Crafting Contours: A Comprehensive Guide to Scrotal Reconstruction
Abstract
1. Introduction
| Category | Specific Causes | Description | References |
|---|---|---|---|
| Infectious diseases | Fournier’s gangrene | Rapidly progressing necrotizing fasciitis of the genital and perineal tissues, often due to bacterial infection in immunocompromised individuals, requiring early diagnosis and aggressive treatment | [3,4,5,6] |
| Tumors | Scrotal cancer, extramammary Paget’s disease | Need to excise lesion and surrounding skin for treatment; higher incidence noted in the European population | [7,8,9,10] |
| Traumatic conditions | Traffic accidents, industrial injuries | Common in developing countries, including degloving injuries from vehicles and machinery | [2,11,12,13,14] |
| Inflammatory disease | Hidradenitis suppurativa | Chronic disease characterized by abscesses, fistulas, and scars, mainly in the axillae, groin, inframammary folds, and perianal regions; requires surgical intervention in severe cases | [15,16,17,23] |
2. Scrotal Anatomy and Functions
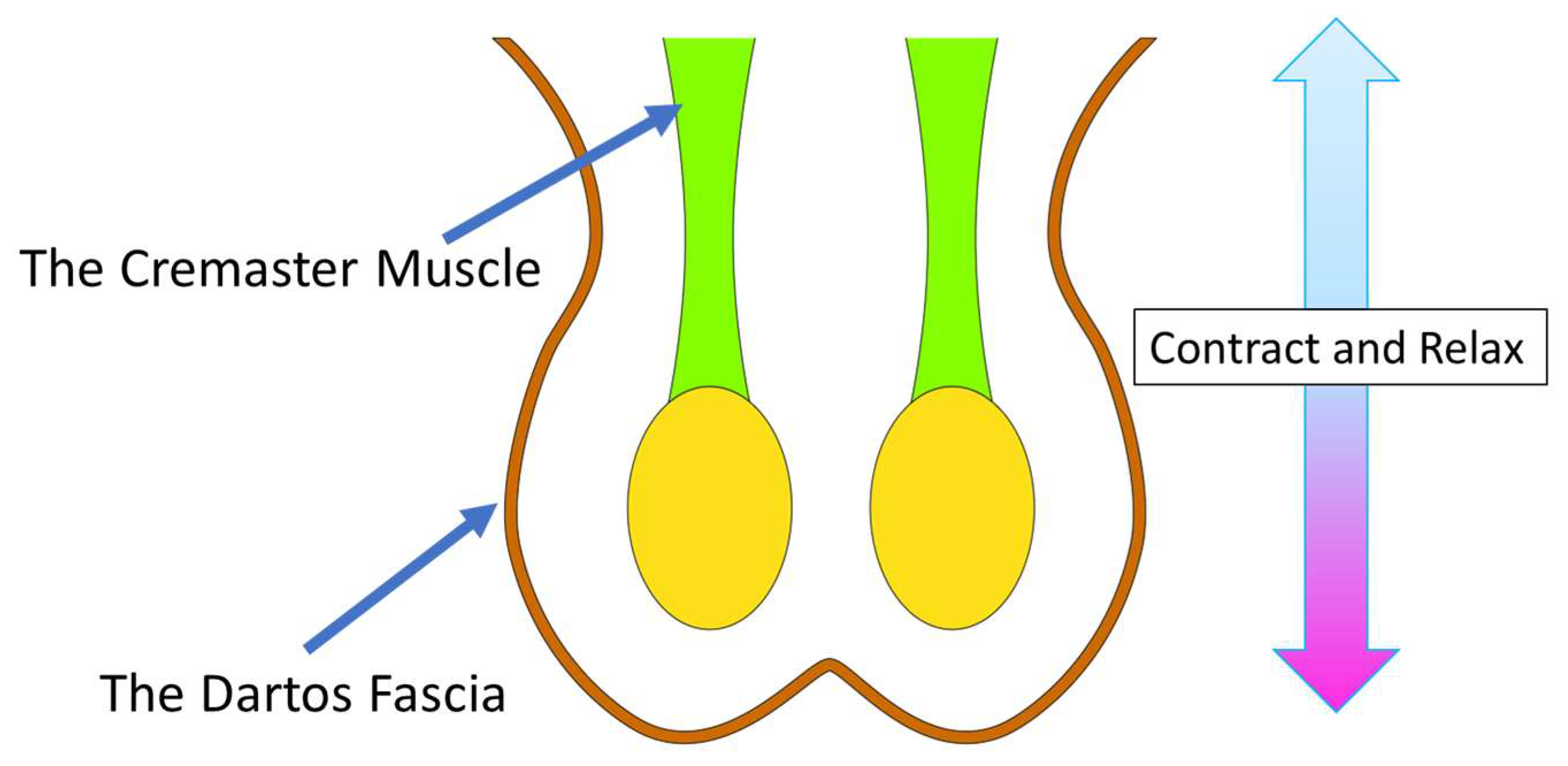
2.1. Scrotal Anatomy
2.2. Scrotal Functions
3. Approaches to Scrotal Reconstruction
3.1. Testicular Transposition
3.2. Tissue Expander
3.3. Split-Thickness Skin Graft
3.4. Local Flaps
3.5. Free Flaps
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chen, S.Y.; Fu, J.P.; Wang, C.H.; Lee, T.P.; Chen, S.G. Fournier gangrene: A review of 41 patients and strategies for reconstruction. Ann. Plast. Surg. 2010, 64, 765–769. [Google Scholar] [CrossRef]
- Maurya, R.; Mir, M.A.; Mahajan, S. Various Options for Scrotal Reconstruction: A Prospective Observational Study. Cureus 2022, 14, e22671. [Google Scholar] [CrossRef]
- Insua-Pereira, I.; Ferreira, P.C.; Teixeira, S.; Barreiro, D.; Silva, Á. Fournier’s gangrene: A review of reconstructive options. Cent. Eur. J. Urol. 2020, 73, 74–79. [Google Scholar] [CrossRef]
- Wroblewska, M.; Kuzaka, B.; Borkowski, T.; Kuzaka, P.; Kawecki, D.; Radziszewski, P. Fournier’s gangrene—Current concepts. Pol. J. Microbiol. 2014, 63, 267–273. [Google Scholar] [CrossRef]
- Shyam, D.C.; Rapsang, A.G. Fournier’s gangrene. Surgeon 2013, 11, 222–232. [Google Scholar] [CrossRef]
- Sorensen, M.D.; Krieger, J.N. Fournier’s Gangrene: Epidemiology and Outcomes in the General US Population. Urol. Int. 2016, 97, 249–259. [Google Scholar] [CrossRef] [PubMed]
- van der Zwan, J.M.; Siesling, S.; Blokx, W.A.; Pierie, J.P.; Capocaccia, R. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur. J. Surg. Oncol. 2012, 38, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Siesling, S.; Elferink, M.A.; van Dijck, J.A.; Pierie, J.P.; Blokx, W.A. Epidemiology and treatment of extramammary Paget disease in the Netherlands. Eur. J. Surg. Oncol. 2007, 33, 951–955. [Google Scholar] [CrossRef]
- Yin, S.; Xu, L.; Wang, S.; Feng, J.; Liu, L.; Liu, G.; Wang, J.; Zhan, S.; Zhao, Z.; Gao, P. Prevalence of extramammary Paget’s disease in urban China: A population-based study. Orphanet J. Rare Dis. 2021, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, S.; Zhang, H.; Shen, Y.; Zhu, Y.; Ye, D. Reconstruction with scrotal skin flaps after wide local resection of penoscrotal extramammary Paget’s disease. BJU Int. 2012, 110 Pt C, E1121–E1124. [Google Scholar] [CrossRef]
