Metabolomic Profile of Arthrospira platensis from a Bulgarian Bioreactor—A Potential Opportunity for Inclusion in Dietary Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
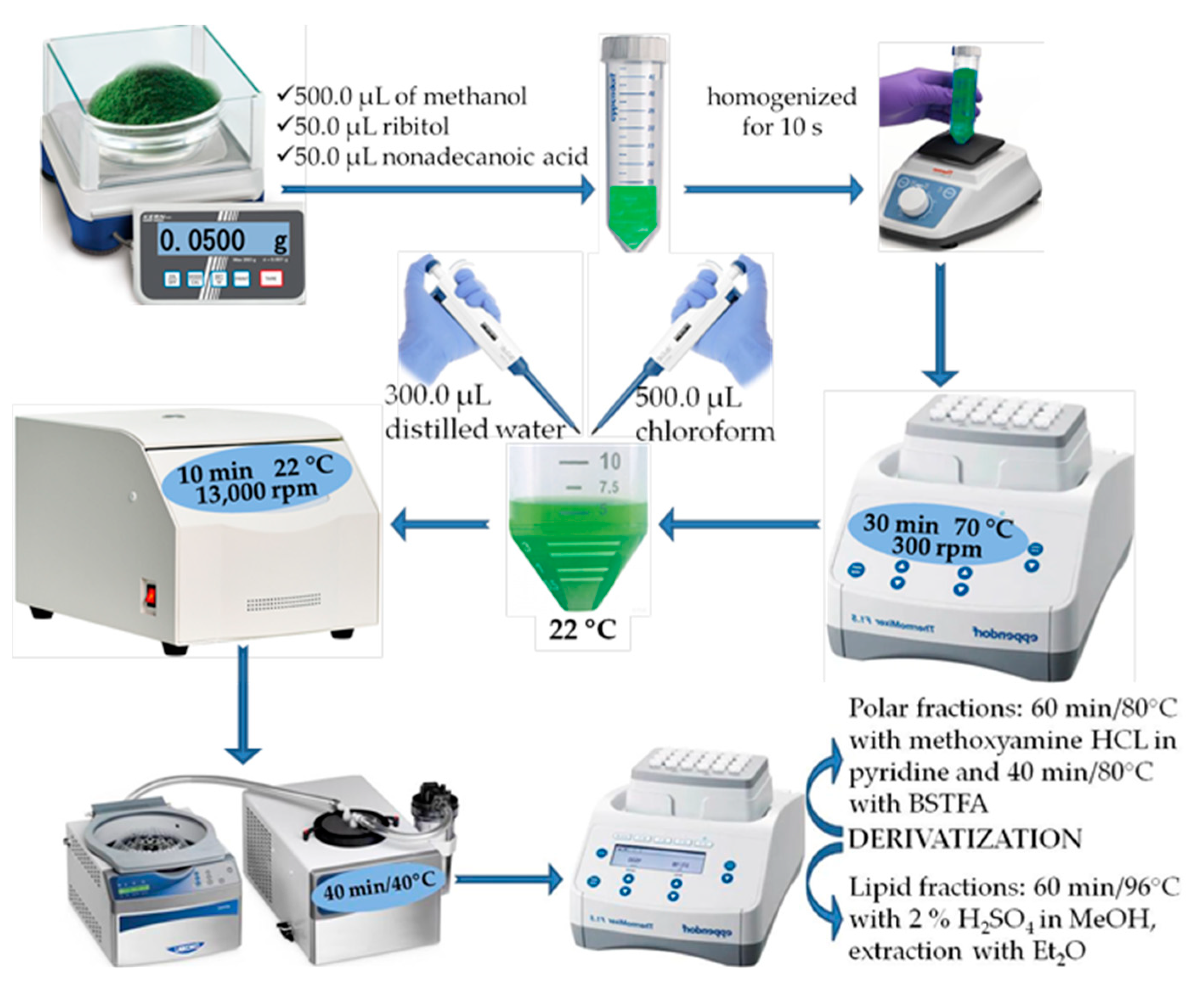
2.2. Sample Preparation
2.2.1. Polar Phase
2.2.2. Nonpolar Phase
2.3. Methods Used
2.3.1. Microcystin Analysis
2.3.2. Gas Chromatography and Mass Spectrometry
2.3.3. Determination of Vitamin Content and Protein
Determination of Proteins
Determination of Vitamin B2
Determination of Vitamin E and Vitamin A
Determination of Vitamin C
2.3.4. Determination of Fat in Arthrospira platensis
Preliminary Acid Hydrolysis
Soxhlet Extraction, after Acid Hydrolysis
2.3.5. Determination of Fatty Acid Composition in Arthrospira platensis
Fat Extraction
Preparation of Methyl Esters of Fatty Acids with 20% Boron Trifluoride in Methanol
Gas Chromatographic Determination of the Fatty Acid Composition
2.3.6. Determination of Dietary Fiber According to AOAC 985.29 Method
2.3.7. Elemental Composition
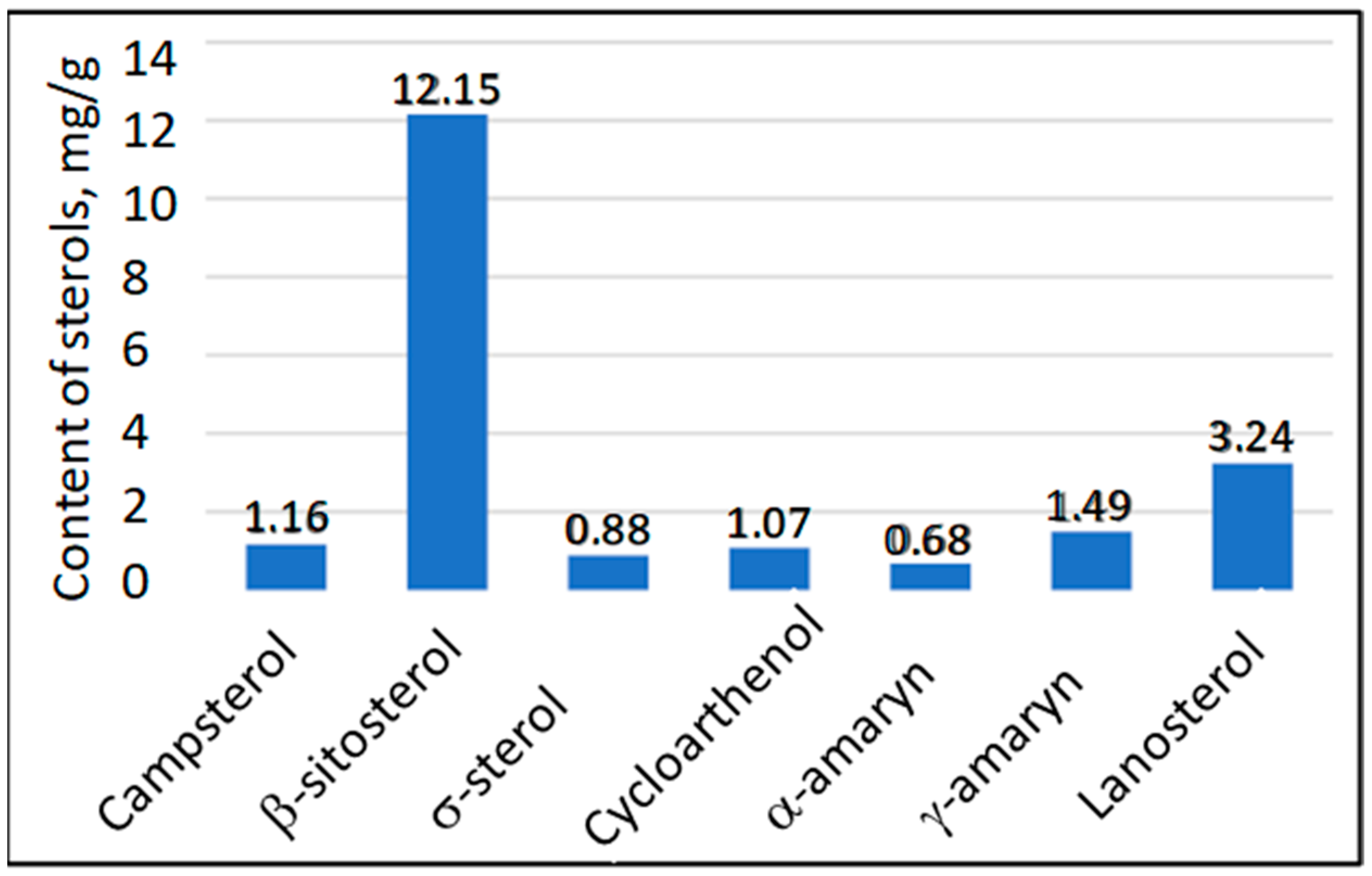
3. Results and Discussion
4. Conclusions
- -
- Pyroglutamic and aspartic acid;
- -
- Vitamins A and C;
- -
- Mannose;
- -
- α-linolenic acid, palmitic acid, and arachidonic acid;
- -
