Effect of Vision and Surface Slope on Postural Sway in Healthy Adults: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
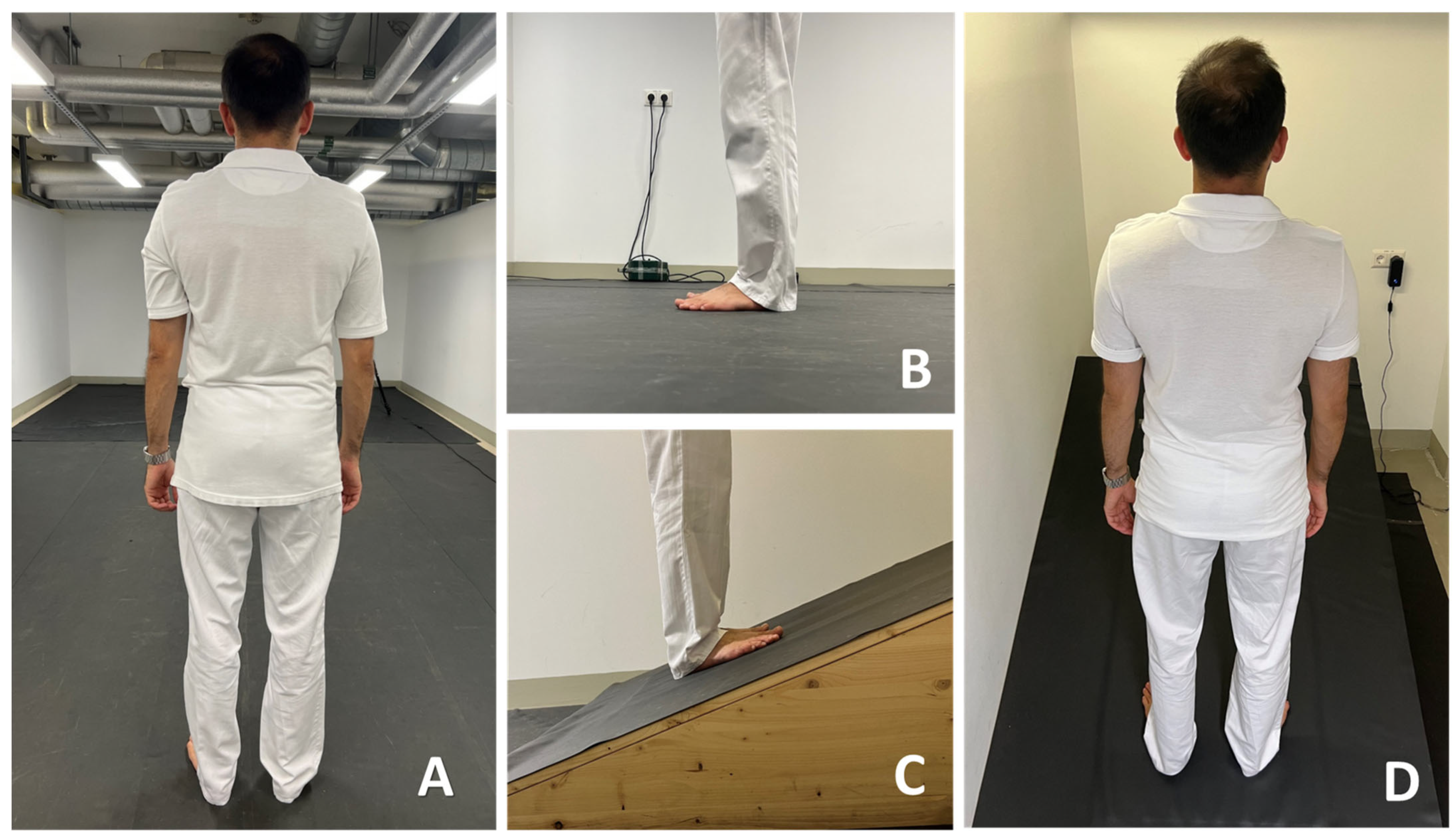
2.1. Sample and Procedure
- C1: standing on flat ground with EO (standard condition);
- C2: standing on flat ground with EC;
- C3: standing uphill with EO;
- C4: standing uphill with EC;
- C5: standing downhill with EO;
- C6: standing downhill with EC.
