Laccases as Effective Tools in the Removal of Pharmaceutical Products from Aquatic Systems
Abstract
1. Introduction
2. Laccase
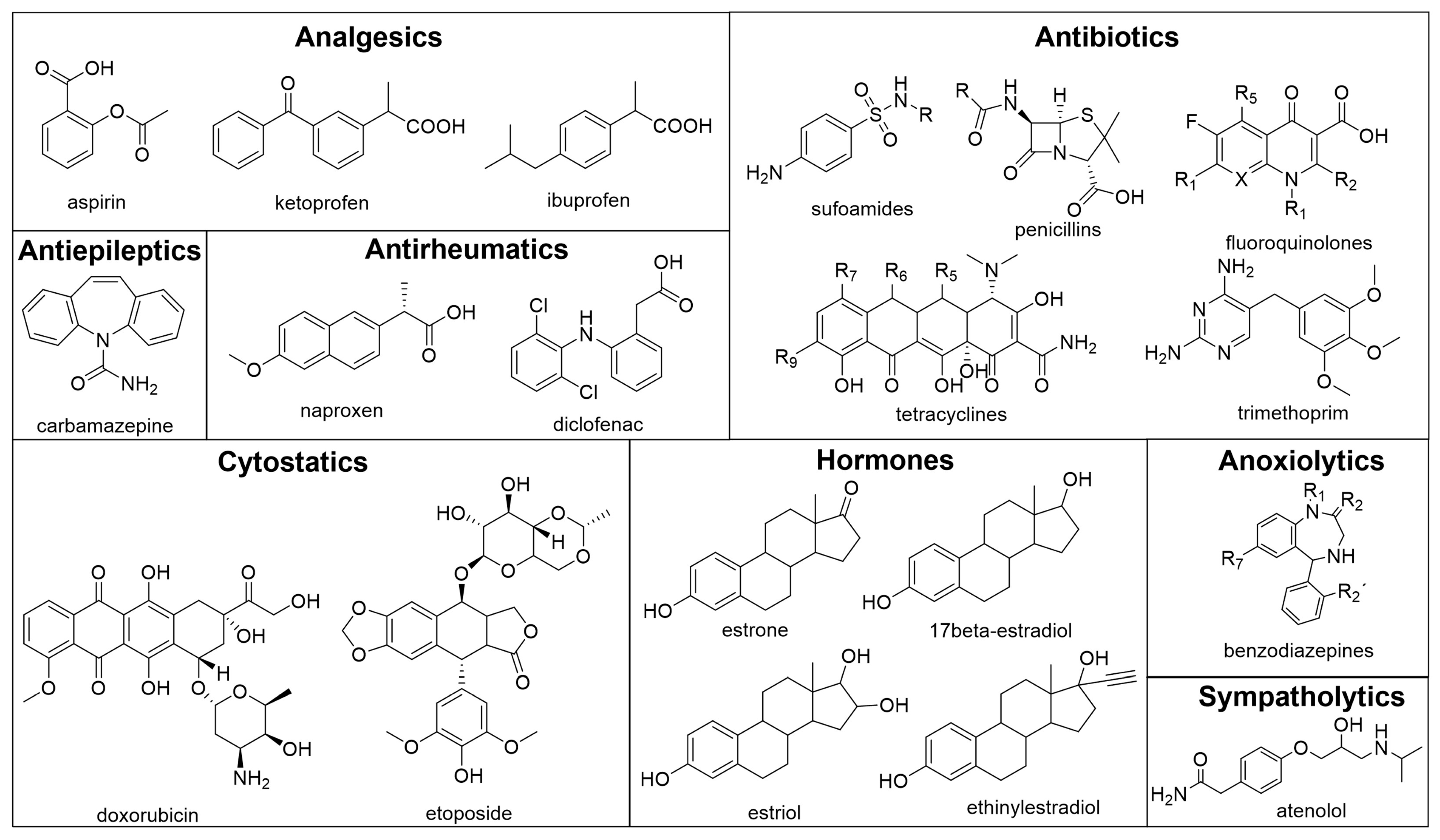
3. Pharmaceutical Products
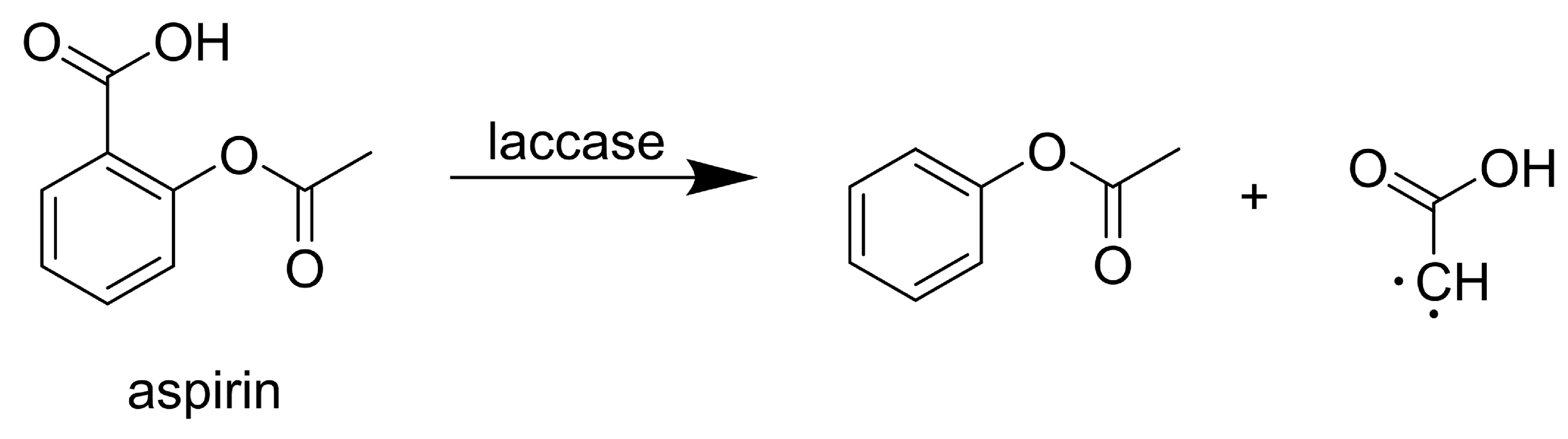
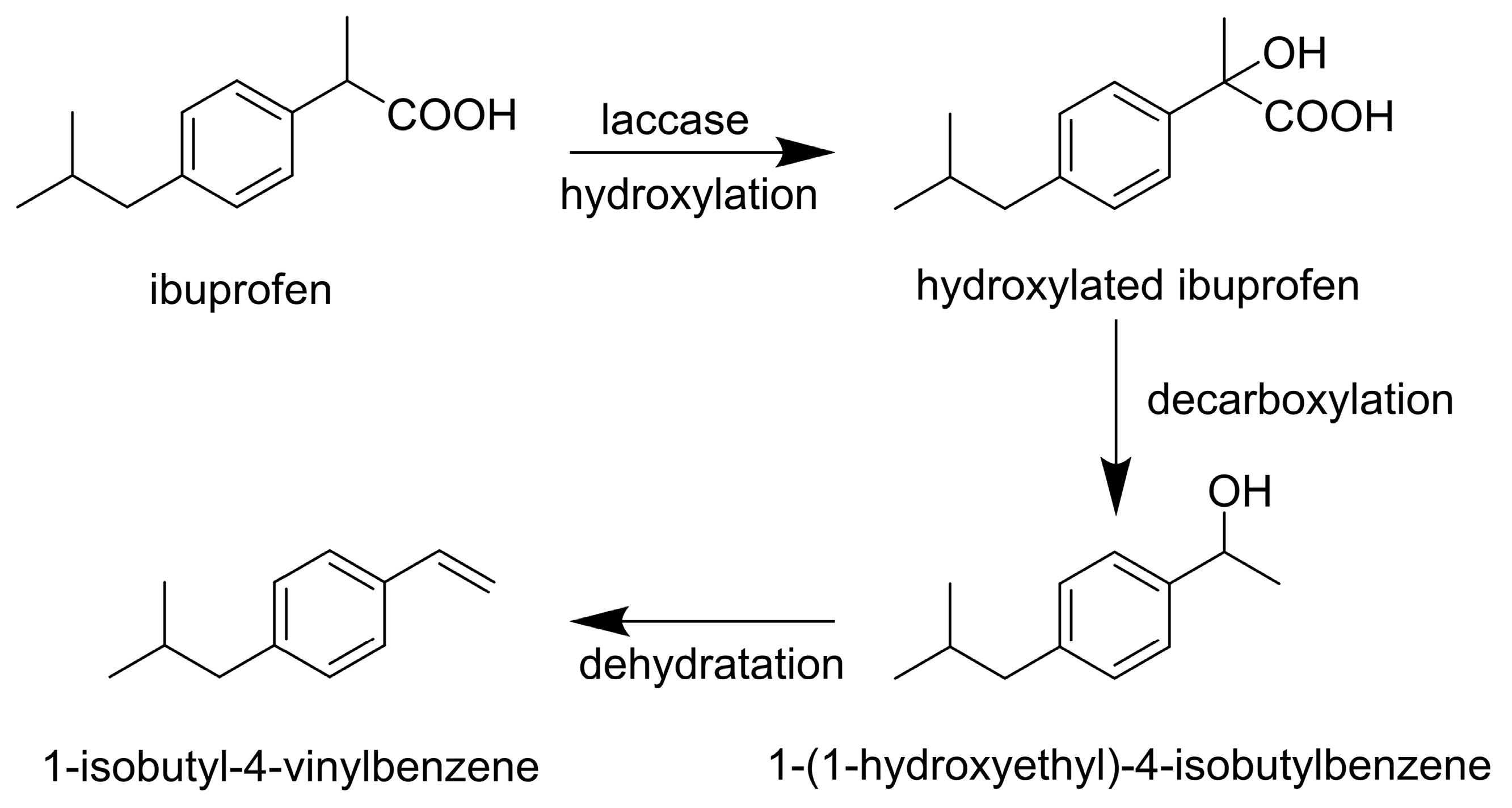
3.1. Analgesics
3.2. Antibiotics
3.3. Antiepileptics
3.4. Antirheumatics
3.5. Cytostatics
3.6. Hormones
3.7. Anxiolytics, Sympatholytics
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Statista. Available online: https://www.statista.com/statistics/263102/pharmaceutical-market-worldwide-revenue-since-2001/ (accessed on 22 December 2023).