- Iafrate, M.; Leone, N.; Mancini, M.; Prayer, T.; Bassetto, F.; Moro, F.D. Domestic trauma with penile and scrotum skin degloving and testicular avulsion. J. Surg. Case Rep. 2021, 2021, rjab175. [Google Scholar] [CrossRef] [PubMed]
- Terrier, J.E.; Paparel, P.; Gadegbeku, B.; Ruffion, A.; Jenkins, L.C.; N’Diaye, A. Genitourinary injuries after traffic accidents: Analysis of a registry of 162,690 victims. J. Trauma Acute Care Surg. 2017, 82, 1087–1093. [Google Scholar] [CrossRef]
- Suresh Kumar Shetty, B.; Jagadish Rao, P.P.; Menezes, R.G. Traumatic degloving lesion of male external genitalia. J. Forensic Leg. Med. 2008, 15, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Zanettini, L.A.; Fachinelli, A.; Fonseca, G.P. Traumatic degloving lesion of penile and scrotal skin. Int. Braz. J. Urol. 2005, 31, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Jemec, G.B.E. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA 2017, 318, 2019–2032. [Google Scholar] [CrossRef]
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol. 2020, 82, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Odom, B.; Santucci, R.A. Surgical management of genitoperineal hidradenitis suppurativa in men. Urology 2014, 83, 1412–1417. [Google Scholar] [CrossRef]
- Younes, M.T.; Abdelmofeed, A.M.; Seif, O.; Abdelhalim, M.H. Versatility of unilateral propeller groin flap for coverage of large scrotal defects and its impact on testicular function. JPRAS Open 2022, 34, 158–167. [Google Scholar] [CrossRef]
- Gencosmanoğlu, R.; Bilkay, U.; Alper, M.; Gürler, T.; Cağdaş, A. Late results of split-grafted penoscrotal avulsion injuries. J. Trauma 1995, 39, 1201–1203. [Google Scholar] [CrossRef]
- Thomas, C.; Navia, A. Aesthetic Scrotoplasty: Systematic Review and a Proposed Treatment Algorithm for the Management of Bothersome Scrotum in Adults. Aesthetic Plast. Surg. 2021, 45, 769–776. [Google Scholar] [CrossRef]
- Por, Y.C.; Tan, B.K.; Hong, S.W.; Chia, S.J.; Cheng, C.W.; Foo, C.L.; Tan, K.C. Use of the scrotal remnant as a tissue-expanding musculocutaneous flap for scrotal reconstruction in Paget’s disease. Ann. Plast. Surg. 2003, 51, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Michael, P.; Peiris, B.; Ralph, D.; Johnson, M.; Lee, W.G. Genital Reconstruction following Fournier’s Gangrene. Sex. Med. Rev. 2022, 10, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Hamad, J.; McCormick, B.J.; Sayed, C.J.; Paci, K.; Overton, M.; Daubert, T.; Figler, B.D. Multidisciplinary Update on Genital Hidradenitis Suppurativa: A Review. JAMA Surg. 2020, 155, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Holstein, A.F.; Orlandini, G.E.; Baumgarten, H.G. Morphological analysis of tissue components in the tunica dartos of man. Cell Tissue Res. 1974, 154, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Sengul, G.; Ertekin, C. Human cremaster muscle and cremasteric reflex: A comprehensive review. Clin. Neurophysiol. 2020, 131, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Qin, D.; Peng, S. Responses and coping methods of different testicular cell types to heat stress: Overview and perspectives. Biosci. Rep. 2021, 41, bsr20210443. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.M. Human spermatozoa and temperature: The elephant in the room. Biol. Reprod. 2015, 93, 97. [Google Scholar] [CrossRef]