2.2. Approval and Consent
2.3. Equipment
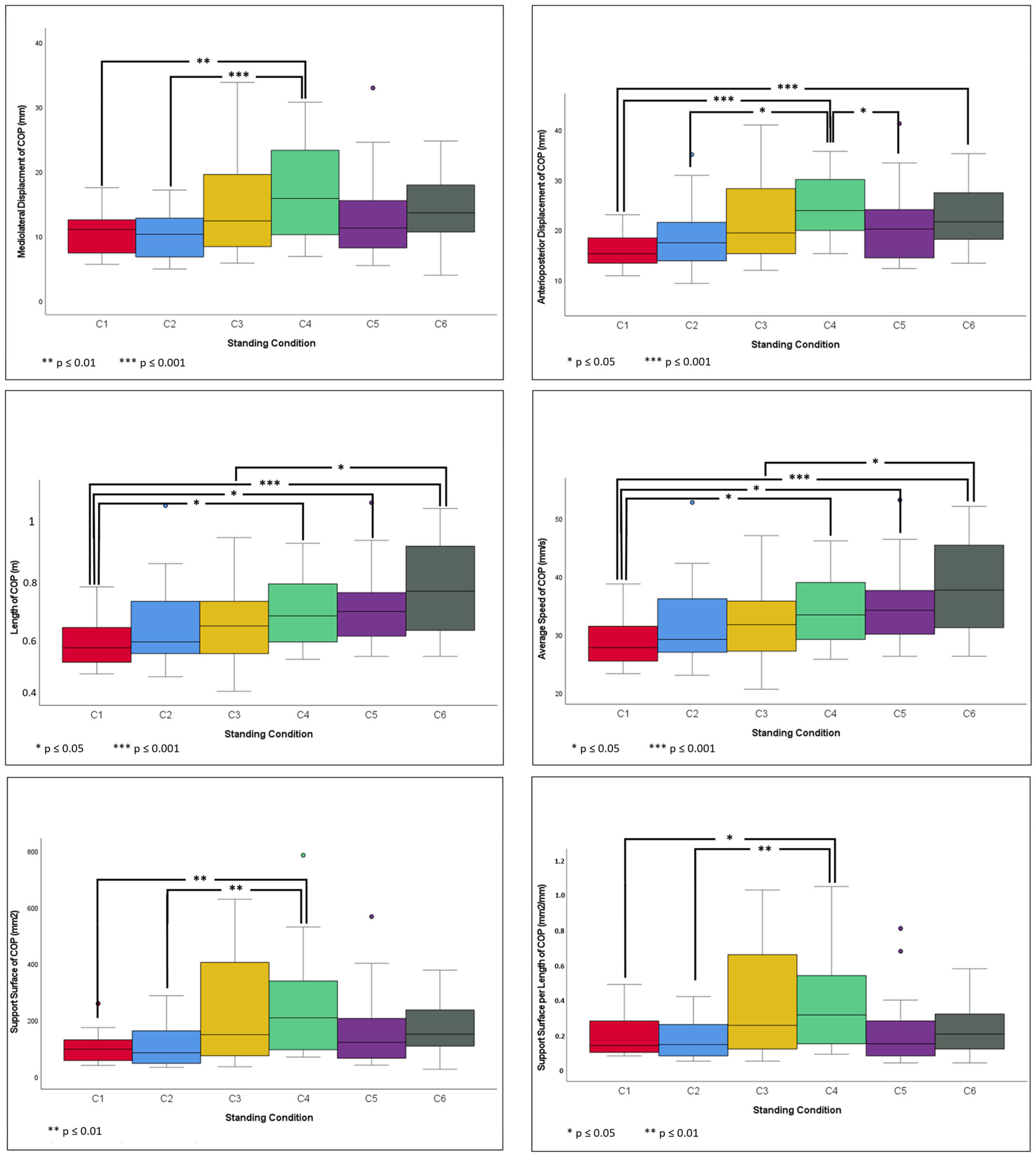
2.4. COP Parameters and Romberg Index
- Mediolateral displacement (MLD) in millimeters.