- Calcium, sulfur, and magnesium.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rautiainen, S.; Manson, J.E.; Lichtenstein, A.H.; Sesso, H.D. Dietary supplements and disease prevention—A global overview. Nat. Rev. Endocrinol. 2016, 12, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Myint, M.W.; Wu, J.; Wong, E.; Chan, S.P.; To, T.S.; Chau, M.W.; Ting, K.H.; Fung, P.M.; Au, K.S. Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: A single blind randomised controlled trial. Age Ageing 2012, 42, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sotiroudis, T.; Sotiroudis, G. Health Aspects of Spirulina (Arthrospira) Microalga Food Supplement. J. Serbian Chem. Soc. 2013, 78, 395–405. [Google Scholar] [CrossRef]
- Wu, J.; Gu, X.; Yang, D.; Xu, S.; Wang, S.; Chen, X.; Wang, Z. Bioactive substances and potentiality of marine microalgae. Food Sci. Nutr. 2021, 9, 5279–5292. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar]
- Hussein, A.; Ibrahim, G.; Kamil, M.; El-Shamarka, M.; Mostafa, S.; Mohamed, D. Spirulina-Enriched Pasta as Functional Food Rich in Protein and Antioxidant. Biointerface Res. Appl. Chem. 2021, 11, 14736–14750. [Google Scholar]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhat, A.G.; OKeefe, J. Effects of Spirulina on weight loss and blood lipids: A review. Open Heart 2020, 7, e001003. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Research and Innovation, Bioeconomy: The European Way to Use Our Natural Resources: Action Plan 2018; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Sorrenti, V.; Castagna, D.A.; Fortinguerra, S.; Buriani, A.; Scapagnini, G.; Willcox, D.C. Spirulina