- Mieusset, R.; Bujan, L.; Mondinat, C.; Mansat, A.; Pontonnier, F.; Grandjean, H. Association of scrotal hyperthermia with impaired spermatogenesis in infertile men. Fertil. Steril. 1987, 48, 1006–1011. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Z.; Sun, G.; Luo, Z. Thin-trimming of the scrotal reconstruction flap: Long-term follow-up shows reversal of spermatogenesis arrest. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, e455–e456. [Google Scholar] [CrossRef]
- Rebourcet, D.; Mackay, R.; Darbey, A.; Curley, M.K.; Jørgensen, A.; Frederiksen, H.; Mitchell, R.T.; O’Shaughnessy, P.J.; Nef, S.; Smith, L.B. Ablation of the canonical testosterone production pathway via knockout of the steroidogenic enzyme HSD17B3, reveals a novel mechanism of testicular testosterone production. Faseb. J. 2020, 34, 10373–10386. [Google Scholar] [CrossRef]
- Li, Y.; Mi, P.; Wu, J.; Tang, Y.; Liu, X.; Cheng, J.; Huang, Y.; Qin, W.; Cheng, C.Y.; Sun, F. High Throughput scRNA-Seq Provides Insights Into Leydig Cell Senescence Induced by Experimental Autoimmune Orchitis: A Prominent Role of Interstitial Fibrosis and Complement Activation. Front. Immunol. 2021, 12, 771373. [Google Scholar] [CrossRef]
- Goyal, A.K.; Saini, J. Steroidogenesis and VEGF Production Doesn’t Alter in Leydig Cells within the Homeostatic Range of Testicular Temperature. J. Hum. Reprod. Sci. 2018, 11, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.T.; Nawar, A.M.; Shoulah, A.A. Outcome of pudendal thigh flap in scrotal reconstruction and its effect on testicular function. Egypt. J. Surg. 2019, 38, 399–405. [Google Scholar] [CrossRef]
- Tiwari, I.N.; Seth, H.P.; Mehdiratta, K.S. Reconstruction of the scrotum by thigh flaps. Plast. Reconstr. Surg. 1980, 66, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Maguiña, P.; Palmieri, T.L.; Greenhalgh, D.G. Split thickness skin grafting for recreation of the scrotum following Fournier’s gangrene. Burns 2003, 29, 857–862. [Google Scholar] [CrossRef]
- Bedford, J.M.; Berrios, M.; Dryden, G.L. Biology of the scrotum. IV. Testis location and temperature sensitivity. J. Exp. Zool. 1982, 224, 379–388. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, H.; Deng, F. Spermatogenesis after scrotal reconstruction. Br. J. Plast. Surg. 2003, 56, 484–488. [Google Scholar] [CrossRef]
- Still, E.F., 2nd; Goodman, R.C. Total reconstruction of a two-compartment scrotum by tissue expansion. Plast. Reconstr. Surg. 1990, 85, 805–807. [Google Scholar] [CrossRef]
- Rapp, D.E.; Cohn, A.B.; Gottlieb, L.J.; Lyon, M.B.; Bales, G.T. Use of tissue expansion for scrotal sac reconstruction after scrotal skin loss. Urology 2005, 65, 1216–1218. [Google Scholar] [CrossRef]
- Hollins, A.; Mundy, L.R.; Atia, A.; Levites, H.; Peterson, A.; Erdmann, D. Tissue Expander Scrotal Reconstruction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2714. [Google Scholar] [CrossRef]