- Product Stewardship Council. Webinar. Global Best Practices for Drug Take-Back Programs—Product Stewardship Institute. Available online: https://www.productstewardship.us/page/20180607_GBPFDTBP (accessed on 22 December 2023).
- López-Serna, R.; Petrović, M.; Barceló, D. Occurrence and distribution of multi-class pharmaceuticals and their active metabolites and transformation products in the Ebro River basin (NE Spain). Sci. Total Environ. 2012, 440, 280–289. [Google Scholar] [CrossRef]
- Mons, M.N.; van Genderen, J.; van Dijk-Looijaard, A.M. Inventory on the Presence of Pharmaceuticals in Dutch Water; KIWA: Nieuwegein, The Netherlands, 2000; Available online: https://www.riwa-rijn.org/wp-content/uploads/2015/06/Inventory_presence_pharmas_dutchwaters.pdf (accessed on 22 December 2023).
- Miao, X.S.; Yang, J.J.; Metcalfe, C.D. Carbamazepine and its metabolites in a municipal wastewater treatment plant. Environ. Sci. Technol. 2005, 39, 7469–7475. [Google Scholar] [CrossRef]
- Morasch, B.; Bonvin, F.; Reiser, H.; Grandjean, D.; De Alencastro, L.F.; Perazzolo, C.; Chevre, N.; Kohn, T. Occurrence and fate of micropollutants in the Vidy Bay of Lake Geneva, Switzerland. Part II: Micropollutant removal between wastewater and raw drinking water. Environ. Toxicol. Chem. 2010, 29, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and ecotoxicological risk assessment of 14 cytostatic drugs in wastewater. Water Air Soil Pollut. 2014, 225, 1896. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Hernández-Arencibia, P.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Simultaneous and systematic analysis of cytostatic drugs in wastewater samples by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2019, 1110–1111, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.K.; Price, W.E.; Kang, J.; Fujioka, T.; Nghiem, L.D. Ozonation of carbamazepine, diclofenac, sulfamethoxazole and trimethoprim and formation of major oxidation products. Desalination Water Treat. 2016, 57, 29340–29351. [Google Scholar] [CrossRef]
- Alharbi, S.K.; Kang, J.; Nghiem, L.D.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E. Photolysis and UV/H2O2 of diclofenac, sulfamethoxazole, carbamazepine, and trimethoprim: Identification of their major degradation products by ESI–LC–MS and assessment of the toxicity of reaction mixtures. Process Saf. Environ. Protect. 2017, 112, 222–234. [Google Scholar] [CrossRef]
- Kudlek, E. Decomposition of contaminants of emerging concern in advanced oxidation processes. Water 2018, 10, 955. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef]
- Yehya, T.; Favier, L.; Audonnet, F.; Fayad, N.; Bahry, H.; Bahrim, G.E.; Vial, C. Towards a better understanding of the removal of carbamazepine by Ankistrodesmus braunii: Investigation of some key parameters. Appl. Sci. 2020, 10, 8034. [Google Scholar] [CrossRef]
- Ji, C.; Hou, J.; Wang, K.; Zhang, Y.; Chen, V. Biocatalytic degradation of carbamazepine with immobilized laccase-mediator membrane hybrid reactor. J. Membr. Sci. 2016, 502, 11–20. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Banerjee, P.; Das, P.; Bhowal, A.; Majumder, S.K.; Ghosh, P. Erratum: Removal of aqueous carbamazepine using graphene oxide nanoplatelets: Process modelling and optimization. Sustain. Environ. Res. 2020, 30, 25. [Google Scholar] [CrossRef]
- Al-Mashaqbeh, O.A.; Snow, D.D.; Alsafadi, D.A.; Alsalhi, L.Z.; Bartelt-Hunt, S.L. Removal of carbamazepine onto modified zeolitic tuff in different water matrices: Batch and continuous flow experiments. Water 2021, 13, 1084. [Google Scholar] [CrossRef]
- Pereira, C.S.; Kelbert, M.; Daronch, N.A.; Michels, C.; de Oliveira, D.; Soares, H.M. Potential of enzymatic process as an innovative technology to remove anticancer drugs in wastewater. Appl. Microbiol. Biotechnol. 2020, 104, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef] [PubMed]
- Chmelová, D.; Ondrejovič, M. Effect of metal ions on triphenylmethane dye decolorization by laccase from Trametes versicolor. Nova Biotechnol. Chim. 2015, 14, 191–200. [Google Scholar] [CrossRef]
- Legerská, B.; Chmelová, D.; Ondrejovič, M. Degradation of synthetic dyes by laccases—A mini-review. Nova Biotechnol. Chim. 2016, 15, 90–106. [Google Scholar] [CrossRef]
- Legerská, B.; Chmelová, D.; Ondrejovič, M. Azonaphthalene dyes decolorization and detoxification by laccase from Trametes versicolor. Nova Biotechnol. Chim. 2018, 17, 172–180. [Google Scholar] [CrossRef][Green Version]
- Legerská, B.; Chmelová, D.; Ondrejovič, M. Decolourization and detoxification of monoazo dyes by laccase from the white-rot fungus Trametes versicolor. J. Biotechnol. 2018, 285, 84–90. [Google Scholar] [CrossRef]
