Microbial Inoculation during the Short-Term Composting Process Enhances the Nutritional and Functional Properties of Oyster Mushrooms (Pleurotus ostreatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microbial Inoculation
2.3. Composting Process and Mushroom Cultivation
2.4. Physicochemical Properties of Composted Substrates
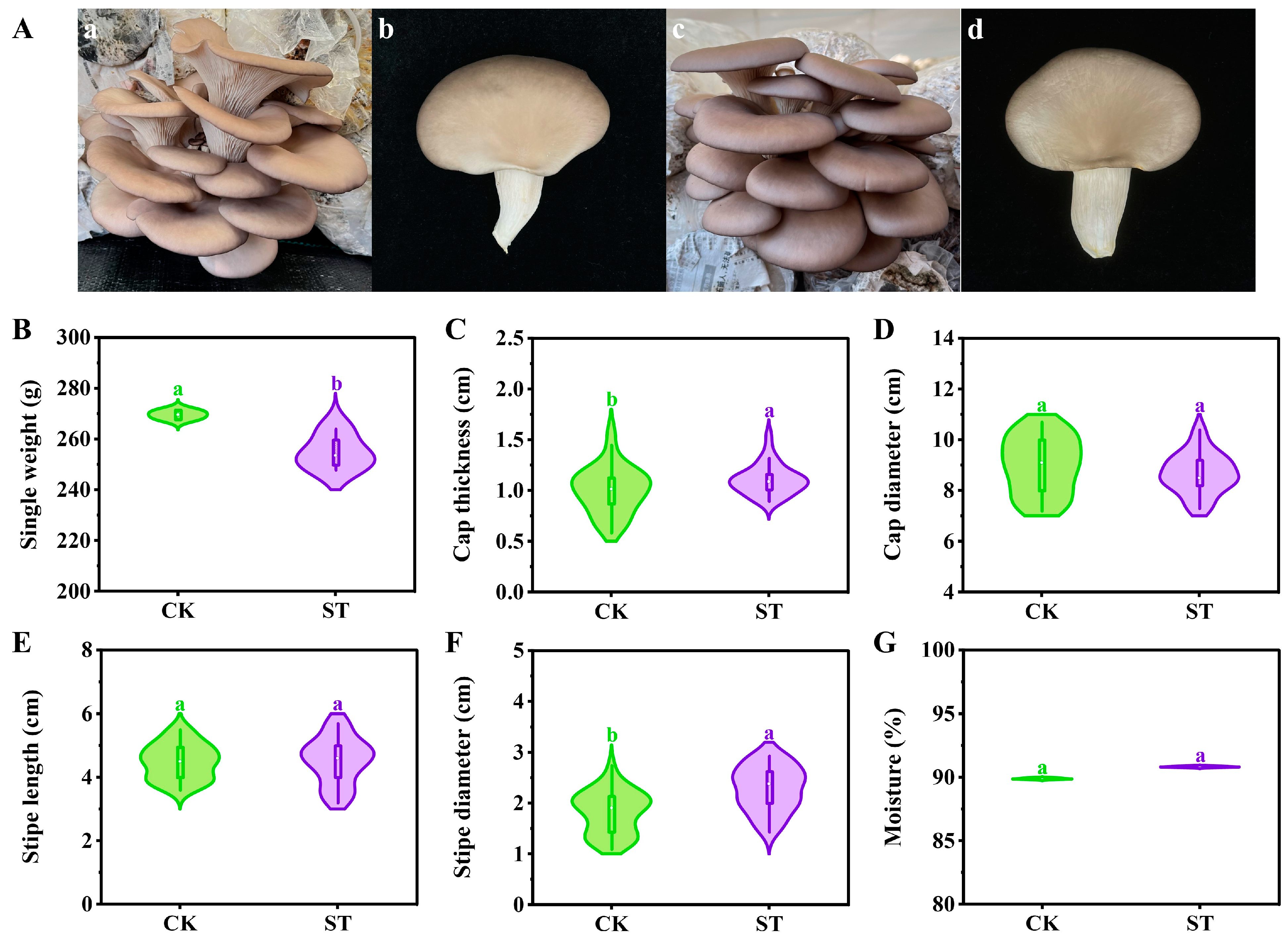
2.5. Agronomic Traits and Contamination Rate
2.6. Main Nutritional Qualities
2.7. Assay of Amino Acid Composition
2.8. Assay of 5’-Nucleotides
2.9. Equivalent Umami Concentration (EUC) Value
2.10. Assay of Antioxidant Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Composted Substrates
3.2. Agronomic Traits and Contamination Rate
3.3. Main Nutritional Qualities
3.4. Amino Acid Composition
3.5. 5’-Nucleotides and Equivalent Umami Concentration (EUC) Value
3.6. Evaluation of Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.R.; Guo, Y.X.; Wang, Q.Y.; Hu, B.Y.; Tian, S.Y.; Yang, Q.Z.; Cheng, Z.A.; Chen, Q.J.; Zhang, G.Q. Impacts of composting duration on physicochemical properties and microbial communities during short-term composting for the substrate for oyster mushrooms. Sci. Total Environ. 2022, 847, 157673. [Google Scholar] [CrossRef]
- Guo, Y.X.; Yang, Y.R.; Qin, Y.; Guan, T.K.; Yang, Q.Z.; Wang, Y.X.; Tang, S.; Zhang, G.Q.; Chen, Q.J. Nutritional qualities and antioxidant activity of Pleurotus floridanus grown on composted peach sawdust substrate with different composting time. Biotechnol. Appl. Biochem. 2023, 70, 210–220. [Google Scholar] [CrossRef]
- Correa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- Rodrigues Barbosa, J.; Dos Santos Freitas, M.M.; da Silva Martins, L.H.; de Carvalho, R.N.J. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohydr. Polym. 2020, 229, 115550. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, G.S.; Ngozi, E.A.; Luo, N.N.; Yu, H.; Wang, M. The yields and quality of golden oyster mushroom cultivated on common reed substrates. J. Food Compos. Anal. 2023, 121, 105331. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, Q.J.; Qin, Y.; Yang, Y.R.; Yang, Q.Z.; Wang, Y.X.; Cheng, Z.A.; Cao, N.; Zhang, G.Q. Succession of the microbial communities and function prediction during short-term peach sawdust-based composting. Bioresour. Technol. 2021, 332, 125079. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ma, N.L.; Mohammad, A.; Sonne, C.; et al. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Sun, B.; Zhang, J.; Zhang, Y.; Gu, L.; Bao, L.; Liu, S. Metagenomic analysis revealed the succession of microbiota and metabolic function in corncob composting for preparation of cultivation medium for Pleurotus ostreatus. Bioresour. Technol. 2020, 306, 123156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kong, W.; Cui, X.; Hu, S.; Shi, Z.; Wu, J.; Zhang, Y.; Qiu, L. Dynamic succession of microbial compost communities and functions during Pleurotus ostreatus mushroom cropping on a short composting substrate. Front. Microbiol. 2022, 13, 946777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Meng, L.; Wang, X.; Zhao, W.; Shi, X.; Wang, W.; Li, Z.; Wang, L. The yield, nutritional value, umami components and mineral contents of the first-flush and second-flush Pleurotus pulmonarius mushrooms grown on three forestry wastes. Food Chem. 2022, 397, 133714. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Tagkouli, D.; Bekiaris, G.; Kaliora, A.; Tsiaka, T.; Tsiantas, K.; Chatzipavlidis, I.; Zoumpoulakis, P.; Kalogeropoulos, N.; Zervakis, G.I. Enhancing the nutritional and functional properties of Pleurotus citrinopileatus mushrooms through the exploitation of winery and olive mill wastes. Food Chem. 2022, 370, 131022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, X.; Luo, G.; Wang, K.; Li, D. Deciphering the dominant components and functions of bacterial communities for lignocellulose degradation at the composting thermophilic phase. Bioresour. Technol. 2022, 348, 126808. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, T.; Wang, J.; Zhao, Y.; Xie, X.; Wei, Z.; Zhang, X.; Song, C.; Song, X. Role of Bacillus inoculation in rice straw composting and bacterial community stability after inoculation: Unite resistance or individual collapse. Bioresour. Technol. 2021, 337, 125464. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Wei, Y.; Chen, W.; Ding, G.; Zhan, Y.; Liu, Y.; Xu, T.; Xiao, J.; Li, J. Impact of inoculation and turning for full-scale composting on core bacterial community and their co-occurrence compared by network analysis. Bioresour. Technol. 2022, 345, 126417. [Google Scholar] [CrossRef]
- Meng, L.; Fu, Y.; Li, D.; Sun, X.; Chen, Y.; Li, X.; Xu, S.; Li, X.; Li, C.; Song, B.; et al. Effects of corn stalk cultivation substrate on the growth of the slippery mushroom (Pholiota microspora). RSC Adv. 2019, 9, 5347–5353. [Google Scholar] [CrossRef]
