadeh et al. (2021) [75] | Iran | 516 | 62.80% | 57.6 | 194 (37.6%) | History of hemodialysis or ESKD | PCR testing of oropharyngeal, nasopharyngeal, or endotracheal specimens, or symptoms consistent with COVID-19 |
| Scarpioni et al. (2021) [76] | Italy | 1701 | 64.30% | 72.8 | 233 (13.7%) | ESKD, kidney transplant, or lack of two consecutive sCr determinations | PCR testing of nasopharyngeal specimen |
| Kilis-Pstrusinska et al. (2021) [77] | Poland | 1958 | 52.10% | 62.3 | 237 (12.1%) | AKI at admission | PCR testing |
| Marques et al. (2021) [78] | Portugal | 544 | 56.30% | 66.2 | 339 (62.3%) | CKD on RRT, discharged or deceased < 1 week after hospital admission | PCR testing of nasopharyngeal specimen |
| Procaccini et al. (2021) [79] | Spain | 3182 | – | 72 | 548 (17.2%) | Dialysis, CKD Stage 5, or on RRT | PCR testing, clinical suspicion based on epidemiological data, blood parameters, and imaging |
| Sullivan et al. (2021) [80] | UK | 41,294 | 62.60% | 68 | 13,000 (31.5%) | Long-term dialysis, nosocomial infection, or readmission to hospital | PCR testing or clinical suspicion |
| Jewell et al. (2021) [81] | UK | 1248 | 58.80% | 69 | 487 (39.0%) | Probable hospital-acquired COVID-19, ESRD requiring RRT, or kidney transplant | Positive nasopharyngeal specimen, symptoms consistent with COVID-19, and a first positive SARS-CoV-2 swab test on, or up to 7 days after admission |
| Wan et al. (2021) [82] | UK | 1855 | 60.50% | 65 | 455 (24.5%) | Lack of sCr data or history of ESKD | PCR testing |
| Strohbehn et al. (2021) [83] | USA | 1091 | 49.50% | 67 | 251 (23.0%) | No recent baseline sCr, eGFR < 15, or on dialysis | PCR testing and hospitalized within 2 weeks of first positive test |
| Hsu et al. (2022) [84] | USA | 4221 | 63.50% | 61 | 2361 (56.0%) | Lack of data, baseline sCr, or kidney function at discharge, on dialysis, or hospitalized at last follow-up | Laboratory-confirmed SARS-CoV-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, C.; How-Volkman, C.; Spencer, M.; El-Shamy, A.; Mohieldin, A.M. The Role of Extracellular Vesicles in SARS-CoV-2-Induced Acute Kidney Injury: An Overview. Life 2024, 14, 163. https://doi.org/10.3390/life14020163
Bernal C, How-Volkman C, Spencer M, El-Shamy A, Mohieldin AM. The Role of Extracellular Vesicles in SARS-CoV-2-Induced Acute Kidney Injury: An Overview. Life. 2024; 14(2):163. https://doi.org/10.3390/life14020163
Chicago/Turabian StyleBernal, Carter, Christiane How-Volkman, Madison Spencer, Ahmed El-Shamy, and Ashraf M. Mohieldin. 2024. "The Role of Extracellular Vesicles in SARS-CoV-2-Induced Acute Kidney Injury: An Overview" Life 14, no. 2: 163. https://doi.org/10.3390/life14020163
APA StyleBernal, C., How-Volkman, C., Spencer, M., El-Shamy, A., & Mohieldin, A. M. (2024). The Role of Extracellular Vesicles in SARS-CoV-2-Induced Acute Kidney Injury: An Overview. Life, 14(2), 163. https://doi.org/10.3390/life14020163