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J. Clean. Prod. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Chmelová, D.; Ondrejovič, M. Purification and characterization of extracellular laccase produced by Ceriporiopsis subvermispora and decolorization of triphenylmethane dyes. J. Basic Microbiol. 2016, 56, 1173–1182. [Google Scholar] [CrossRef]
- Hazuchová, M.; Chmelová, D.; Ondrejovič, M. The optimization of propagation medium for the increase of laccase production by the white-rot fungus Pleurotus ostreatus. Nova Biotechnol. Chim. 2017, 16, 113–123. [Google Scholar] [CrossRef]
- Pecková, V.; Legerská, B.; Chmelová, D.; Horník, M.; Ondrejovič, M. Comparison of efficiency for monoazo dye removal by different species of white-rot fungi. Int. J. Environ. Sci. Technol. 2020, 18, 21–30. [Google Scholar] [CrossRef]
- Chmelová, D.; Legerská, B.; Kunstová, J.; Ondrejovič, M.; Miertuš, S. The production of laccases by white-rot fungi under solid-state fermentation conditions. World J. Microbiol. Biotechnol. 2022, 38, 21. [Google Scholar] [CrossRef] [PubMed]
- Forootanfar, H.; Faramarzi, M.A. Insights into laccase producing organisms, fermentation states, purification strategies, and biotechnological applications. Biotechnol. Prog. 2015, 31, 1443–1463. [Google Scholar] [CrossRef] [PubMed]
- Ivnitski, D.; Atanassov, P. Electrochemical studies of intramolecular electron transfer in laccase from Trametes versicolor. Electroanalysis 2007, 19, 2307–2313. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.R.; Chang, Y.S. Laccase-mediated oxidation of small organics: Bifunctional roles for versatile applications. Trends Biotechnol. 2013, 31, 335–341. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: A review. Enzym. Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Cambria, M.T.; Minniti, Z.; Librando, V.; Cambria, A. Degradation of polycyclic aromatic hydrocarbons by Rigidoporus lignosus and its laccase in the presence of redox mediators. Appl. Biochem. Biotechnol. 2018, 149, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Basheer, S.; Rashid, N.; Akram, M.S.; Akhtar, M. A highly stable laccase from Bacillus subtilis strain R5: Gene cloning and characterization. Biosci. Biotechnol. Biochem. 2019, 83, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Lončar, N.; Božić, N.; Lopez-Santin, J.; Vujčić, Z. Bacillus amyloliquefaciens laccase—From soil bacteria to recombinant enzyme for wastewater decolorization. Bioresour. Technol. 2013, 147, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, S.; Li, X.; Feng, F.; Lu, L. High-level expression of Bacillus amyloliquefaciens laccase and construction of its chimeric variant with improved stability by domain substitution. Bioprocess Biosyst. Eng. 2020, 43, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rawat, S.; Waseem, M.; Gupta, S.; Lynn, A.; Nitin, M.; Ramchiary, N.; Sharma, K.K. Molecular modeling and simulation studies of recombinant laccase from Yersinia enterocolitica suggests significant role in the biotransformation of non-steroidal anti-inflammatory drugs. Biochem. Biophys. Res. Commun. 2016, 469, 306–312. [Google Scholar] [CrossRef]
- Kumar, A.; Ahlawat, S.; Mohan, H.; Sharma, K.K. Stabilization–destabilization and redox properties of laccases from medicinal mushroom Ganoderma lucidum and human pathogen Yersinia enterocolitica. Int. J. Biol. Macromol. 2021, 167, 369–381. [Google Scholar] [CrossRef]
- Mot, A.C.; Parvu, M.; Damian, G.; Irimie, F.D.; Darula, Z.; Medzihradszky, L.F.; Brem, B.; Silaghi-Dumitrescu, R. A “yellow” laccase with “blue” spectroscopic features, from Sclerotinia sclerotiorum. Process Biochem. 2012, 47, 968–975. [Google Scholar] [CrossRef]
- Hollmann, F.; Gumulya, Y.; Tölle, C.; Liese, A.; Thum, O. Evaluation of the laccase from Myceliophthora thermophila as industrial biocatalyst for polymerization reactions. Macromolecules 2008, 41, 8520–8524. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Lú-Chau, T.A.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Laccase-catalyzed degradation of anti-inflammatories and estrogens. Biochem. Eng. J. 2010, 51, 124–131. [Google Scholar] [CrossRef]
- Lucas, M.F.; Monza, E.; Jorgensen, L.J.; Ernst, H.A.; Piontek, K.; Bjerrum, M.J.; Martinez, A.T.; Camarero, S.; Guallar, V. Simulating substrate recognition and oxidation in laccases: From description to design. J. Chem. Theory Comput. 2017, 13, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.J.; Zhang, B.; Martinez, M.; Kuruba, B.; Brozik, J.; Kang, C.; Zhang, X. Structural studies of Myceliophthora thermophila laccase in the presence of deep eutectic solvents. Enzym. Microb. Technol. 2021, 150, 109890. [Google Scholar] [CrossRef]