- Liu, Q.; Bau, T.; Jin, R.; Cui, X.; Zhang, Y.; Kong, W. Comparison of different drying techniques for shiitake mushroom (Lentinus edodes): Changes in volatile compounds, taste properties, and texture qualities. LWT—Food Sci. Technol. 2022, 164, 113651. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Q.; Pei, F.; Mariga, A.M.; Yang, W. Influence of different storage conditions on physical and sensory properties of freeze-dried Agaricus bisporus slices. LWT—Food Sci. Technol. 2018, 97, 164–171. [Google Scholar]
- Zhang, S.K.; Cheng, X.X.; Fu, Q.B.; Li, Y.J.; Wu, P.; Qiao, Y.H.; Yan, J.F.; Si, L.; Waterhouse, G.I.N.; Li, H.S.; et al. Pectin-nanolignin composite films with water resistance, UV resistance, and antibacterial activity. Food Hydrocolloid. 2023, 143, 108783. [Google Scholar] [CrossRef]
- Zhang, S.; He, Z.; Cheng, Y.; Xu, F.; Cheng, X.; Wu, P. Physicochemical characterization and emulsifying properties evaluation of RG-I enriched pectic polysaccharides from Cerasus humilis. Carbohydr. Polym. 2021, 260, 117824. [Google Scholar] [CrossRef] [PubMed]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Avila, S.; Hornung, P.S.; Junior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Z.; Xu, C.; Pan, J.; Zhang, H.; Hu, Q.; Zou, Y. Evaluation of corn stalk as a substrate to cultivate king oyster mushroom (Pleurotus eryngii). Horticulturae 2023, 9, 319. [Google Scholar] [CrossRef]
- Zhu, N.; Zhu, Y.; Li, B.; Jin, H.; Dong, Y. Increased enzyme activities and fungal degraders by Gloeophyllum trabeum inoculation improve lignocellulose degradation efficiency during manure-straw composting. Bioresour. Technol. 2021, 337, 125427. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Du, F.; Hu, Q.; Yuan, X.; Dai, D.; Zhu, M. Integration of Pleurotus tuoliensis cultivation and biogas production for utilization of lignocellulosic biomass as well as its benefit evaluation. Bioresour. Technol. 2020, 317, 124042. [Google Scholar] [CrossRef]
- Sardar, H.; Anjum, M.A.; Hussain, S.; Ali, S.; Shaheen, M.R.; Ahsan, M.; Ejaz, S.; Ahmad, K.S.; Naz, S.; Shafique, M. Deciphering the role of moringa leaf powder as a supplement in the cotton waste substrate for the growth and nutrition of king oyster mushroom. Sci. Hortic. 2022, 293, 110694. [Google Scholar] [CrossRef]
- Lucas de Jesus, G.; Jose Lavoranti, O.; Schafer, G.; Dias de Oliveira, G.; Scheffer de Andrade Silva, R.; Lorena Cuquel, F. Nutrient uptake in supplemented substrate by oyster mushroom. World J. Microbiol. Biotechnol. 2023, 39, 175. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, M.; Peng, C.; Yan, F.; Jia, Y.; Li, X.; Li, M.; Wu, B.; Xu, H.; Qiu, Z. Bacterial dynamics and functions driven by a novel microbial agent to promote kitchen waste composting and reduce environmental burden. J. Clean. Prod. 2022, 3337, 130491. [Google Scholar] [CrossRef]