- Anterior–posterior displacement (APD) in millimeters.
- Total length (L) in meters; defined as the total excursion of the COP during the measurement time calculated by summing the distance among the successive locations of the COP.
- AS in millimeters per second; defined as the mean speed of the COP movement.
- SS in square millimeters; area of the ellipse containing 90% of all COP points.
- Quotient of SS in square millimeters per unit length in millimeters (LFS).
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Relationship between COP Parameters and Standing Conditions, Gender, and BMI
3.3. Pairwise Comparison of Standing Conditions
3.3.1. C1 vs. Other Standing Conditions
3.3.2. C2 vs. Other Standing Conditions
3.3.3. C3 vs. Other Standing Conditions
3.3.4. C4 vs. Other Standing Conditions
3.3.5. C5 vs. Other Standing Conditions
3.3.6. C6 vs. Other Standing Conditions
3.4. Romberg Index
4. Discussion
4.1. Flat Surface vs. Sloped Surface
4.2. Inclined Slope vs. Declined Slope
4.3. Effect of BMI and Gender
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | eyes open |
| EC | eyes closed |
| COP | center of pressure |
| COM | center of mass |
| BOS | base of support |
| COG | center of gravity |
| BMI | Body Mass Index |
| MLD | mediolateral displacement |
| APD | anterior–posterior displacement |
| L | total length |
| AS | average speed |
| SS | support surface |
| LFS | quotient of support surface in square millimeters per unit length |
| C1 | standing on flat ground with EO (standard condition) |
| C2 | standing on flat ground with EC |
| C3 | standing uphill with EO |
| C4 | standing uphill with EC |
| C5 | standing downhill with EO |
| C6 | standing downhill with EC |
| RI | Romberg Index |
References
- Palmieri, R.M.; Ingersoll, C.D.; Stone, M.B.; Krause, B.A. Center-of-Pressure Parameters Used in the Assessment of Postural Control. J. Sport. Rehabil. 2002, 11, 51–66. [Google Scholar] [CrossRef]
- Scherder, E.; Eggermont, L.; Swaab, D.; van Heuvelen, M.; Kamsma, Y.; de Greef, M.; van Wijck, R.; Mulder, T. Gait in ageing and associated dementias; its relationship with cognition. Neurosci. Biobehav. Rev. 2007, 31, 485–497. [Google Scholar] [CrossRef]
- Faraldo-García, A.; Santos-Pérez, S.; Crujeiras-Casais, R.; Labella-Caballero, T.; Soto-Varela, A. Influence of age and gender in the sensory analysis of balance control. Eur. Arch. Otorhinolaryngol. 2012, 269, 673–677. [Google Scholar] [CrossRef]
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Anson, D.; Haller, S. Postural sway biofeedback: Its effect on reestablishing stance stability in hemiplegic patients. Arch. Phys. Med. Rehabil. 1988, 69, 395–400. [Google Scholar]
- Lawson, T.; Morrison, A.; Blaxland, S.; Wenman, M.; Schmidt, C.G.; Hunt, M.A. Laboratory-based measurement of standing balance in individuals with knee osteoarthritis: A systematic review. Clin. Biomech. 2015, 30, 330–342. [Google Scholar] [CrossRef]
- de Andrade, L.P.; Gobbi, L.T.B.; Coelho, F.G.M.; Christofoletti, G.; Costa, J.L.R.; Stella, F. Benefits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with Alzheimer’s disease: A controlled trial. J. Am. Geriatr. Soc. 2013, 61, 1919–1926. [Google Scholar] [CrossRef]
- Frames, C.; Soangra, R.; Lockhart, T.E. Assessment of Postural Stability using Inertial Measurement Unit on Inclined Surfaces in Healthy Adults. Biomed. Sci. Instrum. 2013, 49, 234–242. [Google Scholar] [PubMed]