- Balakrishnan, C. Scrotal avulsion: A new technique of reconstruction by split-skin graft. Br. J. Plast. Surg. 1956, 9, 38–42. [Google Scholar] [CrossRef]
- Yamakawa, S.; Fujioka, M.; Fukui, K.; Matsuo, H.; Noguchi, M.; Kugiyama, T.; Sugimi, S.; Fukuda, H.; Hayashida, K. Fournier’s Gangrene With Subcutaneous Emphysema of the Thigh Caused by Air Inflow Associated With a Rectovaginal Fistula: A Case Report of Pseudo-gas Gangrene. Wounds 2021, 33, E10–E13. [Google Scholar]
- Mortada, H.; Alhablany, T.; Alkahtani, D.; Rashidi, M.E.; Altamimi, A. Meshed Versus Sheet Skin Graft for Scrotum and Perineal Skin Loss: A Retrospective Comparative Study. Cureus 2021, 13, e18348. [Google Scholar] [CrossRef]
- Lembo, F.; Cecchino, L.R.; Parisi, D.; Portincasa, A. A combined protocol for improving reconstruction of scrotal skin avulsions: The experience of the University Hospital Center of Foggia. Urologia 2022, 89, 623–628. [Google Scholar] [CrossRef]
- Ye, J.; Xie, T.; Wu, M.; Ni, P.; Lu, S. Negative pressure wound therapy applied before and after split-thickness skin graft helps healing of Fournier gangrene: A case report (CARE-Compliant). Medicine 2015, 94, e426. [Google Scholar] [CrossRef]
- Zhao, J.C.; Xian, C.J.; Yu, J.A.; Shi, K. Reconstruction of infected and denuded scrotum and penis by combined application of negative pressure wound therapy and split-thickness skin grafting. Int. Wound J. 2013, 10, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Rose, L.F.; Wu, J.C.; Carlsson, A.H.; Tucker, D.I.; Leung, K.P.; Chan, R.K. Recipient wound bed characteristics affect scarring and skin graft contraction. Wound Repair. Regen. 2015, 23, 287–296. [Google Scholar] [CrossRef]
- Demir, Y.; Aktepe, F.; Kandal, S.; Sancaktar, N.; Turhan-Haktanir, N. The effect of scrotal reconstruction with skin flaps and skin grafts on testicular function. Ann. Plast. Surg. 2012, 68, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Konofaos, P.; Hickerson, W.L. A Technique for Improving Cosmesis After Primary Scrotum Reconstruction With Skin Grafts. Ann. Plast. Surg. 2015, 75, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Hayashida, K. Scrotal Reconstruction Using a Free Ulnar Forearm Flap: A Case Report. J. Plast. Reconstr. Surg. 2022, 1, 26–30. [Google Scholar] [CrossRef]
- Sapino, G.; Gonvers, S.; Cherubino, M.; di Summa, P.G. The “Sombrero-Shape” Super-Thin Pedicled ALT Flap for Complete Scrotal Reconstruction Following Fournier’s Gangrene. Arch. Plast. Surg. 2022, 49, 453–456. [Google Scholar] [CrossRef]
- Teven, C.M.; Yu, J.W.; Zhao, L.C.; Levine, J.P. Extended medial sural artery perforator free flap for groin and scrotal reconstruction. Arch. Plast. Surg. 2020, 47, 354–359. [Google Scholar] [CrossRef]
- Kumar, S.; Saha, A.; Kumar, S.; Singh, P.; Singh, K.K. Giant Scrotal Lymphoedema: A Case Series. Cureus 2023, 15, e48248. [Google Scholar] [CrossRef] [PubMed]