- Chi, C.P.; Chu, S.; Wang, B.; Zhang, D.; Zhi, Y.; Yang, X.; Zhou, P. Dynamic bacterial assembly driven by Streptomyces griseorubens JSD-1 inoculants correspond to composting performance in swine manure and rice straw co-composting. Bioresour. Technol. 2020, 313, 123692. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Huang, C.; Zhang, Z.; Gao, W. Isolation and identification of pigments from oyster mushrooms with black, yellow and pink caps. Food Chem. 2022, 372, 131171. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hou, Q.; Niu, T. Effect of cultivating Pleurotus ostreatus on substrates supplemented with herb residues on yield characteristics, substrates degradation, and fruiting bodies’ properties. J. Sci. Food Agric. 2020, 100, 4901–4910. [Google Scholar] [CrossRef]
- Mandeel, Q.; Al-Laith, A.; Mohamed, S. Cultivation of oyster mushrooms (Pleurotus spp.) on various lignocellulosic wastes. World J. Microb. Biot. 2005, 21, 601–607. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically active polysaccharide from edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Boadu, K.B.; Nsiah-Asante, R.; Antwi, R.T.; Obirikorang, K.A.; Anokye, R.; Ansong, M. Influence of the chemical content of sawdust on the levels of important macronutrients and ash composition in Pearl oyster mushroom (Pleurotus ostreatus). PLoS ONE 2023, 18, e0287532. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary nutrients in edible mushroom, Agaricus bisporus and their radical scavenging, antibacterial, and antifungal effects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Gao, H.Y.; Wu, W.J.; Chen, H.J.; Fang, X.J.; Han, Y.C.; Mu, H.L. Effects of fermentation with different strains on the umami taste of Shiitake mushroom (Lentinus edodes). LWT—Food Sci. Technol. 2021, 141, 110889. [Google Scholar] [CrossRef]
- Yang, J.H.; Lin, H.C.; Mau, J.L. Non-volatile taste components of several commercial mushrooms. Food Chem. 2001, 72, 465–471. [Google Scholar] [CrossRef]
- Manninen, H.; Rotola-Pukkila, M.; Aisala, H.; Hopia, A.; Laaksonen, T. Free amino acids and 5′-nucleotides in Finnish forest mushrooms. Food Chem. 2018, 247, 23–28. [Google Scholar] [CrossRef]