Microalgae and Brain Health: A Scoping Review of Experimental and Clinical Evidence. Mar. Drugs 2021, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxidative Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef]
- Calella, P.; Cerullo, G.; Di Dio, M.; Liguori, F.; Di Onofrio, V.; Gallè, F.; Liguori, G. Antioxidant, anti-inflammatory and immunomodulatory effects of Spirulina in exercise and sport: A systematic review. Front. Nutr. 2022, 9, 1048258. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Di Dio, M.; Cerullo, G.; Di Onofrio, V.; Gallé, F.; Liguori, G. Antioxidant, immunomodulatory, and anti-inflammatory effects of Spirulina in disease conditions: A systematic review. Int. J. Food Sci. Nutr. 2022, 73, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Gentscheva, G.; Milkova-Tomova, I.; Pehlivanov, I.; Gugleva, V.; Nikolova, K.; Petkova, N.; Andonova, V.; Buhalova, D.; Pisanova, E. Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity. Appl. Sci. 2022, 12, 9935. [Google Scholar] [CrossRef]
- EN 12822:2014; Foodstuffs—Determination of Vitamin E by High-Performance Liquid Chromatography—Measurement of α-, β-, γ- and δ-Tocopherol. CEN: Brussels, Belgium, 2014. Available online: https://standards.iteh.ai/catalog/standards/cen/71b18a31-b392-46a4-85e1-bf94949565b8/en-12822-2014 (accessed on 18 January 2024).