- Ehrl, D.; Heidekrueger, P.I.; Giunta, R.E.; Wachtel, N. Giant Penoscrotal Lymphedema-What to Do? Presentation of a Curative Treatment Algorithm. J. Clin. Med. 2023, 12, 7586. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Akita, S.; Mitsukawa, N. Fertility-sparing Scrotal Reconstruction with a Superficial Circumflex Iliac Perforator Flap. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5015. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zheng, D.; Wen, J.; Li, J.; Lu, M.; Xu, M.; Zhang, K.; Jiang, Y.; Wang, Z. Reconstruction of Major Scrotal Defects by Anterolateral Thigh Flap. Cell Biochem. Biophys. 2014, 70, 1331–1335. [Google Scholar] [CrossRef]
- Dayan, J.H.; Clarke-Pearson, E.M.; Dayan, E.; Smith, M.L. Aesthetic scrotal reconstruction following extensive Fournier’s gangrene using bilateral island pedicled sensate anterolateral thigh flaps: A case report. Can. Urol. Assoc. J. 2014, 8, E114–E117. [Google Scholar] [CrossRef] [PubMed]
- Kashiyama, K.; Nakano, M.; Higashi, A.; Ashizuka, S.; Moriuchi, Y.; Iwao, A.; Tanaka, K. Reconstruction of a Scrotum by Combining Two Skin Flaps in a Ball Shape. Case Rep. Urol. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Mopuri, N.; O’Connor, E.F.; Iwuagwu, F.C. Scrotal reconstruction with modified pudendal thigh flaps. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 278–283. [Google Scholar] [CrossRef]
- Aydin, T.; Feyzi, K.; Tayfun, T.; Berna, T. Reconstruction of wide scrotal defect using groin fasciocutaneous island flap combined with a strip of deep fascia. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1394–1395. [Google Scholar] [CrossRef]
- El-Sabbagh, A.H. Coverage of the scrotum after Fournier’s gangrene. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2018, 7, Doc01. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, G.; Rui, Y.J.; Mi, J.Y. Bilateral femoral posterior neurocutaneous perforater flap successfully treating Fournier gangrene: A case report. Medicine 2017, 96, e8720. [Google Scholar] [CrossRef] [PubMed]
- Han, H.H.; Ahn, M.R.; Lee, J.H. Penoscrotal reconstruction with superficial circumflex iliac artery perforator propeller flap. Microsurgery 2019, 39, 688–695. [Google Scholar] [CrossRef]
- Bhogesya, S.; Khanna, A.; Taylor, D. Pedicled Superficial Circumflex Iliac Artery Perforator (SCIP) flap for perineo-scrotal reconstruction following Fournier’s gangrene. ANZ J. Surg. 2023, 93, 276–280. [Google Scholar] [CrossRef]
- Han, H.H.; Lee, J.H.; Kim, S.M.; Jun, Y.J.; Kim, Y.J. Scrotal reconstruction using a superficial circumflex iliac artery perforator flap following Fournier’s gangrene. Int. Wound J. 2016, 13, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, M.F.; Meroni, M.; Fritsche, E. Pedicled superficial circumflex iliac artery perforator flap for male genital reconstruction: A case series. Microsurgery 2022, 42, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, M.F.; Chen, Y.C.; Yang, J.C. Posteromedial thigh (PMT) propeller flap for perineoscrotal reconstruction: A case report. Microsurgery 2015, 35, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Daigeler, A.; Behr, B.; Mikhail, B.D.; Lehnhardt, M.; Wallner, C. Bilateral pedicled gracilis flap for scrotal reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, e195–e196. [Google Scholar] [CrossRef]