- Gago, M.F.; Fernandes, V.; Ferreira, J.; Yelshyna, D.; Silva, H.D.; Rodrigues, M.L.; Rocha, L.; Bicho, E.; Sousa, N. Role of the Visual and Auditory Systems in Postural Stability in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 46, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Pomarino, D.; Nawrath, A.; Beyer, J. Altersabhängige Messungen zur posturalen Stabilität gesunder Probanden. Orthopädische Unfallchirurgische Prax. 2013, 9, 420–425. [Google Scholar] [CrossRef]
- Benvenuti, F.; Mecacci, R.; Gineprari, I.; Bandinelli, S.; Benvenuti, E.; Ferrucci, L.; Baroni, A.; Rabuffetti, M.; Hallett, M.; Dambrosia, J.M.; et al. Kinematic characteristics of standing disequilibrium: Reliability and validity of a posturographic protocol. Arch. Phys. Med. Rehabil. 1999, 80, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T.; Noé, F. Techniques and Methods for Testing the Postural Function in Healthy and Pathological Subjects. Biomed. Res. Int. 2015, 2015, 891390. [Google Scholar] [CrossRef]
- Quijoux, F.; Nicolaï, A.; Chairi, I.; Bargiotas, I.; Ricard, D.; Yelnik, A.; Oudre, L.; Bertin-Hugault, F.; Vidal, P.-P.; Vayatis, N.; et al. A review of center of pressure (COP) variables to quantify standing balance in elderly people: Algorithms and open-access code. Physiol. Rep. 2021, 9, e15067. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bonal, A.; Marshak, A. Approximate Entropy and Sample Entropy: A Comprehensive Tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef]
- Viseu, J.-P.; Yiou, E.; Morin, P.-O.; Olivier, A. Sport dependent effects on the sensory control of balance during upright posture: A comparison between professional horseback riders, judokas and non-athletes. Front. Hum. Neurosci. 2023, 17, 1213385. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.; Weimar, W.; Luttgens, K. The Center of Gravity and Stability. In Kinesiology: Scientific Basis of Human Motion; McGraw-Hill Humanities: New York, NY, USA, 2007; p. 656. ISBN 978-0072972979. [Google Scholar]
- Melzer, I.; Benjuya, N.; Kaplanski, J. Age-Related Changes of Postural Control: Effect of Cognitive Tasks. Gerontology 2001, 47, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Sternad, D. Complexity of human postural control in young and older adults during prolonged standing. Exp. Brain Res. 2008, 191, 265–276. [Google Scholar] [CrossRef]
- Kido, T.; Tabara, Y.; Igase, M.; Ochi, N.; Uetani, E.; Nagai, T.; Yamamoto, M.; Taguchi, K.; Miki, T.; Kohara, K. Postural instability is associated with brain atrophy and cognitive impairment in the elderly: The J-SHIPP study. Dement. Geriatr. Cogn. Disord. 2010, 29, 379–387. [Google Scholar] [CrossRef]
- Leirós-Rodríguez, R.; Romo-Pérez, V.; García-Soidán, J.L.; García-Liñeira, J. Percentiles and Reference Values for the Accelerometric Assessment of Static Balance in Women Aged 50–80 Years. Sensors 2020, 20, 940. [Google Scholar] [CrossRef]
- Allan, L.M.; Ballard, C.G.; Burn, D.J.; Kenny, R.A. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J. Am. Geriatr. Soc. 2005, 53, 1681–1687. [Google Scholar] [CrossRef]
- Gago, M.F.; Fernandes, V.; Ferreira, J.; Silva, H.; Rocha, L.; Bicho, E.; Sousa, N. Postural stability analysis with inertial measurement units in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. Extra 2014, 4, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, N.; Perry, M.; Hill, K.D.; Kaur, M.; Hale, L. Postural Stability in Older Adults with Alzheimer Disease. Phys. Ther. 2017, 97, 290–309. [Google Scholar] [CrossRef] [PubMed]