- Forootanfar, H.; Faramarzi, M.A.; Shahverdi, A.R.; Yazdi, M.T. Purification and biochemical characterization of extracellular laccase from the ascomycete Paraconiothyrium variabile. Bioresour. Technol. 2011, 102, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Bronikowski, A.; Hagedoorn, P.L.; Koschorreck, K.; Urlacher, V.B. Expression of a new laccase from Moniliophthora roreri at high levels in Pichia pastoris and its potential application in micropollutant degradation. AMB Express 2017, 7, 73. [Google Scholar] [CrossRef]
- Shi, L.; Ma, F.; Han, Y.; Zhang, X.; Yu, H. Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators. J. Hazard. Mater. 2014, 279, 203–211. [Google Scholar] [CrossRef]
- Aydemir, T.; Güler, S. Characterization and immobilization of Trametes versicolor laccase on magnetic chitosan–clay composite beads for phenol removal. Artif. Cells Nanomed. Biotechnol. 2015, 43, 425–432. [Google Scholar] [CrossRef]
- Tran, H.N.; Urase, T.; Kusakabe, O. Biodegradation characteristics of pharmaceutical substances by whole fungal culture Trametes versicolor and its laccase. J. Water Environ. Technol. 2010, 8, 125–140. [Google Scholar] [CrossRef]
- Spina, F.; Cordero, C.; Sgorbini, B.; Schilirò, T.; Gilli, G.; Bicchi, C.; Varese, G.C. Endocrine disrupting chemicals [EDCs] in municipal wastewaters: Effective degradation and detoxification by fungal laccases. Chem. Eng. Trans. 2013, 32, 391–396. [Google Scholar]
- Praveenkumarreddy, Y.; Vimalkumar, K.; Ramaswamy, B.R.; Kumar, V.; Singhal, R.K.; Basu, H.; Gopal, C.M.; Vandana, K.E.; Bhat, K.; Udayashankar, H.N.; et al. Assessment of nonsteroidal anti-inflammatory drugs from selected wastewater treatment plants of Southwestern India. Emerg. Contam. 2021, 7, 43–51. [Google Scholar] [CrossRef]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef]
- Al-sareji, O.J.; Meiczniger, M.; Salman, J.M.; Al-Juboori, R.A.; Hashim, K.S.; Somogyi, V.; Jakab, M. Ketoprofen and aspirin removal by laccase immobilized on date stones. Chemosphere 2023, 311, 137133. [Google Scholar] [CrossRef]
- Papagiannaki, D.; Morgillo, S.; Bocina, G.; Calza, P.; Binetti, R. Occurrence and human health risk assessment of pharmaceuticals and hormones in drinking water sources in the metropolitan area of Turin in Italy. Toxics 2021, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Tyumina, E.; Subbotina, M.; Polygalov, M.; Tyan, S.; Ivshina, I. Ketoprofen as an emerging contaminant: Occurrence, ecotoxicity and [bio]removal. Front. Microbiol. 2023, 14, 1200108. [Google Scholar] [CrossRef] [PubMed]
- Marchlewicz, A.; Guzik, U.; Wojcieszyńska, D. Over-the-counter monocyclic non-steroidal anti-inflammatory drugs in environment–sources, risks, biodegradation. Water Air Soil Pollut. 2015, 226, 355. [Google Scholar] [CrossRef]
- Huppert, N.; Würtele, M.; Hahn, H.H. Determination of the plasticizer N-butylbenzenesulfonamide and the pharmaceutical Ibuprofen in wastewater using solid phase microextraction [SPME]. Fresenius’ J. Anal. Chem. 1998, 362, 529–536. [Google Scholar] [CrossRef]
- Fang, T.-H.; Nan, F.-H.; Chin, T.-S.; Feng, H.-M. The occurrence and distribution of pharmaceutical compounds in the effluents of a major sewage treatment plant in Northern Taiwan and the receiving coastal waters. Mar. Pollut. Bull. 2012, 64, 1435–1444. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, 04087. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Q.; Chen, J.; Lu, Y.; Ren, X.; Lio, Q.; Wen, L.; Mateen, M. Enhanced removal of ibuprofen in water using dynamic dialysis of laccase catalysis. J. Water Process Eng. 2022, 47, 102791. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Mutuku, C.; Gazdag, Z.; Melegh, S. Occurrence of antibiotics and bacterial resistance genes in wastewater: Resistance mechanisms and antimicrobial resistance control approaches. World J. Microbiol. Biotechnol. 2022, 38, 152. [Google Scholar] [CrossRef] [PubMed]
- Sahay, R. Fungal laccase mediator and its biocatalytic potential applications: A review. Biotechnol. Apl. 2020, 38, 1101–1109. [Google Scholar]
- Becker, D.; Giustina, S.V.D.; Rodriquez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.S.; Ku, K.-L.; Lai, H.-T. The implication of mediators for enhancement of laccase oxidation of sulfonamide antibiotics. Bioresour. Technol. 2012, 113, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.K.; Nghiem, L.D.; van de Merwe, J.P.; Leusch, F.D.L.; Asif, M.B.; Hai, F.I.; Price, W.E. Degradation of diclofenac, trimethoprim, carbamazepine, and sulfamethoxazole by laccase from Trametes versicolor: Transformation products and toxicity of treated effluent. Biocatal. Biotransformation 2019, 37, 399–408. [Google Scholar] [CrossRef]
- Zhang, C.; You, S.; Zhang, J.; Qi, W.; Su, R.; He, Z. An effective in-situ method for laccase immobilization: Excellent activity, effective antibiotic removal rate and low potential ecological risk for degradation products. Bioresour. Technol. 2020, 308, 123271. [Google Scholar] [CrossRef]