- EN ISO 12966-1:2015; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. ISO: Geneva, Switzerland, 2015.
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Ester—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- Nikolova, K.; Eftimov, T.; Pashev, A.; Karadjov, M.; Tzvetkova, C.; Gentscheva, G.; Brabant, D.; Fouzar, S. Correlation between chemical characteristics and optical spectra of Spirulina commercially available on the Bulgarian market. TCSA J. Turk. Chem. Soc. Sect. A Chem. 2023, 10, 465–474. [Google Scholar] [CrossRef]
- Petrov, G. Organic Chemistry, 4th ed.; University Edition of St. Clement of Ohridski: Sofia, Bulgaria, 2006. [Google Scholar]
- Fais, G.; Manca, A.; Bolognesi, F.; Borselli, M.; Concas, A.; Busutti, M.; Broggi, G.; Sanna, P.; Castillo-Aleman, Y.M.; Rivero-Jiménez, R.A.; et al. Wide Range Applications of Spirulina: From Earth to Space Missions. Mar. Drugs 2022, 20, 299. [Google Scholar] [CrossRef]
- Dillon, J.C.; Phuc, A.P.; Dubacq, J.P. Nutritional Value of the Alga Spirulina. World Rev. Nutr. Diet. 1995, 77, 32–46. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina-From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Colla, L.M.; Oliveira Reinehr, C.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Vaz, B.S.; Moreira, J.B.; Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplement. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- De Morais, M.G.; da Silva Vaz, B.; Greque de Morais, E.; Vieira Costa, J.A. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- Gager, L.; Lalegerie, F.; Connan, S.; Stiger-Pouvreau, V. Marine Algal Derived Phenolic Compounds and Their Biological Activities for Medicinal and Cosmetic Applications. In Recent Advances in Micro and Macroalgal Processing; Rajauria, G., Yuan, Y.V., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 278–334. ISBN 978-1-119-54265-0. [Google Scholar]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Braune, S.; Jung, C.H.G.; Krüger-Genge, A.; Waldeck, P.; Petrick, I.; Küpper, J.H. Lipophilic and Hydrophilic Compounds from Arthrospira platensis and Its Effects on Tissue and Blood Cells-An Overview. Life 2022, 12, 1497. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Francois, R.A.; Yamauchi, T.; Perez, M.; Sebti, S.M.; Malafa, M.P. Vitamin E δ-Tocotrienol Augments the Antitumor Activity of Gemcitabine and Suppresses Constitutive NF-KB Activation in Pancreatic Cancer. Mol. Cancer Ther. 2011, 10, 2363–2372. [Google Scholar] [CrossRef]