- Schmit, J.M.; Riley, M.A.; Dalvi, A.; Sahay, A.; Shear, P.K.; Shockley, K.D.; Pun, R.Y.K. Deterministic center of pressure patterns characterize postural instability in Parkinson’s disease. Exp. Brain Res. 2006, 168, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.W.; Orawiec, R. Assessment of postural control in patients with Parkinson’s disease: Sway ratio analysis. Hum. Mov. Sci. 2011, 30, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Davids, K.; Kingsbury, D.; George, K.; O’Connell, M.; Stock, D. Interacting Constraints and the Emergence of Postural Behavior in ACL-Deficient Subjects. J. Mot. Behav. 1999, 31, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Tropp, H.; Ekstrand, J.A.; Gillquist, J.A. Stabilometry in functional instability of the ankle and its value in predicting injury. Med. Sci. Sports Exerc. 1984, 16, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, M.W.; Murrell, P. Postural sway following inversion sprain of the ankle. J. Am. Podiatr. Med. Assoc. 1991, 81, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Goldie, P.A.; Evans, O.M.; Bach, T.M. Postural control following inversion injuries of the ankle. Arch. Phys. Med. Rehabil. 1994, 75, 969–975. [Google Scholar] [CrossRef]
- Jarnlo, G.B.; Thorngren, K.G. Standing balance in hip fracture patients. 20 middle-aged patients compared with 20 healthy subjects. Acta Orthop. Scand. 1991, 62, 427–434. [Google Scholar] [CrossRef]
- Ingersoll, C.D.; Armstrong, C.W. The effects of closed-head injury on postural sway. Med. Sci. Sports Exerc. 1992, 24, 739–743. [Google Scholar] [CrossRef]
- Geurts, A.C.; Ribbers, G.M.; Knoop, J.A.; van Limbeek, J. Identification of static and dynamic postural instability following traumatic brain injury. Arch. Phys. Med. Rehabil. 1996, 77, 639–644. [Google Scholar] [CrossRef]
- Hue, O.; Simoneau, M.; Marcotte, J.; Berrigan, F.; Doré, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Body weight is a strong predictor of postural stability. Gait Posture 2007, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Menegoni, F.; Tacchini, E.; Bigoni, M.; Vismara, L.; Priano, L.; Galli, M.; Capodaglio, P. Mechanisms underlying center of pressure displacements in obese subjects during quiet stance. J. Neuroeng. Rehabil. 2011, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.C.; Luna, N.M.S.; Mochizuki, L.; Barbieri, F.; Santos, S.; Greve, J.M.D. The influence of anthropometric factors on postural balance: The relationship between body composition and posturographic measurements in young adults. Clinics 2012, 67, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwon, Y.; Chung, H.-Y.; Kim, C.-S.; Eom, G.-M.; Jun, J.-H.; Park, B.K. Relationship between body factors and postural sway during natural standing. Int. J. Precis. Eng. Manuf. 2012, 13, 963–968. [Google Scholar] [CrossRef]
- Lockhart, T.E.; Frames, C.W.; Soangra, R.; Lieberman, A. Effects of obesity and fall risk on gait and posture of community-dwelling older adults. Int. J. Progn. Health Manag. 2019, 10, 019. [Google Scholar] [CrossRef]
- Jeronymo, B.F.; Silva, P.R.d.O.; Mainenti, M.; Felicio, L.R.; Ferreira, A.d.S.; de Carvalho, T.L.; Vigário, P. The Relationship between Postural Stability, Anthropometry Measurements, Body Composition, and Sport Experience in Judokas with Visual Impairment. Asian J. Sports Med. 2020, 11, e103030. [Google Scholar] [CrossRef]