- Zou, M.; Tian, W.; Chu, M.; Lu, Z.; Liu, B.; Xu, D. Magnetically separable laccase-biochar composite enable highly efficient adsorption-degradation of quinolone antibiotics: Immobilization, removal, performance and mechanisms. Sci. Total Environ. 2023, 879, 163057. [Google Scholar] [CrossRef]
- Jafari-Nodoushan, H.; Fazeli, M.R.; Faramarzi, M.A.; Samadi, N. Hierarchically-structured laccase@Ni3[PO4]2 hybrid nanoflowers for antibiotic degradation: Application in real wastewater effluent and toxicity evaluation. Int. J. Biol. Macromol. 2023, 234, 123574. [Google Scholar] [CrossRef]
- Han, Z.; Wang, H.; Zheng, J.; Wang, S.; Yu, S.; Lu, L. Ultrafast synthesis of laccase-copper phosphate hybrid nanoflowers for efficient degradation of tetracycline antibiotics. Environ. Res. 2023, 216, 114690. [Google Scholar] [CrossRef] [PubMed]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, F.; Zhang, B. Elimination of tetracyclines in seawater by laccase-mediator system. Chemosphere 2023, 333, 138916. [Google Scholar] [CrossRef] [PubMed]
- Van den Brandhof, E.-J.; Montforts, M. Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol. Environ. Saf. 2010, 73, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, M.L.; Lesaoana, M.; Kotze, I.; Richards, H.L. Zeolitic imidazolate frameworks as effective crystalline supports for Aspergillus-based laccase immobilization for the biocatalytic degradation of carbamazepine. Chemosphere 2023, 311, 137142. [Google Scholar] [CrossRef]
- Hata, T.; Shintate, H.; Kawai, S.; Okamura, H.; Nishida, T. Elimination of carbamazepine by repeated treatment with laccase in the presence of 1-hydroxybenzotriazole. J. Hazard. Mater. 2010, 181, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Jelic, A.; Cruz-Morató, C.; Marco-Urrea, E.; Sarrà, M.; Perez, S.; Vicent, T.; Petrović, M.; Barcelo, D. Degradation of carbamazepine by Trametes versicolor in an air pulsed fluidized bed bioreactor and identification of intermediates. Water Res. 2012, 46, 955–964. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Guzik, U. Naproxen in the environment: Its occurrence, toxicity to non-target organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgola-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C 2019, 103, 109789. [Google Scholar] [CrossRef]
- Apriceno, A.; Astolfi, M.L.; Girelli, A.M.; Scuto, F.R. A new laccase-mediator system facing the biodegradation challenge: Insight into NSAIDs removal. Chemosphere 2019, 215, 535–542. [Google Scholar] [CrossRef]
- Coman, C.; Hadade, N.; Pesek, S.; Silaghi-Dumitrescu, R.; Mot, A.C. Removal and degradation of sodium diclofenac via radical-based using S. sclerotiorum laccase. J. Inorg. Biochem. 2023, 249, 112400. [Google Scholar] [CrossRef]
- Kelbert, M.; Pereira, C.S.; Daronch, N.A.; Cesca, K.; Michels, C.; de Oliveira, D.; Soares, H.M. Laccase as an efficacious approach to remove anticancer drugs: A study of doxorubicin degradation, kinetic parameters, and toxicity assessment. J. Hazard. Mater. 2021, 409, 124520. [Google Scholar] [CrossRef]
- Jinga, L.I.; Tudose, M.; Ionita, P. Laccase-TEMPO as an efficient system for doxorubicin removal from wastewaters. Int. J. Environ. Res. Public Health 2022, 19, 6645. [Google Scholar] [CrossRef]
- Pereira, S.C.; Kelbert, M.; Daronch, N.A.; Cordeiro, A.P.; Cesca, K.; Michels, C.; de Oliveira, D.; Soares, H.M. Laccase-assisted degradation of anticancer drug etoposide: By-products and cytotoxicity. Bioenergy Res. 2023; in press. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Perez-Trujillo, M.; Vicent, T.; Caminal, G. Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 2009, 74, 765–772. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.F.; Kang, J.G.; Leusch, F.D.L.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters, industrial chemicals and pesticides by Trametes versicolor: Role of biosorption and biodegradation. Int. Biodeterior. Biodegrad. 2014, 88, 169–175. [Google Scholar] [CrossRef]
- Negreira, N.; de Alda, M.L.; Barceló, D. Cytostatic drugs and metabolites in municipal and hospital wastewaters in Spain: Filtration, occurrence, and environmental risk. Sci. Total Environ. 2014, 497–498, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Shao, B.; Zhang, J.; Li, K. A preliminary study on the occurrence of cytostatic drugs in hospital effluents in Beijing, China. Bull. Environ. Contam. Toxicol. 2010, 84, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Perko, S.; Žigon, D.; Heath, E. Fluorouracil in the environment: Analysis, occurrence, degradation and transformation. J. Chromatogr. A 2013, 1290, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Development of a UPLC-MS/MS method for the determination of ten anticancer drugs in hospital and urban wastewaters, and its application for the screening of human metabolites assisted by information-dependent acquisition tool [IDA] in sewage samples. Anal. Bioanal. Chem. 2013, 405, 5937–5952. [Google Scholar] [CrossRef] [PubMed]