- Kayikçioğlu, A. A new technique in scrotal reconstruction: Short gracilis flap. Urology 2003, 61, 1254–1256. [Google Scholar] [CrossRef]
- Karsidag, S.; Akcal, A.; Sirvan, S.S.; Guney, S.; Ugurlu, K. Perineoscrotal reconstruction using a medial circumflex femoral artery perforator flap. Microsurgery 2011, 31, 116–121. [Google Scholar] [CrossRef]
- Mellors, T.R.; Rees, C.A.; Franchina, F.A.; Burklund, A.; Patel, C.; Hathaway, L.J.; Hill, J.E. The volatile molecular profiles of seven Streptococcus pneumoniae serotypes. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1096, 208–213. [Google Scholar] [CrossRef]
- Mishra, J.K.; Sahu, S.A.; De, M.; Saha, A. Pedicled anterolateral thigh flap: A versatile flap for complex regional defect reconstruction. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2023, 12, Doc04. [Google Scholar] [CrossRef]
- Zelken, J.A.; AlDeek, N.F.; Hsu, C.C.; Chang, N.J.; Lin, C.H.; Lin, C.H. Algorithmic approach to lower abdominal, perineal, and groin reconstruction using anterolateral thigh flaps. Microsurgery 2016, 36, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.F.; Helene Júnior, A. Scrotal reconstruction with superomedial fasciocutaneous thigh flap. Rev. Col. Bras. Cir. 2018, 45, e1389. [Google Scholar] [CrossRef] [PubMed]
- Oufkir, A.A.; Tazi, M.F.; El Alami, M.N. The superomedial thigh flap in scrotal reconstruction: Technical steps to improve cosmetic results. Indian. J. Urol. 2013, 29, 360–362. [Google Scholar] [CrossRef]
- Khanal, B.; Agrawal, S.; Gurung, R.; Sah, S.; Gupta, R.K. Pudendal flap-a good option for creating neo-scrotum after Fournier’s gangrene: A case series. J. Surg. Case Rep. 2020, 2020, rjaa414. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.R.; Weber, J.; Neeff, H.P.; Manegold, P.; Fichtner-Feigl, S.; Stark, G.B.; Eisenhardt, S.U. Reconstruction of Perineal Defects: A Comparison of the Myocutaneous Gracilis and the Gluteal Fold Flap in Interdisciplinary Anorectal Tumor Resection. Front. Oncol. 2020, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; Sun, S.H.; Ben-Nakhi, M. Modified superficial circumflex iliac artery perforator flap and supermicrosurgery technique for lower extremity reconstruction: A new approach for moderate-sized defects. Ann. Plast. Surg. 2013, 71, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.L.H.; Park, S.W.; Cho, J.Y.; Choi, J.W.; Hong, J.P. The search for the ideal thin skin flap: Superficial circumflex iliac artery perforator flap--a review of 210 cases. Plast. Reconstr. Surg. 2015, 135, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.C.; Tsai, W.H.; Ting, P.S.; Hsueh, J.H.; Chen, L.W.; Lin, Y.S. Comparison between anterolateral thigh, radial forearm, and peroneal artery flap donor site thickness in Asian patients-A sonographic study. Microsurgery 2017, 37, 655–660. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Yang, G.; Qin, X.; Xiong, P. Correlation Between Body Mass Index and Anterolateral Thigh Flap Thickness: A Retrospective Study From a Single Center in China. Front. Surg. 2021, 8, 748799. [Google Scholar] [CrossRef]