- Garcia, P.A.; de Queiroz, L.L.; Caetano, M.B.D.; Silva, K.H.C.V.E.; Da Hamu, T.C.D.S. Obesity is associated with postural balance on unstable surfaces but not with fear of falling in older adults. Braz. J. Phys. Ther. 2021, 25, 311–318. [Google Scholar] [CrossRef] [PubMed]
- García-Liñeira, J.; Leirós-Rodríguez, R.; Romo-Pérez, V.; García-Soidán, J.L. Sex differences in postural control under unstable conditions in schoolchildren with accelerometric assessment. Gait Posture 2021, 87, 81–86. [Google Scholar] [CrossRef]
- Nashner, L.M.; Black, F.O.; Wall, C. Adaptation to altered support and visual conditions during stance: Patients with vestibular deficits. J. Neurosci. 1982, 2, 536–544. [Google Scholar] [CrossRef]
- Juodžbalienė, V.; Kazimieras, M. The influence of the degree of visual impairment on psychomotor reaction and equilibrium maintenance of adolescents. Medicina 2006, 42, 49–56. [Google Scholar] [PubMed]
- Dozza, M.; Horak, F.B.; Chiari, L. Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp. Brain Res. 2007, 178, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Nardone, A.; de Nunzio, A.M.; Schmid, M.; Schieppati, M. Equilibrium during static and dynamic tasks in blind subjects: No evidence of cross-modal plasticity. Brain 2007, 130, 2097–2107. [Google Scholar] [CrossRef]
- Horlings, C.G.C.; Küng, U.M.; Bloem, B.R.; Honegger, F.; van Alfen, N.; van Engelen, B.G.M.; Allum, J.H.J. Identifying deficits in balance control following vestibular or proprioceptive loss using posturographic analysis of stance tasks. Clin. Neurophysiol. 2008, 119, 2338–2346. [Google Scholar] [CrossRef]
- Mergner, T.; Schweigart, G.; Fennell, L.; Maurer, C. Posture control in vestibular-loss patients. Ann. N. Y. Acad. Sci. 2009, 1164, 206–215. [Google Scholar] [CrossRef]
- Almansba, R.; Sterkowicz-Przybycień, K.; Sterkowicz, S.; Mahdad, D.; Boucher, J.P.; Calmet, M.; Comtois, A.S. Postural balance control ability of visually impaired and unimpaired judoists. Arch. Budo 2012, 8, 153–158. [Google Scholar] [CrossRef]
- Black, F.O.; Wall, C.; Nashner, L.M. Effects of Visual and Support Surface Orientation References Upon Postural Control in Vestibular Deficient Subjects. Acta Oto-Laryngol. 1983, 95, 199–210. [Google Scholar] [CrossRef]
- Mezzarane, R.A.; Kohn, A.F. Control of upright stance over inclined surfaces. Exp. Brain Res. 2007, 180, 377–388. [Google Scholar] [CrossRef]
- King, A.C.; Patton, J.; Dutt-Mazumder, A.; Newell, K.M. Center-of-pressure dynamics of upright standing as a function of sloped surfaces and vision. Neurosci. Lett. 2020, 737, 135334. [Google Scholar] [CrossRef]
- Wade, C.; Davis, J. Postural sway following prolonged exposure to an inclined surface. Saf. Sci. 2009, 47, 652–658. [Google Scholar] [CrossRef]
- Isableu, B.; Hlavackova, P.; Diot, B.; Vuillerme, N. Regularity of Center of Pressure Trajectories in Expert Gymnasts during Bipedal Closed-Eyes Quiet Standing. Front. Hum. Neurosci. 2017, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Silva, P.R.; Felício, L.R.; Mainenti, M.R.; Miranda, H.L.; Paz, G.A.; Lemos, T.; Ferreira, A.d.S.; Vigário, P.S. Postural control in football players with vision impairment: Effect of sports adaptation or visual input restriction? Mot. Rev. Educ. Fis. 2022, 28, e1022001082. [Google Scholar] [CrossRef]
- Wade, C.; Davis, J. Experience on an Elevated Inclined Surface and Postural Control. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2005, 49, 1311–1314. [Google Scholar] [CrossRef]