- Zounková, R.; Odráška, P.; Doležalová, L.; Hilscherová, K.; Maršálek, B.; Bláha, L. Ecotoxicity and genotoxicity assessment of cytostatic pharmaceuticals. Environ. Toxicol. Chem. 2007, 26, 2208–2214. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, J.; Olsen, P.; Bach, K.; Barlebo, H.C.; Ingerslev, F.; Hansen, M.; Sørensen, B.H. Leaching of estrogenic hormones from manure-treated structure soils. Environ. Sci. Technol. 2007, 41, 3911–3917. [Google Scholar] [PubMed]
- Fernández, L.; Louvado, A.; Esteves, V.I.; Gomes, N.C.; Almeida, A.; Cunha, Â. Biodegradation of 17β-estradiol by bacteria isolated from deep sea sediments in aerobic and anaerobic media. J. Hazard. Mater. 2017, 323, 359–366. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Persistence and impact of steroidal estrogens on the environment and their laccase-assisted removal. Sci. Total Environ. 2019, 690, 447–459. [Google Scholar] [CrossRef]
- Zofair, S.F.F.; Hasmi, M.A.; Faridi, I.H.; Rasool, F.; Magani, S.K.J.; Khan, M.A.; Younus, H. Immobilization of laccase on poly-L-lysine modified silver nanoparticles formed by green synthesis for enhanced stability, suppressed estrogenic activity of 17β-estradiol, biocompatibility and anti-cancer action: An in vitro and in silico study. J. Mol. Liq. 2023, 392, 123502. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Strybel, U.; Marczak, L.; Nguyen, L.N.; Oleskowicz-Popiel, P.; Jesionowski, T. Bioremoval of estrogens by laccase immobilized onto polyacrylonitrile/polyethersulfone material: Effect of inhibitors and mediators, process characterization and catalytic pathways determination. J. Hazard. Mater. 2022, 432, 128688. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Kang, J.; Leusch, F.D.L.; Roddick, F.; van de Merwe, J.P.; Magram, S.F.; Nghiem, L.D. Degradation of a broad spectrum of trace organic contaminants by an enzymatic membrane reactor: Complementary role of membrane retention and enzymatic degradation. Int. Biodeterm. Biodegrad. 2015, 99, 115–122. [Google Scholar] [CrossRef]
- Beck, S.; Berry, E.; Duke, S.; Milliken, A.; Patterson, H.; Prewett, D.L.; Rae, T.C.; Sridhar, V.; Wendland, N.; Gregory, B.W.; et al. Characterization of Trametes versicolor laccase-catalyzed degradation of estrogenic pollutants: Substrate limitation and product identification. Int. Biodeterior. Biodegrad. 2018, 127, 146–159. [Google Scholar] [CrossRef]
- Sun, K.; Hong, D.; Liu, J.; Latif, A.; Li, S.; Chu, G.; Qin, W.; Si, Y. Trametes versicolor laccase-assisted oxidative coupling of estrogens: Conversion kinetics, linking mechanisms, and practical applications in water purification. Sci. Total Environ. 2021, 782, 146917. [Google Scholar] [CrossRef]
- Kosjek, T.; Perko, S.; Zupanc, M.; Zanoski Hren, M.; Landeka Dragicevic, T.; Zigon, D.; Kompare, B.; Heath, E. Environmental occurrence, fate and transformation of benzodiazepines in water treatment. Water Res. 2012, 46, 355–368. [Google Scholar] [CrossRef]
- Ternes, T.; Bonerz, M.; Schmidt, T. Determination of neutral pharmaceuticals in wastewater and rivers by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. A 2001, 938, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Ostadhadi-Dehkordi, S.; Tabatabaei-Sameni, M.; Forootanfar, H.; Kolahdouz, S.; Ghazi-Khansari, M.; Faramarzi, M.A. Degradation of some benzodiazepines by a laccase-mediated system in aqueous solution. Bioresour. Technol. 2012, 125, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Cabana, H. Towards high potential magnetic biocatalysts for on-demand elimination of pharmaceuticals. Bioresour. Technol. 2016, 200, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Khan, I.A.; Foran, C.M.; Schlenk, D. Determination of beta-adrenergic receptor blocking pharmaceuticals in United States wastewater effluent. Environ. Pollut. 2003, 121, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, H.; Zhang, Y.; Alder, A.C. Occurrence and enantiomer profiles of beta-blockers in wastewater and a receiving water body and adjacent soil in Tianjin, China. Sci. Total Environ. 2019, 650, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Cleuvers, M. Initial risk assessment for three beta-blockers found in the aquatic environment. Chemosphere 2005, 59, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.K.; Ramirez, A.J.; Mottaleb, M.; Chambliss, C.K.; Brooks, B.W. Enantiospecific toxicity of the beta-blocker propranolol to Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2006, 25, 1780–1786. [Google Scholar] [CrossRef]