- Husain, K.; Francois, R.A.; Hutchinson, S.Z.; Neuger, A.M.; Lush, R.; Coppola, D.; Sebti, S.; Malafa, M.P. Vitamin E Delta-Tocotrienol Levels in Tumor and Pancreatic Tissue of Mice after Oral Administration. Pharmacology 2009, 83, 157–163. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The Role of Oxidative Stress, Antioxidants and Vascular Inflammation in Cardiovascular Disease (a Review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Kirsh, V.A.; Hayes, R.B.; Mayne, S.T.; Chatterjee, N.; Subar, A.F.; Dixon, L.B.; Albanes, D.; Andriole, G.L.; Urban, D.A.; Peters, U.; et al. Supplemental and Dietary Vitamin E, Beta-Carotene, and Vitamin C Intakes and Prostate Cancer Risk. J. Natl. Cancer Inst. 2006, 98, 245–254. [Google Scholar] [CrossRef]
- Pyne, S.K.; Bhattacharjee, P.; Srivastav, P.P. Microalgae (Spirulina platensis) and Its Bioactive Molecules: Review. Indian J. Nutr. 2017, 4, 160. [Google Scholar]
- D’Aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cells Int. 2017, 2017, 8936156. [Google Scholar] [CrossRef]
- Carcea, M.; Sorto, M.; Batello, C.; Narducci, V.; Aguzzi, A.; Azzini, E.; Fantauzzi, P.; Finotti, E.; Gabrielli, P.; Galli, V.; et al. Nutritional Characterization of Traditional and Improved Dihé, Alimentary Blue-Green Algae from the Lake Chad Region in Africa. LWT Food Sci. Technol. 2015, 62, 753–763. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Masten Rutar, J.; Jagodic Hudobivnik, M.; Nečemer, M.; Vogel Mikuš, K.; Arčon, I.; Ogrinc, N. Nutritional Quality and Safety of the Spirulina Dietary Supplements Sold on the Slovenian Market. Foods 2022, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Lope, V.; Guerrero-Zotano, Á.; Casas, A.; Baena-Cañada, J.M.; Bermejo, B.; Pérez-Gómez, B.; Criado-Navarro, I.; Antolín, S.; Sánchez-Rovira, P.; Ramos-Vázquez, M.; et al. Serum Phospholipids Fatty Acids and Breast Cancer Risk by Pathological Subtype. Nutrients 2020, 12, 3132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, T.; Zhai, S.; Li, W.; Meng, Q. Linoleic Acid and Breast Cancer Risk: A Meta-Analysis. Public Health Nutr. 2016, 19, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Muhling, M.; Belay, A.; Whitton, B.A. Variation in fatty acid composition of Arthrospira (Spirulina) strains. J. Appl. Phycol. 2005, 17, 137–146. [Google Scholar] [CrossRef]