- Dutt-Mazumder, A.; Slobounov, S.M.; Challis, J.H.; Newell, K.M. Postural Stability Margins as a Function of Support Surface Slopes. PLoS ONE 2016, 11, e0164913. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Han, S.; Hopkins, J.T. Altered Visual Reliance Induced by Stroboscopic Glasses during Postural Control. Int. J. Environ. Res. Public Health 2022, 19, 2076. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, P.I.; Hsiao, H.; Dotson, B.W.; Ammons, D.E. Control and perception of balance at elevated and sloped surfaces. Hum. Factors 2003, 45, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Le Clair, K.; Riach, C. Postural stability measures: What to measure and for how long. Clin. Biomech. 1996, 11, 176–178. [Google Scholar] [CrossRef]
- Lacour, M.; Barthelemy, J.; Borel, L.; Magnan, J.; Xerri, C.; Chays, A.; Ouaknine, M. Sensory strategies in human postural control before and after unilateral vestibular neurotomy. Exp. Brain Res. 1997, 115, 300–310. [Google Scholar] [CrossRef]
- Tjernström, F.; Björklund, M.; Malmström, E.-M. Romberg ratio in quiet stance posturography—Test to retest reliability. Gait Posture 2015, 42, 27–31. [Google Scholar] [CrossRef]
- Cuisinier, R.; Olivier, I.; Vaugoyeau, M.; Nougier, V.; Assaiante, C. Reweighting of Sensory Inputs to Control Quiet Standing in Children from 7 to 11 and in Adults. PLoS ONE 2011, 6, e19697. [Google Scholar] [CrossRef]
- Kirby, R.L.; Price, N.A.; MacLeod, D.A. The influence of foot position on standing balance. J. Biomech. 1987, 20, 423–427. [Google Scholar] [CrossRef]
- Uimonen, S.; Laitakari, K.; Sorri, M.; Bloigu, R.; Palva, A. Effect of positioning of the feet in posturography. J. Vestib. Res. 1992, 2, 349–356. [Google Scholar] [PubMed]
- Ekdahl, C.; Jarnlo, G.B.; Andersson, S.I. Standing Balance in Healthy Subjects. Evaluation of a Quantitative Test Battery on a Force Platform. J. Rehabil. Med. 1989, 21, 187–195. [Google Scholar] [CrossRef]
- Baloh, R.W.; Jacobson, K.M.; Enrietto, J.A.; Corona, S.; Honrubia, V. Balance disorders in older persons: Quantification with posturography. Otolaryngol. Head. Neck Surg. 1998, 119, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Dutt-Mazumder, A.; King, A.C.; Newell, K.M. Recurrence dynamics reveals differential control strategies to maintain balance on sloped surfaces. Gait Posture 2019, 69, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Patla, A.E.; Prince, F.; Ishac, M.; Gielo-Perczak, K. Stiffness control of balance in quiet standing. J. Neurophysiol. 1998, 80, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Laughton, C.A.; Slavin, M.; Katdare, K.; Nolan, L.; Bean, J.F.; Kerrigan, D.C.; Phillips, E.; Lipsitz, L.A.; Collins, J.J. Aging, muscle activity, and balance control: Physiologic changes associated with balance impairment. Gait Posture 2003, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Martoni, M.; Esposito, M.J.; Brighetti, G.; Natale, V. Postural control after a night without sleep. Neuropsychologia 2006, 44, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, G.L. Postural sway increases with attentional demands of concurrent cognitive task. Gait Posture 2003, 18, 29–34. [Google Scholar] [CrossRef]