- Sun, L.; Liu, F.; Chen, H.; Wang, S.; Lin, X.; Chi, J.; Zhu, Q.; Fu, Z. Transcriptional responses in adult zebrafish (Danio rerio) exposed to propranolol and metoprolol. Ecotoxicology 2015, 24, 1352–1361. [Google Scholar] [CrossRef]
- Feng, Y.; Shen, M.; Wang, Z.; Liu, G. Transformation of atenolol by a laccase-mediator system: Efficiencies, effect of water constituents, and transformation pathways. Ecotoxicol. Environ. Saf. 2019, 183, 109555. [Google Scholar] [CrossRef]
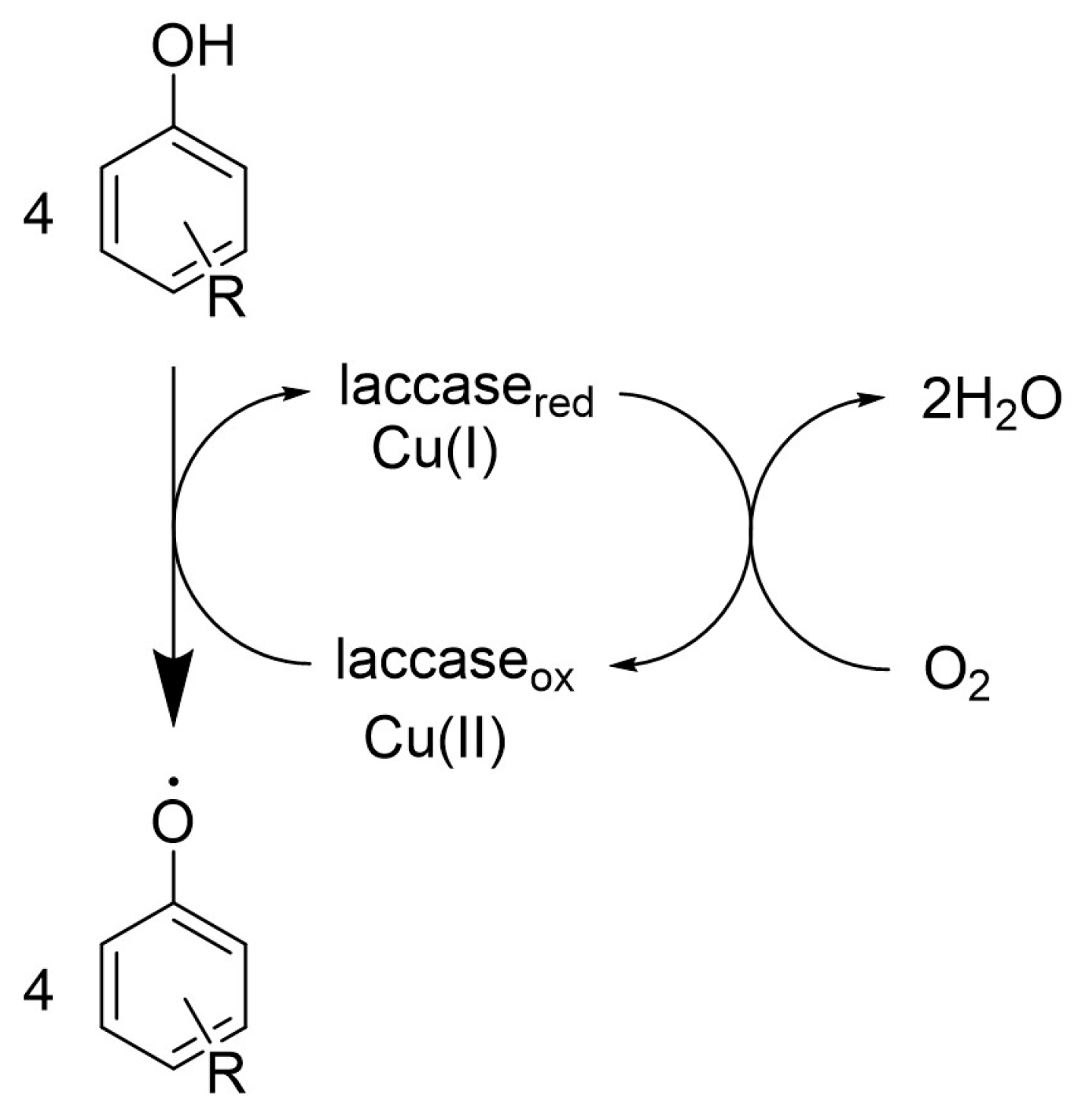

| Producer | Redox Potential (V vs. NHE) | Biochemical Properties | Ref. | ||
|---|---|---|---|---|---|
| pH | Temperature (°C) | Kinetic Parameters | |||
| Bacillus subtilis [B] | 0.440 | 7.0 | 55 | Km = 2070 μM | [36,37] |
| Vmax = 6500 U/mL | |||||
| (SYR) | |||||
| Bacillus amyloliquefaciens [B] | n.d. | 4.0 | 65 | Km = 436.8 μM | [38,39] |
| (ABTS) | |||||
| Yersinia enterocolitica a [B] | 0.27–0.432 | 9.0 | 70 | Km = 2070 μM | [40,41] |
| Vmax = 6500 U/mL | |||||
| (guaiacol) | |||||
| Sclerotinia sclerotiorum [F] | n.d. | 4.0 | 60–70 | Km = 85.8 μM | [42] |
| Vmax = 18.64 U/mL | |||||
| (ABTS) | |||||
| Myceliophthora Thermophila b [F] | 0.460 | 4.0 | 50 | Km = 52.151 μM | [43,44,45,46] |
| Vmax = 11.493 mU | |||||
| (ABTS) | |||||
| Paraconiothyrium variabile [F] | n.d. | 4.8 | 50 | Km = 203 μM | [47] |
| Vmax = 40 U/mg | |||||
| [ABTS] | |||||
| Moniliophthora roreri c [F] | 0.58 | 4.0 | n.d. | Km = 24.13 μM | [48] |
| (ABTS) | |||||
| Echinodontium taxodii [F] | n.d. | 3.0 | 60 | Km = 41.4 μM | [49] |
| Vmax = 5.9 U/mL | |||||
| (ABTS) | |||||
| Trametes versicolor [F] | 0.990 | 4.0 | 40 | Km = 297 μM | [29,50] |
| Vmax = 26.96 U/mg | |||||
| (ABTS) | |||||
| Drug | Concentration [mg/L] | Laccase Producer | Laccase Activity [U/L] | Enzyme Reaction | Degradation/Transformation Efficiency * | Ref. |
|---|---|---|---|---|---|---|
| Aspirin | 5.0 | Yersinia enterocolitica | 100 | 45 °C, pH 9.0, 24 h | 100% (BT) | [40] |
| 25.0 | Trametes versicolor | 40 | 35 °C, pH 4.0, 6 h | 72% (n.s.) | [55] | |
| Ketoprofen | 25.0 | 40 | 35 °C, pH 4.0, 6 h | 70% (n.s.) | ||
| Ibuprofen | 515.7 | 29 | 40 °C, pH 7.0, 8 h | 76% (BT) | [62] |
| Drug | Concentration [mg/L] | Laccase Producer | Laccase Activity [U/L] | Concentration of Redox Mediator [mM] | Enzyme Reaction | Degradation/Transformation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Sulfamonomethoxine | 50 | Perenniporia TFRI 707 | 600 | ABTS [1.0] | 30 °C, pH 4.0, 0.5 h | 100% (BT) | [67] |
| VA [1.0] | 100% (BT) | ||||||
| 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 5.4% (n.s.) | [66] | |
| SYR [1.0] | 96.1% (n.s.) | ||||||
| Sulfadimethoxine | 50 | Perenniporia TFRI 707 | 600 | ABTS [1.0] | 30 °C, pH 4.0, 0.5 h | 100% (BT) | [67] |
| VA [1.0] | 100% (BT) | ||||||
| Sulfadiazine | 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 11.2% (n.s.) | [66] |
| SYR [1.0] | 99.7% (n.s.) | ||||||
| 50 | Echinodontium taxodii | 200 | SA [1.0] | 30 °C, pH 5.0, 0.5 h | 100% (BT) | [49] | |
| Sulfamethoxazole | 50 | Echinodontium taxodii | 200 | SA [1.0] | 30 °C, pH 5.0, 0.5 h | 100% (BT) | [49] |
| 5.0 | Trametes versicolor | 430–460 | - | 25 °C, pH 6.8–6.9, 8 h | 56% (n.s.) | [68] | |
| 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 14.2% (n.s.) | [66] | |
| SYR [1.0] | 97.2% (n.s.) | ||||||
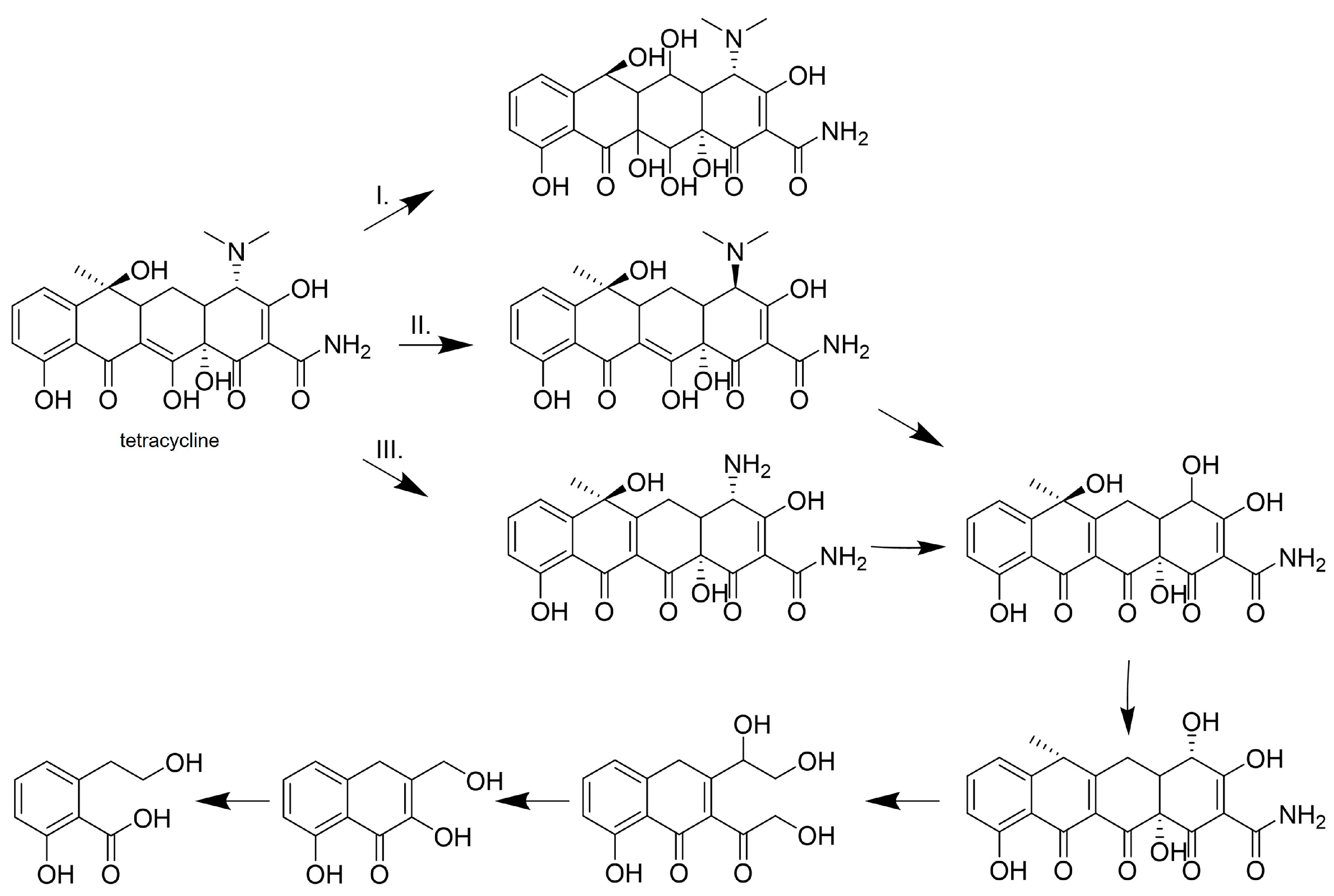
| Tetracycline | 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 26.0% (n.s.) | [66] |
| SYR [0.01] | 85.2% (n.s.) | ||||||
| 100 | Bacillus subtilis | 34.3 | - | 25 °C, pH 5.5, 2 h | 100% (D) | [69] | |
| 100 | Bacillus amyloliquefaciens | 2000 | - | 30 °C, pH 7.0, 2 h | 86.1% (D) | [72] | |
| 0.002 | Myceliophthora thermophila | 200 | SA [0.001] | 15 °C, pH 8.0, 2 h | 100% (D) | [74] | |
| Doxycycline | 100 | Bacillus amyloliquefaciens | 500 | - | 30 °C, pH 7.0, 2 h | 96.5% (D) | [72] |
| 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 30.4% (n.s.) | [66] | |
| SYR [0.01] | 89.1% (n.s.) | ||||||
| Tigecycline | 100 | Bacillus amyloliquefaciens | 500 | - | 30 °C, pH 7.0, 2 h | 81.0% (D) | [72] |
| Norfloxacin | 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 58.1% (n.s.) | [66] |
| SYR [1.0] | 82.4% (n.s.) | ||||||
| Enrofloxacin | 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 50.1% (n.s.) | [66] |
| SYR [1.0] | 76.6% (n.s.) | ||||||
| Ciprofloxacin | 10 | Trametes versicolor | n.s. | p-CA [3.0] | 45 °C, pH 6.8, 6 h | 57% (n.s.) | [71] |
| HBT [3.0] | 81% (n.s.) | ||||||
| Amoxicillin | 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 96.6% (n.s.) | [66] |
| SYR [1.0] | 94.7% (n.s.) | ||||||
| Ampicillin | 0.01 | Trametes versicolor | n.s. | - | 25 °C, pH 7.0, 24 h | 88.6% (n.s.) | [66] |
| SYR [1.0] | 99.9% (n.s.) | ||||||
| 100 | Bacillus subtilis | 34.3 | - | 25 °C, pH 5.5, 2 h | 100% (D) | [69] | |