- Saxena, R.; Rodríguez-Jasso, R.M.; Chávez-Gonzalez, M.L.; Aguilar, C.N.; Quijano, G.; Ruiz, H.A. Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content. Fermentation 2022, 8, 374. [Google Scholar] [CrossRef]
- Taiti, C.; Di Vito, M.; Di Mercurio, M.; Costantini, L.; Merendino, N.; Sanguinetti, M.; Bugli, F.; Garzoli, S. Detection of Secondary Metabolites, Proximate Composition and Bioactivity of Organic Dried Spirulina (Arthrospira platensis). Appl. Sci. 2024, 14, 67. [Google Scholar] [CrossRef]
- Phélippéa, M.; Gonçalvesa, O.; Thouanda, G.; Cognea, G.; Larocheb, C. Characterization of the polysaccharides chemical diversity of the cyanobacteria Arthrospira platensis. Algal Res. 2019, 38, 101426. [Google Scholar] [CrossRef]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and Therapeutics of Omega-3 Polyunsaturated Fatty Acids in Chronic Inflammatory Disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, V.Z. Determination of Biologically Active Substances in Black Sea Algae. Ph.D. Thesis, MU Varna, Varna, Bulgaria, 2014. [Google Scholar]
- Campanella, L.; Crescentini, G.; Avino, P.; Moauro, A. Determination of macrominerals and trace elements in the alga Spirulina platensis. Analusis 1998, 26, 210–214. [Google Scholar] [CrossRef]
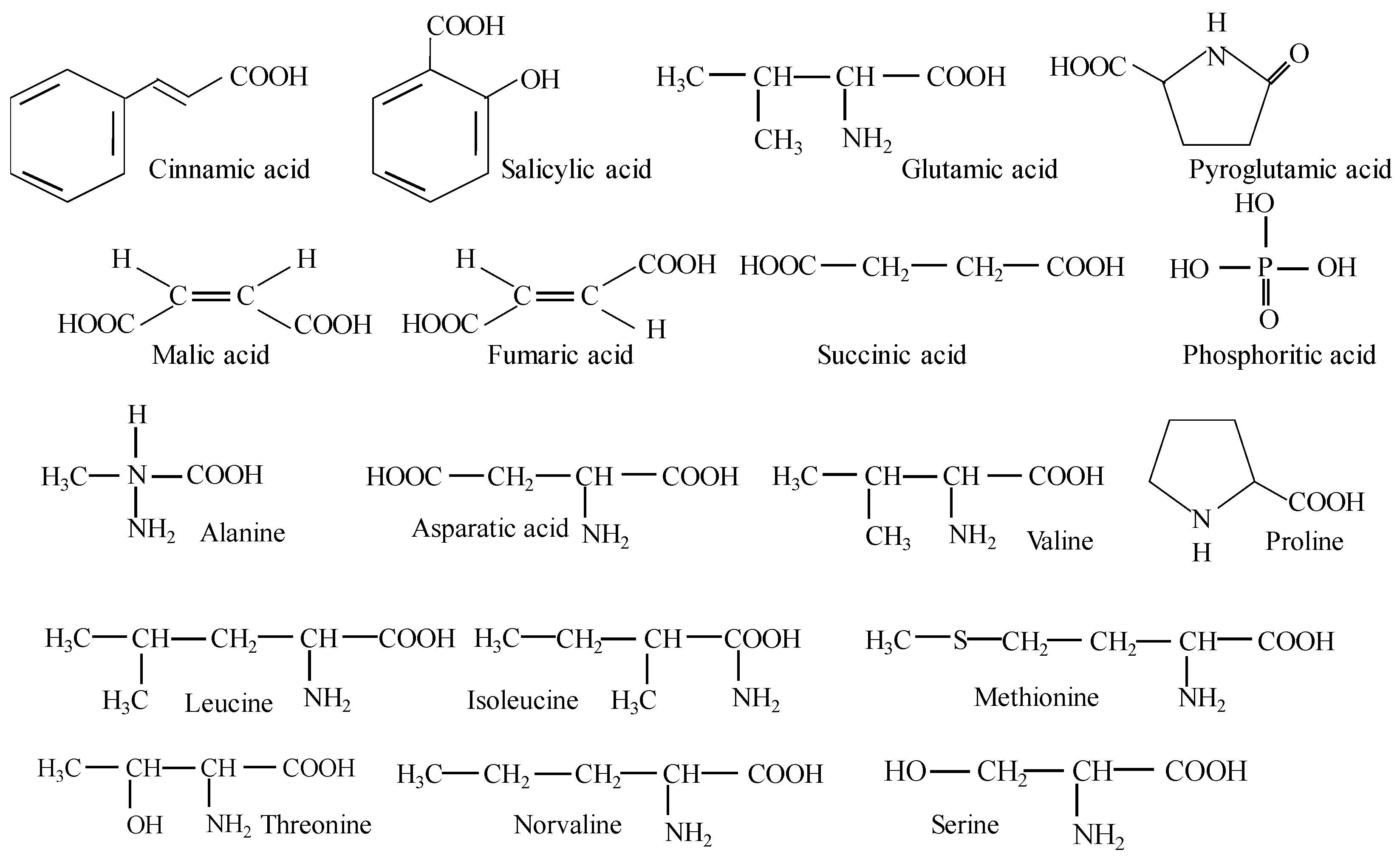
| Our Data | Value from the Literature | Refs. | |
|---|---|---|---|
| Content ± SD, mg g−1 Dry Mass | |||
| Phenolic acids | |||
| Cinnamic acid | 2.27 ± 0.34 | ||
| Salicylic acid | 0.94 ± 0.14 | ||
| Carboxylic acids | |||
| Pyroglutamic acid | 9.51 ± 1.41 | ||
| Fumaric acid | 0.69 ± 0.10 | ||
| Malic acid | 2.41 ± 0.36 | ||
| Succinic acid | 2.40 ± 0.35 | ||
| Phosphoritic acid | 0.60 ± 0.09 | ||
| Non-essential amino acids | |||
| Alanine | 0.98 ± 0.05 | ||
| Asparatic acid | 5.25 ± 0.78 | ||
| Essential amino acids | |||
| Valine | 0.82 ± 0.12 | 0.35 | [21,22] |
| Leucine | 0.78 ± 0.12 | 0.49 | [21,22] |
| Isoleucine | 0.57 ± 0.08 | 0.32 | [21,22] |
| Methionine | 0.64 ± 0.09 | 0.12 | [21,22] |
| Threonine | 0.62 ± 0.09 | 0.30 | [21,22] |
| Conditional non-essential amino acids | |||
| Norvaline | 0.50 ± 0.07 | ||
| Proline | 0.96 ± 0.14 | ||
| Serine | 0.68 ± 0.10 | ||
| Vitamin Content | Mean Value | Standard Deviation |
|---|---|---|
| Vitamin B2 (riboflavin), mg/100 g | 1.01 | 0.25 |
| Vitamin C (Ascorbic acid), mg/100 g | 9.43 | 0.80 |
| Vitamin E (α-tocopherol), mg/100 g | 3.57 | 0.55 |