- Choi, S.D.; Fredericks, T.K. Surface slope effects on shingling frequency and postural balance in a simulated roofing task. Ergonomics 2008, 51, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Chiari, L.; Rocchi, L.; Cappello, A. Stabilometric parameters are affected by anthropometry and foot placement. Clin. Biomech. 2002, 17, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Angyán, L.; Téczely, T.; Angyán, Z. Factors affecting postural stability of healthy young adults. Acta Physiol. Hung. 2007, 94, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Chen, H.; Prabhu, M.; Trogdon, J.G.; Corso, P.S. The relationship between obesity and injuries among U.S. adults. Am. J. Health Promot. 2007, 21, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.W.; Beck, M.; Sadowska, D. Assessment of postural stability in young healthy subjects based on directional features of posturographic data: Vision and gender effects. Acta Neurobiol. Exp. 2014, 74, 433–442. [Google Scholar] [CrossRef]
- Jiang, L.; Kasahara, S.; Ishida, T.; Koshino, Y.; Chiba, A.; Yoshimi, K.; Wei, Y.; Samukawa, M.; Tohyama, H. Change in sensory integration and regularity of postural sway with the suspensory strategy during static standing balance. Front. Neurol. 2023, 14, 1290986. [Google Scholar] [CrossRef]
- Bryant, E.C.; Trew, M.E.; Bruce, A.M.; Kuisma, R.M.E.; Smith, A.W. Gender differences in balance performance at the time of retirement. Clin. Biomech. 2005, 20, 330–335. [Google Scholar] [CrossRef]
| Standing Condition | COP Parameter (Mean ± SD) | |||||
|---|---|---|---|---|---|---|
| MLD (mm) | APD (mm) | L (m) | AS (mm/s) | SS (mm2) | LFS (mm2/mm) | |
| C1 | 10.57 ± 3.64 # | 16.32 ± 3.64 #§ | 0.58 ± 0.08 #†§ | 28.84 ± 4.25 #†§ | 111.07 ± 61.97 # | 0.20 ± 0.12 # |
| C2 | 9.93 ± 3.65 † | 18.70 ± 6.78 † | 0.64 ± 0.15 | 32.12 ± 7.33 | 109.88 ± 72.13 † | 0.18 ± 0.12 † |
| C3 | 15.26 ± 8.05 | 21.98 ± 8.52 | 0.64 ± 0.12 ¥ | 31.94 ± 5.95 ¥ | 230.61 ± 193.20 | 0.37 ± 0.31 |
| C4 | 16.55 ± 7.08 #† | 24.93 ± 6.62 #†¥ | 0.68 ± 0.12 # | 34.11 ± 6.07 # | 240.56 ± 173.88 #† | 0.35 ± 0.24 #† |
| C5 | 12.68 ± 6.52 | 20.33 ± 7.24 ¥ | 0.80 ± 0.36 † | 39.79 ± 18.12 † | 160.03 ± 131.60 | 0.23 ± 0.20 |
| C6 | 14.19 ± 5.44 | 22.38 ± 5.98 § | 0.80 ± 0.25 §¥ | 39.94 ± 12.31 §¥ | 179.97 ± 102.46 | 0.25 ± 0.17 |
| RI | COP Parameter (Mean ± SD) | |||||
|---|---|---|---|---|---|---|
| MLD (mm) | APD (mm) | L (m) | AS (mm/s) | SS (mm2) | LFS (mm2/mm) | |
| RI 1 | 95.52 ± 22.12 | 114.0 ± 27.91 | 110.58 ± 14.80 | 110.87 ± 14.79 | 100.87 ± 48.0 | 94.13 ± 49.79 |
| RI 2 | 121.75 ± 59.81 | 122.04 ± 36.53 | 107.60 ± 10.39 | 107.42 ± 10.24 | 147.25 ± 114.47 | 135.33 ± 101.31 |
| RI 3 | 122.65 ± 47.23 | 116.03 ± 31.31 | 106.24 ± 26.02 | 106.35 ± 26.01 | 139.78 ± 76.55 | 130.84 ± 56.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghapour, M.; Affenzeller, N.; Peham, C.; Lutonsky, C.; Tichy, A.; Bockstahler, B. Effect of Vision and Surface Slope on Postural Sway in Healthy Adults: A Prospective Cohort Study. Life 2024, 14, 227. https://doi.org/10.3390/life14020227
Aghapour M, Affenzeller N, Peham C, Lutonsky C, Tichy A, Bockstahler B. Effect of Vision and Surface Slope on Postural Sway in Healthy Adults: A Prospective Cohort Study. Life. 2024; 14(2):227. https://doi.org/10.3390/life14020227
Chicago/Turabian StyleAghapour, Masoud, Nadja Affenzeller, Christian Peham, Christiane Lutonsky, Alexander Tichy, and Barbara Bockstahler. 2024. "Effect of Vision and Surface Slope on Postural Sway in Healthy Adults: A Prospective Cohort Study" Life 14, no. 2: 227. https://doi.org/10.3390/life14020227