- Zhao, X.M.; Wang, Y.D.; Zhang, Z.Y.; Sun, L.B.; Wei, Y.Y.; Bao, X.J.; Xin, G. Postharvest short-time partial dehydration affects shiitake mushroom (Lentinus edodes) storage quality and umami taste. Sci. Hortic. 2021, 287, 110274. [Google Scholar] [CrossRef]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- Maity, G.N.; Maity, P.; Khatua, S.; Acharya, K.; Dalai, S.; Mondal, S. Structural features and antioxidant activity of a new galactoglucan from edible mushroom Pleurotus djamor. Int. J. Biol. Macromol. 2021, 168, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Fan, J.; Zhu, M.; Tong, H. Antioxidant effects of a water-soluble proteoglycan isolated from the fruiting bodies of Pleurotus ostreatus. J. Taiwan Inst. Chem. Eng. 2011, 42, 402–407. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Investigation of chemical and biological properties of an acidic polysaccharide fraction from Pleurotus eous (Berk.) Sacc. Food Biosci. 2021, 42, 101209. [Google Scholar] [CrossRef]

| Treatment | pH | EC (mS/cm) | TC (%) | TN (%) | C/N Ratio | MC (%) |
|---|---|---|---|---|---|---|
| CK | 6.22 ± 0.05 a | 1.32 ± 0.04 a | 47.99 ± 0.07 a | 1.75 ± 0.00 a | 27.49 ± 0.06 a | 64.24 ± 0.54 a |
| ST | 6.23 ± 0.03 a | 1.35 ± 0.01 a | 47.38 ± 0.07 b | 1.74 ± 0.00 a | 27.16 ± 0.04 b | 61.96 ± 0.78 b |
| Treatment | Flush | Yield (kg/100 Bags) | BE (%) | Total Yield (kg/100 Bags) | Total BE (%) | CR (%) |
|---|---|---|---|---|---|---|
| CK | FF | 38.80 ± 1.36 Ab | 38.84 ± 1.36 Ab | 106.49 ± 1.35 b | 106.60 ± 1.35 b | 3.51 ± 0.27 a |
| SF | 34.91 ± 0.50 Bb | 34.95 ± 0.50 Ba | ||||
| TF | 32.78 ± 0.74 Ca | 32.81 ± 0.74 Ca | ||||
| ST | FF | 42.71 ± 0.96 Aa | 41.68 ± 0.94 Aa | 113.71 ± 0.75 a | 110.97 ± 0.56 a | 2.94 ± 0.10 b |
| SF | 37.09 ± 1.09 Ba | 36.19 ± 1.06 Ba | ||||
| TF | 33.92 ± 0.45 Ca | 33.10 ± 0.44 Ca |
| Treatment | Flush | Crude Protein (%) | Crude Polysaccharide (%) | Crude Fiber (%) | Crude Fat (%) |
|---|---|---|---|---|---|
| CK | FF | 24.07 ± 0.21 Ab | 9.65 ± 0.60 Ab | 18.11 ± 0.29 Aa | 1.39 ± 0.07 Aa |
| SF | 22.84 ± 0.21 Bb | 5.86 ± 0.55 Ba | 14.85 ± 1.04 Ba | 1.32 ± 0.08 Aa | |
| TF | 22.70 ± 0.75 Ba | 5.47 ± 0.59 Ba | 11.26 ± 0.50 Ca | 1.29 ± 0.06 Aa | |
| ST | FF | 25.22 ± 0.23 Aa | 10.70 ± 0.43 Aa | 17.90 ± 1.88 Aa | 1.36 ± 0.08 Aa |
| SF | 25.16 ± 0.23 Aa | 5.70 ± 0.34 Ba | 13.31 ± 1.00 Ba | 1.32 ± 0.06 Aa | |
| TF | 22.87 ± 0.22 Ba | 5.37 ± 0.47 Ba | 11.81 ± 0.50 Ba | 1.24 ± 0.09 Aa |
| Amino Acid | Content (mg/g Dry Weight) | |||||
|---|---|---|---|---|---|---|
| CK | ST | |||||
| FF | SF | TF | FF | SF | TF | |
| a,f Glu | 29.91 ± 3.56 Ab | 27.07 ± 0.49 ABb | 25.24 ± 0.82 Ba | 43.67 ± 0.72 Aa | 29.97 ± 0.10 Ba | 25.02 ± 0.79 Ca |