| Trimethoprim | 5.0 | Trametes versicolor | 430–460 | - | 25 °C, pH 6.8–6.9, 24 h | 95% (n.s.) | [68] |
| 0.01 | n.s. | - | 25 °C, pH 7.0, 24 h | 26.6% (n.s.) | [66] | ||
| SYR [1.0] | 66.8% (n.s.) |
| Drug | Concentration [mg/L] | Laccase Producer | Laccase Activity [U/L] | Concentration of Redox Mediator [mM] | Enzyme Reaction | Degradation/Transformation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
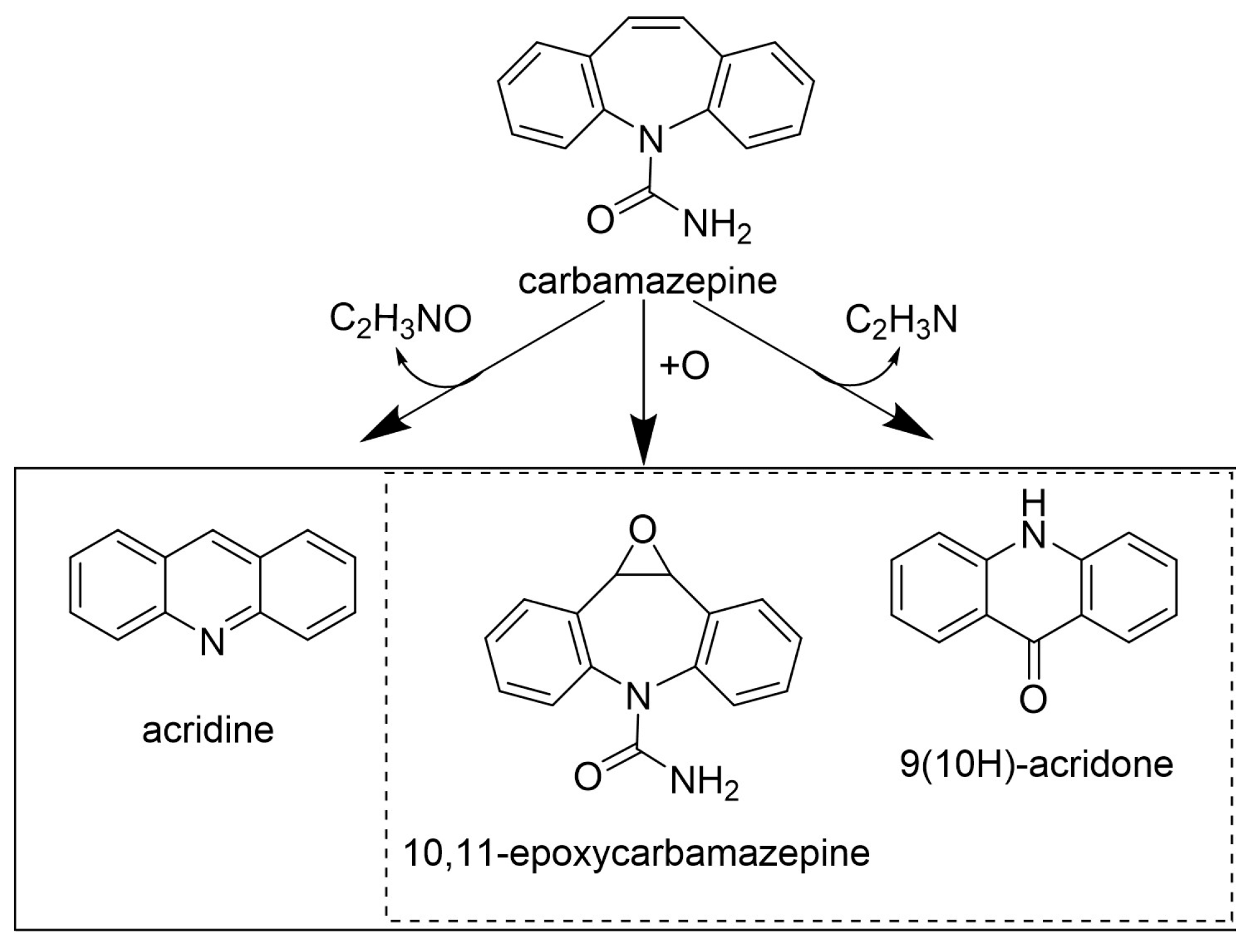
| Carbamazepine | 5.0 | Myceliophthora thermophila | n.s. | - | LT, pH 7.0, 24 h | 46% (BT) | [76] |
| 5.0 | Trametes versicolor | 430–460 | - | 25 °C, pH 6.8–6.9, 48 h | 82% (BT) | [68] | |
| 5.0 | 500 | - | LT, pH 7.0, 96 h | 5% (BT) | [14] | ||
| p-CA [2.0] | 60% (BT) | ||||||
| 4.7 | 600 | - | 30 °C, pH 4.5, 48 h | 39% (BT) | [77] | ||
| HBT [0.2] | 60% (BT) |
| Drug | Concentration [mg/L] | Laccase Producer | Laccase Activity [U/L] | Concentration of Redox Mediator [mM] | Enzyme Reaction | Degradation/Transformation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
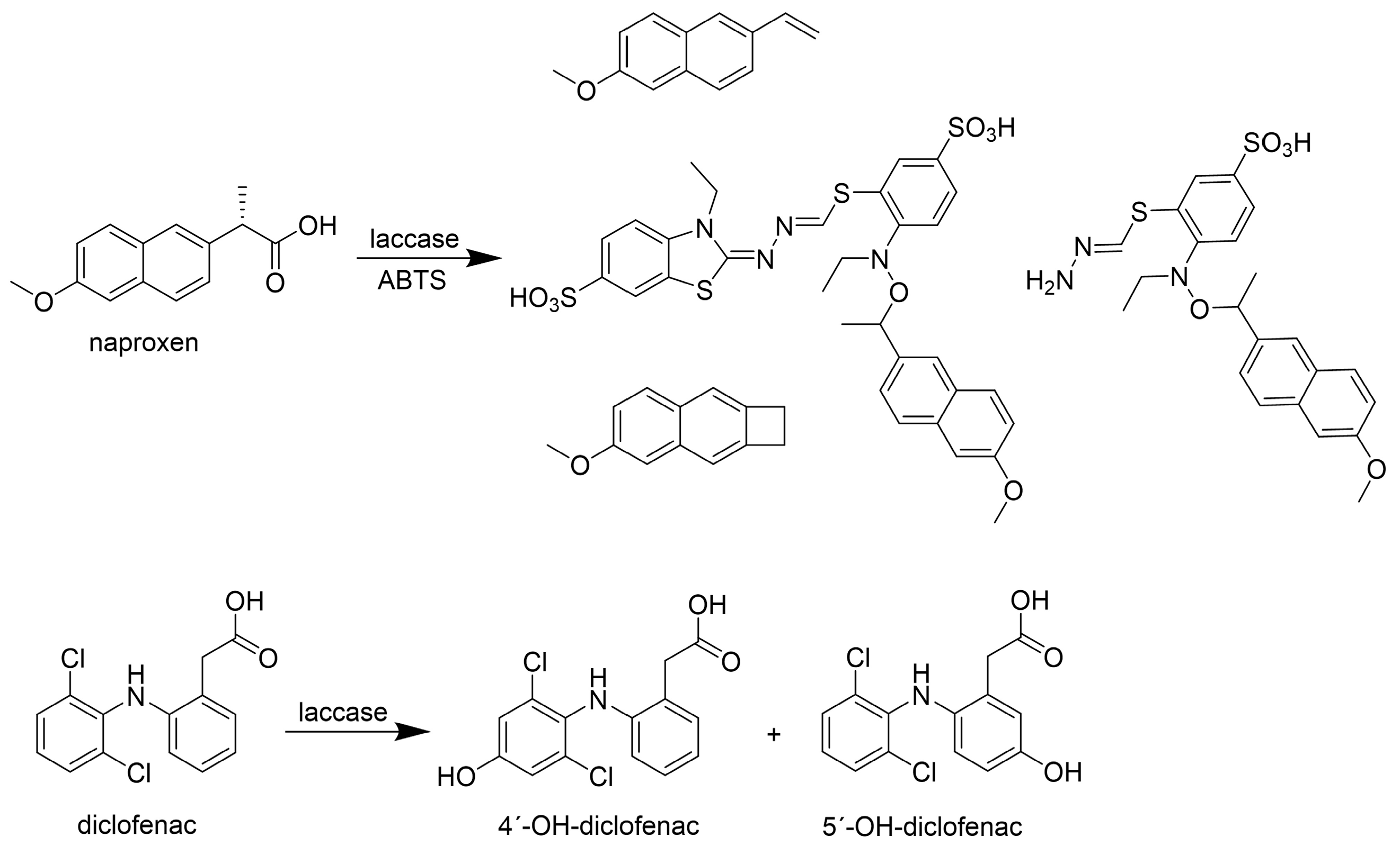
| Naproxen | 5.0 | Myceliophthora thermophila | 2000 | - | LT, pH 4.0, 24 h | - | [44] |
| HBT [1.0] | 68% (n.s.) | ||||||
| VA [1.0] | 36% (n.s.) | ||||||
| 1.0 | Trametes versicolor | 250 | ABTS [0.1] | 25 °C, pH 5.0, 24 h | 95% (D) | [80] | |
| Diclofenac | 29.6 | Sclerotinia sclerotiorum | 456 | - | LT, pH 5.0, 30 h | 96% (BT) | [82] |
| 5.0 | Yersinia enterocolitica | 100 | - | 45 °C, pH 9.0, 24 h | 100% (BT) | [40] | |
| 5.0 | Myceliophthora thermophila | 2000 | - | LT, pH 4.0, 8 h | 100% (n.s.) | [44] | |
| HBT [1.0] | LT, pH 7.0, 1 h | 100% (n.s.) | |||||
| 30.0 | Moniliophthora roreri | 20,000 | - | LT, pH 7.0, 20 h | 58% (n.s.) | [48] | |
| 4.6 | Trametes versicolor | 10 | - | LT, pH 3.0, 4 h | 90% (D) | [81] | |
| 5.0 | 430–460 | - | 25 °C, pH 6.8–6.9, 8 h | 100% (D) | [68] | ||
| 1.0 | 250 | - | 25 °C, pH 5.0, 24 h | 95% (D) | [80] | ||
| Naproxen | 5.0 | Myceliophthora thermophila | 2000 | - | LT, pH 4.0, 24 h | - | [44] |
| HBT [1.0] | 68% (n.s.) | ||||||
| VA [1.0] | 36% (n.s.) | ||||||
| Doxorubicin | 0.25 | Trametes versicolor | 900 | - | 30 °C, pH 7.0, 2 h | 100% (BT) | [83] |
| 25 | 210 | TEMPO [250] | n.s., pH 7.0, 24 h | 100% (n.s.) | [84] | ||
| 50 | 100% (n.s.) | ||||||
| 75 | 65% (n.s.) | ||||||
| Etoposide | 0.5 | Trametes versicolor | 1100 | - | 30 °C, pH 6.0, 1 h | 100% (BT) | [85] |
| 55 | 68% (BT) |
| Drug | Concentration [mg/L] | Laccase Producer | Laccase Activity [U/L] | Concentration of Redox Mediator [mM] | Enzyme Reaction | Degradation/Transformation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Estrone | 5.0 | Myceliophthora thermophila | 2000 | - | LT, pH 4.0, 24 h | 65% (n.s.) | [44] |
| VA [1.0] | LT, pH 4.0, 8 h | 100% (n.s.) | |||||
| 0.1 | 180 | - | 25 °C, pH 6.8 ± 0.2, 24 h | 94.8% (n.s.) | [98] | ||
| 27.0 | Moniliophthora roreri | 20,000 | - | LT, pH 7.0, 20 h | 100% (n.s.) | [48] | |
| 17β-estradiol | 5.0 | Myceliophthora thermophila | 2000 | - | LT, pH 4.0, 3 h | 100% (n.s.) | [44] |
| 0.1 | 180 | - | 25 °C, pH 6.8 ± 0.2, 24 h | 98.5% (n.s.) | [98] | ||
| 27.4 | Moniliophthora roreri | 20,000 | - | LT, pH 7.0, 0.5 h | 100% (n.s.) | [48] | |
| 1.0 | Trametes versicolor | 1500 | - | 25 °C, pH 5.0, 24 h | 92% (D) | [97] | |
| 27.4 | Trametes versicolor | 1000 | - | 25 °C, pH 5.0, 5 h | 95.3% (n.s.) | [96] | |
| Estriol | 0.1 | Myceliophthora thermophila | 180 | - | 25 °C, pH 6.8 ± 0.2, 24 h | 98.5% (n.s.) | [98] |
| 28.8 | Moniliophthora roreri | 20,000 | - | LT, pH 7.0, 0.5 h | 100% (n.s.) | [48] | |
| Ethinylestradiol | 0.1 | Myceliophthora thermophila | 180 | - | 25 °C, pH 6.8 ± 0.2, 24 h | 98.2% (n.s.) | [98] |
| 5.0 | 2000 | - | LT, pH 4.0, 5 h | 100% (n.s.) | [44] | ||
| 5.0 | Trametes versicolor | 1500 | - | 25 °C, pH 5.0, 24 h | 100% (D) | [97] | |
| 29.4 | Moniliophthora roreri | 20,000 | - | LT, pH 7.0, 0.5 h | 100% (n.s.) | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmelová, D.; Ondrejovič, M.; Miertuš, S. Laccases as Effective Tools in the Removal of Pharmaceutical Products from Aquatic Systems. Life 2024, 14, 230. https://doi.org/10.3390/life14020230
Chmelová D, Ondrejovič M, Miertuš S. Laccases as Effective Tools in the Removal of Pharmaceutical Products from Aquatic Systems. Life. 2024; 14(2):230. https://doi.org/10.3390/life14020230
Chicago/Turabian StyleChmelová, Daniela, Miroslav Ondrejovič, and Stanislav Miertuš. 2024. "Laccases as Effective Tools in the Removal of Pharmaceutical Products from Aquatic Systems" Life 14, no. 2: 230. https://doi.org/10.3390/life14020230
APA StyleChmelová, D., Ondrejovič, M., & Miertuš, S. (2024). Laccases as Effective Tools in the Removal of Pharmaceutical Products from Aquatic Systems. Life, 14(2), 230. https://doi.org/10.3390/life14020230