- Ferreira, P.C.; Reis, J.C.; Amarante, J.M.; Silva, A.C.; Pinho, C.J.; Oliveira, I.C.; da Silva, P.N. Fournier’s gangrene: A review of 43 reconstructive cases. Plast. Reconstr. Surg. 2007, 119, 175–184. [Google Scholar] [CrossRef]
- Antony, A.K.; Hootnick, J.L.; Antony, A.K. Ulnar forearm free flaps in head and neck reconstruction: Systematic review of the literature and a case report. Microsurgery 2014, 34, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, X.; Yu, Y.; Zhong, G.; Tang, M.; Mei, J. Vascular anatomy and clinical application of the free proximal ulnar artery perforator flaps. Plast. Reconstr. Surg. Glob. Open 2014, 2, e179. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Liu, J. Prospective Comparison of Donor-Site Morbidity following Radial Forearm and Ulnar Artery Perforator Flap Harvest. Plast. Reconstr. Surg. 2020, 145, 1267–1274. [Google Scholar] [CrossRef]
- Hakim, S.G.; Trenkle, T.; Sieg, P.; Jacobsen, H.C. Ulnar artery-based free forearm flap: Review of specific anatomic features in 322 cases and related literature. Head. Neck 2014, 36, 1224–1229. [Google Scholar] [CrossRef]
- Sieg, P.; Bierwolf, S. Ulnar versus radial forearm flap in head and neck reconstruction: An experimental and clinical study. Head. Neck 2001, 23, 967–971. [Google Scholar] [CrossRef]
- Hakim, S.G. Median antebrachial vein for drainage of the ulnar free forearm flap. Br. J. Oral. Maxillofac. Surg. 2014, 52, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.A.; Rehman, S.U.; Haq, A.U.; Riaz, A.; Saleem, M.; Rabbani, M.J.; Amin, M.; Mujahid, A.M.; Alvi, H.F.; Tarar, M.N. Medial Sural Artery Perforator Flap: A Versatile Option For Soft Tissue Reconstruction Of Head And Neck And Limbs. J. Ayub Med. Coll. Abbottabad 2018, 30, 155–158. [Google Scholar]
- Kao, H.K.; Chang, K.P.; Chen, Y.A.; Wei, F.C.; Cheng, M.H. Anatomical basis and versatile application of the free medial sural artery perforator flap for head and neck reconstruction. Plast. Reconstr. Surg. 2010, 125, 1135–1145. [Google Scholar] [CrossRef]
- Daar, D.A.; Abdou, S.A.; Cohen, J.M.; Wilson, S.C.; Levine, J.P. Is the Medial Sural Artery Perforator Flap a New Workhorse Flap? A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2019, 143, 393e–403e. [Google Scholar] [CrossRef]
- Taufique, Z.M.; Daar, D.A.; Cohen, L.E.; Thanik, V.D.; Levine, J.P.; Jacobson, A.S. The medial sural artery perforator flap: A better option in complex head and neck reconstruction? Laryngoscope 2019, 129, 1330–1336. [Google Scholar] [CrossRef]
- He, Y.; Jin, S.F.; Zhang, Z.Y.; Feng, S.Q.; Zhang, C.P.; Zhang, Y.X. A prospective study of medial sural artery perforator flap with computed tomographic angiography-aided design in tongue reconstruction. J. Oral. Maxillofac. Surg. 2014, 72, 2351–2365. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.; Tang, C.B.; Kadirkamanathan, S.S.; Tare, M. Scrotal reconstruction with a free greater omental flap: A case report. Microsurgery 2010, 30, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Nasr, R.W.; Karami, R.A.; Atallah, G.M.; Abou Heidar, N.F.; Ibrahim, A.E. Free omental flap for the treatment of chronic scrotal lymphedema: A case report. Eur. J. Plast. Surg. 2021, 44, 279–284. [Google Scholar] [CrossRef]