| Vitamin A (retinol), μg/100 g | 10.30 | 2.80 |
| Carbohydrate Content | Mean Value, mg g−1 | Standard Deviation, mg g−1 |
|---|---|---|
| Fructose | 2.62 | 0.38 |
| Glucose | 3.61 | 0.54 |
| Mannose | 137.02 | 14.44 |
| Sucrose | 4.86 | 0.72 |
| Maltose | 50.61 | 7.78 |
| Maltitol | 88.87 | 14.42 |
| Fatty Acids | Mean Value mg g−1 | Standard Deviation, mg g−1 | Value % |
|---|---|---|---|
| -Linolenic acid (C18:3 n-3) | 15.59 | 2.44 | 25.28 |
| Palmitic acid (C16:0) | 13.91 | 3.07 | 22.55 |
| Oleic acid (C18:1) | 1.12 | 0.24 | 1.82 |
| Linoleic acid (C18:2 n-6) | 4.22 | 0.91 | 6.84 |
| Cerotic acid | 4.14 | 0.89 | 6.71 |
| Lingoseric acid (C24:0) | 2.83 | 0.61 | 4.59 |
| Behenic acid (C22:0) | 2.16 | 0.47 | 3.50 |
| Arachidic acid (C20:4 n-6) | 12.87 | 1.88 | 20.87 |
| Stearic acid (C18:0) | 3.11 | 0.67 | 5.04 |
| Myristic acid (C14:0) | 1.52 | 0.33 | 2.46 |
| Margaric acid (C17:0) | 0.22 | 0.05 | 0.37 |
| 27.88 | - | 45.21 | |
| 33.80 | - | 54.79 | |
| 15.59 | - | 25.28 | |
| 17.09 | - | 27.71 | |
| n-6/n-3 | 1.10 | - | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, K.; Gentscheva, G.; Gyurova, D.; Pavlova, V.; Dincheva, I.; Velikova, M.; Gerasimova, A.; Makedonski, L.; Gergov, G. Metabolomic Profile of Arthrospira platensis from a Bulgarian Bioreactor—A Potential Opportunity for Inclusion in Dietary Supplements. Life 2024, 14, 174. https://doi.org/10.3390/life14020174
Nikolova K, Gentscheva G, Gyurova D, Pavlova V, Dincheva I, Velikova M, Gerasimova A, Makedonski L, Gergov G. Metabolomic Profile of Arthrospira platensis from a Bulgarian Bioreactor—A Potential Opportunity for Inclusion in Dietary Supplements. Life. 2024; 14(2):174. https://doi.org/10.3390/life14020174
Chicago/Turabian StyleNikolova, Krastena, Galia Gentscheva, Desislava Gyurova, Vera Pavlova, Ivayla Dincheva, Margarita Velikova, Anelia Gerasimova, Lubomir Makedonski, and Georgi Gergov. 2024. "Metabolomic Profile of Arthrospira platensis from a Bulgarian Bioreactor—A Potential Opportunity for Inclusion in Dietary Supplements" Life 14, no. 2: 174. https://doi.org/10.3390/life14020174
APA StyleNikolova, K., Gentscheva, G., Gyurova, D., Pavlova, V., Dincheva, I., Velikova, M., Gerasimova, A., Makedonski, L., & Gergov, G. (2024). Metabolomic Profile of Arthrospira platensis from a Bulgarian Bioreactor—A Potential Opportunity for Inclusion in Dietary Supplements. Life, 14(2), 174. https://doi.org/10.3390/life14020174