| c,e Val | 19.51± 5.88 Ab | 19.46 ± 2.40 Aa | 15.27 ± 0.50 Ab | 29.91 ± 0.37 Aa | 18.55 ± 0.06 Ba | 17.92 ± 0.54 Ca |
| a,f Asp | 17.04 ± 1.98 Ab | 17.78 ± 0.32 Ab | 13.94 ± 0.46 Bb | 29.68 ± 0.42 Aa | 20.45 ± 0.08 Ba | 17.46 ± 0.56 Ca |
| b,f Ser | 10.87 ± 1.26 Ab | 10.74 ± 0.22 Ab | 9.89 ± 0.33 Aa | 15.17 ± 0.06 Aa | 12.35 ± 0.07 Ba | 7.87 ± 0.25 Cb |
| c,e Leu | 10.64 ± 1.35 Ab | 10.41 ± 0.12 Ab | 10.20 ± 0.35 Aa | 18.51 ± 0.29 Aa | 12.43 ± 0.05 Ba | 10.51 ± 0.33 Ca |
| c,f Arg | 10.04 ± 1.22 Ab | 9.98 ± 0.21 Ab | 8.48 ± 0.30 Bb | 16.58 ± 0.28 Aa | 11.34 ± 0.03 Ba | 9.77 ± 0.31 Ca |
| b,f Ala | 9.60 ± 1.17 Ab | 9.30 ± 0.17 Ab | 9.01 ± 0.30 Aa | 15.79 ± 0.17 Aa | 10.73 ± 0.05 Ba | 9.45 ± 0.29 Ca |
| d,e Lys | 9.09 ± 1.12 Ab | 8.95 ± 0.17 Ab | 8.20 ± 0.28 Ab | 15.56 ± 0.23 Aa | 10.46 ± 0.03 Ba | 9.21 ± 0.29 Ca |
| b,f Gly | 7.44 ± 0.90 Ab | 7.25 ± 0.13 Ab | 7.27 ± 0.24 Aa | 12.28 ± 0.10 Aa | 8.22 ± 0.04 Ba | 7.18 ± 0.21 Ca |
| b,e Thr | 7.19 ± 0.82 Ab | 6.99 ± 0.13 Ab | 6.42 ± 0.20 Aa | 10.83 ± 0.15 Aa | 7.98 ± 0.03 Ba | 6.21 ± 0.19 Ca |
| c,e Phe | 6.88 ± 0.84 Ab | 6.66 ± 0.13 Ab | 6.25 ± 0.20 Ab | 11.47 ± 0.15 Aa | 7.87 ± 0.02 Ba | 6.79 ± 0.21 Ca |
| b,f Pro | 6.41 ± 0.87 Ab | 6.48 ± 0.12 Ab | 6.09 ± 0.21 Ab | 11.60 ± 0.24 Aa | 7.62 ± 0.03 Ba | 6.41 ± 0.23 Ca |
| c,e Ile | 6.16 ± 0.76 Ab | 5.97 ± 0.08 Ab | 5.82 ± 0.20 Ab | 11.37 ± 0.18 Aa | 6.97 ± 0.03 Ba | 6.55 ± 0.20 Ca |
| d,f Tyr | 4.51 ± 0.55 Ab | 4.09 ± 0.11 Ab | 2.83 ± 0.12 Bb | 5.57 ± 0.08 Aa | 5.01 ± 0.02 Ba | 3.27 ± 0.11 Ca |
| c,e Met | 4.27 ± 0.80 Aa | 3.61 ± 0.86 Ab | 4.18 ± 0.17 Aa | 3.41 ± 0.08 Bb | 7.19 ± 0.07 Aa | 1.89 ± 0.06 Cb |
| c,e His | 3.77 ± 0.46 Ab | 3.43 ± 0.08 Ab | 2.82 ± 0.09 Bb | 6.44 ± 0.06 Aa | 4.27 ± 0.04 Ba | 3.85 ± 0.14 Ca |
| f Cys | 0.56 ± 0.05 Aa | 0.57 ± 0.01 ABb | 0.45 ± 0.01 Ba | 0.58 ± 0.01 Ba | 0.66 ± 0.01 Aa | 0.39 ± 0.01 Ca |
| Umami | 46.95 ± 5.54 Ab | 44.86 ± 0.81 Ab | 39.18 ± 1.28 Bb | 73.36 ± 1.15 Aa | 50.42 ± 0.17 Ba | 42.48 ± 1.35 Ca |
| Sweetness | 41.51 ± 5.01 Ab | 40.77 ± 0.76 Ab | 38.68 ± 1.27 Aa | 65.66 ± 0.64 Aa | 46.90 ± 0.22 Ba | 37.11 ± 1.17 Ca |
| Bitterness | 61.26 ± 9.79 Ab | 59.52 ± 1.89 Ab | 53.01 ± 1.80 Ab | 97.69 ± 1.41 Aa | 68.62 ± 0.22 Ba | 57.30 ± 1.78 Ca |
| Tasteless | 13.61 ± 1.68 Ab | 13.05 ± 0.28 Ab | 11.04 ± 0.47 Bb | 21.14 ± 0.30 Aa | 15.47 ± 0.06 Ba | 12.48 ± 0.40 Ca |
| EAA | 67.50 ± 10.51 Ab | 65.48 ± 1.96 Ab | 59.15 ± 1.97 Ab | 107.50 ± 1.50 Aa | 75.72 ± 0.25 Ba | 62.95 ± 1.95 Ca |
| NEAA | 96.38 ± 11.56 Ab | 93.28 ± 1.77 ABb | 83.20 ± 2.77 Ba | 150.92 ± 1.99 Aa | 106.35 ± 0.41 Ba | 86.81 ± 2.76 Ca |