- Kamei, Y.; Aoyama, H.; Yokoo, K.; Fujii, K.; Kondo, C.; Sato, T.; Onishi, S. Composite gastric seromuscular and omental pedicle flap for urethral and scrotal reconstruction after Fournier’s gangrene. Ann. Plast. Surg. 1994, 33, 565–568. [Google Scholar] [CrossRef]
- Shash, H.; Al-Halabi, B.; Aldekhayel, S.; Dionisopoulos, T. Laparoscopic Harvesting of Omental Flaps for Breast Reconstruction-A Review of the Literature and Outcome Analysis. Plast. Surg. 2018, 26, 126–133. [Google Scholar] [CrossRef]
- Albrecht, P.; Eimer, C.; Kasten, E. The scrotum: A comparison of men’s and women’s aesthetic assessments. J. Cosmet. Dermatol. 2023, 22, 2273–2282. [Google Scholar] [CrossRef]


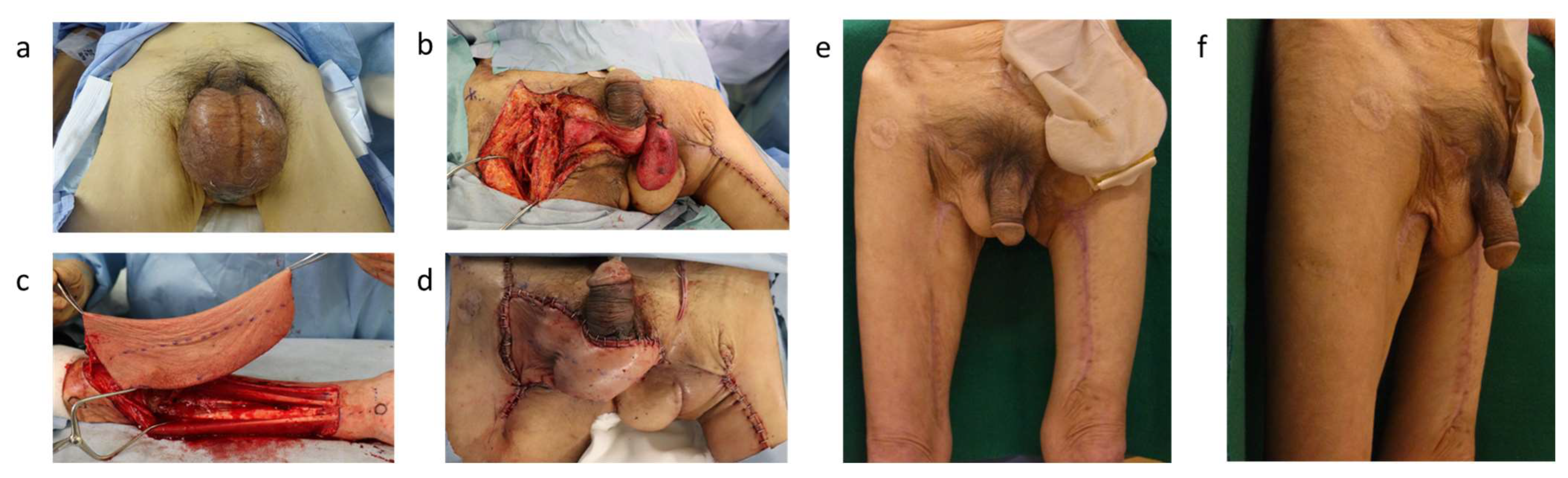
| Advantages | Disadvantages | Unilateral Side | Bilateral Sides | |
|---|---|---|---|---|
| SCIP flap (groin flap) | Thinnest flap in the perineal area (5–7 mm); minimal subcutaneous fat, making it suitable for preserving spermatogenic function. Postoperative improvement in spermatogenic function has been demonstrated. Ideal for bilateral use in scrotal reconstruction. | Limited tissue availability; may not be sufficient in cases of extensive debridement. Its thinness may be a limitation in patients requiring more voluminous tissue coverage. | [63,64,65,66] | [33,55,60] |
| ALT flap | Can be raised as either a fasciocutaneous or a myocutaneous flap; long pedicle with extensive reach; allows tailored reconstruction based on defect requirements; generally well-tolerated tissue transfer; low morbidity at the donor site. | Average thickness is about 9.8 mm in Asians, which is thicker than SCIP flaps. Thickness correlates with BMI, potentially limiting its use in obese patients. In cases where thin flaps are preferred for preserving spermatogenic function, ALT flaps may not be the ideal choice. | [51,56,72,73] | [57] |
| Medial thigh flap | Good option when SCIP flap is not viable; offers adequate tissue for reconstruction; can provide color and texture match similar to native scrotal tissue; if the muscular component is attached, useful for filling the dead space and for separating the anus from the perineum. | Tends to accumulate subcutaneous fat, making thickness dependent on obesity. The relative thickness compared to SCIP flaps may compromise the preservation of spermatogenic function. | [33,61,67] | [33,58,59,62,68,69,70,74,75,76,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suda, S.; Hayashida, K. Crafting Contours: A Comprehensive Guide to Scrotal Reconstruction. Life 2024, 14, 223. https://doi.org/10.3390/life14020223
Suda S, Hayashida K. Crafting Contours: A Comprehensive Guide to Scrotal Reconstruction. Life. 2024; 14(2):223. https://doi.org/10.3390/life14020223
Chicago/Turabian StyleSuda, Shota, and Kenji Hayashida. 2024. "Crafting Contours: A Comprehensive Guide to Scrotal Reconstruction" Life 14, no. 2: 223. https://doi.org/10.3390/life14020223
APA StyleSuda, S., & Hayashida, K. (2024). Crafting Contours: A Comprehensive Guide to Scrotal Reconstruction. Life, 14(2), 223. https://doi.org/10.3390/life14020223