| TAA | 163.88 ± 22.05 Ab | 158.76 ± 3.39 Ab | 142.35 ± 4.74 Aa | 258.42 ± 3.50 Aa | 182.08 ± 0.66 Ba | 149.75 ± 4.71 Ca |
| 5′-Nucleotides | Content (mg/g Dry Weight) | |||||
|---|---|---|---|---|---|---|
| CK | ST | |||||
| FF | SF | TF | FF | SF | TF | |
| 5′-AMP | 3.028 ± 0.085 Ab | 3.159 ± 0.097 Ab | 2.856 ± 0.012 Ba | 3.710 ± 0.155 Aa | 3.850 ± 0.071 Aa | 2.986 ± 0.064 Ba |
| 5′-GMP | 0.014 ± 0.001 Ba | 0.018 ± 0.001 Ab | 0.003 ± 0.000 Ca | 0.015 ± 0.001 Ba | 0.027 ± 0.001 Aa | 0.003 ± 0.000 Ca |
| 5′-IMP | 0.003 ± 0.000 Aa | 0.002 ± 0.000 Cb | 0.002 ± 0.000 Ba | 0.003 ± 0.000 Aa | 0.002 ± 0.000 Ba | 0.002 ± 0.000 Ba |
| 5′-UMP | 0.526 ± 0.18 Aa | 0.308 ± 0.003 Ba | 0.296 ± 0.015 Ba | 0.255 ± 0.001 Ab | 0.209 ± 0.009 Bb | 0.144 ± 0.016 Cb |
| 5′-CMP | nd | nd | nd | nd | nd | nd |
| 5′-XMP | nd | nd | nd | nd | nd | nd |
| Total | 3.570 ± 0.079 Ab | 3.486 ± 0.098 Ab | 3.157 ± 0.026 Bb | 3.983 ± 0.156 Aa | 4.087 ± 0.081 Aa | 3.135 ± 0.053 Bb |
| EUC (g MSG/100 g) | 219.79 ± 17.91 Ab | 211.90 ± 7.61 Ab | 167.42 ± 4.78 Ba | 394.68 ± 11.71 Aa | 290.85 ± 4.67 Ba | 175.40 ± 6.48 Ca |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhao, M.; Wang, Y.; Xie, Z.; Zhao, S.; You, S.; Chen, Q.; Zhang, W.; Qin, Y.; Zhang, G. Microbial Inoculation during the Short-Term Composting Process Enhances the Nutritional and Functional Properties of Oyster Mushrooms (Pleurotus ostreatus). Life 2024, 14, 201. https://doi.org/10.3390/life14020201
Wang Q, Zhao M, Wang Y, Xie Z, Zhao S, You S, Chen Q, Zhang W, Qin Y, Zhang G. Microbial Inoculation during the Short-Term Composting Process Enhances the Nutritional and Functional Properties of Oyster Mushrooms (Pleurotus ostreatus). Life. 2024; 14(2):201. https://doi.org/10.3390/life14020201
Chicago/Turabian StyleWang, Qiuying, Minrui Zhao, Yiyang Wang, Zhenfei Xie, Shunyin Zhao, Shuning You, Qingjun Chen, Weiwei Zhang, Yong Qin, and Guoqing Zhang. 2024. "Microbial Inoculation during the Short-Term Composting Process Enhances the Nutritional and Functional Properties of Oyster Mushrooms (Pleurotus ostreatus)" Life 14, no. 2: 201. https://doi.org/10.3390/life14020201
APA StyleWang, Q., Zhao, M., Wang, Y., Xie, Z., Zhao, S., You, S., Chen, Q., Zhang, W., Qin, Y., & Zhang, G. (2024). Microbial Inoculation during the Short-Term Composting Process Enhances the Nutritional and Functional Properties of Oyster Mushrooms (Pleurotus ostreatus). Life, 14(2), 201. https://doi.org/10.3390/life14020201






