Different Lengths of Diet Supplementation with 10% Flaxseed Alter the Hormonal Profile and the Follicular Fluid Fatty Acid Content of Fattening Gilts
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Diets
2.2. Blood Collection and Necropsy
2.3. Preparation and Processing of Ovarian Fragment Culture
2.4. Immunoassays
2.5. Follicular Fluid Collection and Gas Chromatography
2.6. Statistical Analyses
3. Results
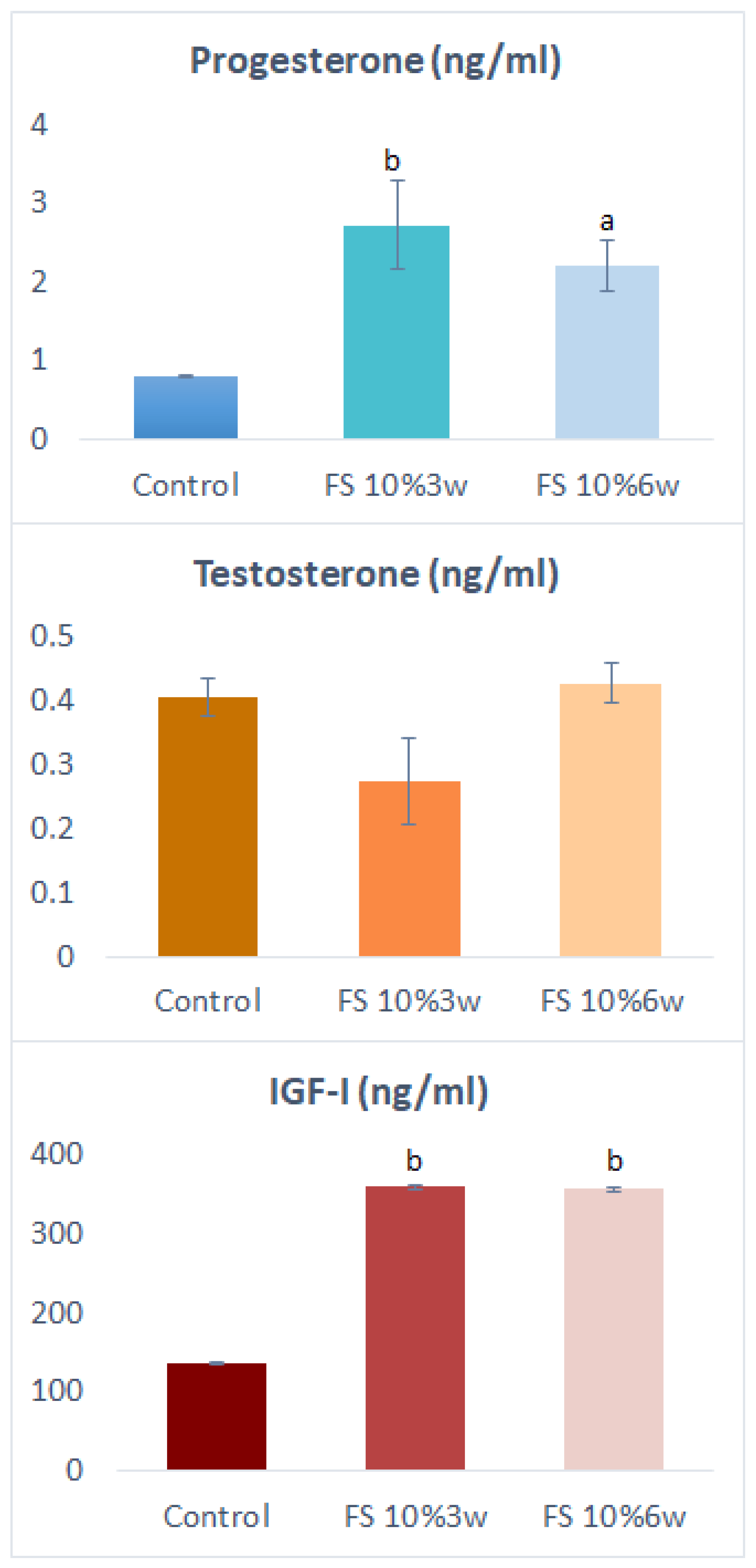
3.1. Serum Hormones
3.2. Culture Media Hormones
3.2.1. Hormone Release without Any Regulatory Hormone Addition
3.2.2. Hormone Release with the Addition of FSH
3.2.3. Hormone Release with the Addition of LH
3.2.4. Hormone Release with the Addition of IGF-I
3.3. Follicular Fluid Fatty Acids
3.3.1. Fatty Acids in 3 mm Follicles
3.3.2. Fatty Acids in 5 mm Follicles
4. Discussion
4.1. Can the Length of a Flaxseed Supplementing Diet Affect the Levels of Serum Hormones?
4.2. Can the Length of a Flaxseed Supplementation Period Modify the Hormone Secretion by the Ovaries?
4.3. Can Gonadotropins and IGF-I Modify the Release of Ovarian Hormones?
4.4. Can Supplementary Flaxseed Modify the Release of Ovarian Hormones in Response to Gonadotropins and IGF-I?
4.5. Can the Length of a Flaxseed Supplementary Period Modify the Fatty Acid Composition of Follicular Fluid in Developing Follicles?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrejčáková, Z.; Sopková, D.; Vlčková, R.; Kulichová, L.; Gancarčíková, S.; Almášiová, V.; Holovská, K.; Petrilla, V.; Krešáková, L. Synbiotics suppress the release of lactate dehydrogenase promote non-specific immunity and integrity of jejunum mucosa in piglets. Anim. Sci. J. 2016, 87, 1157–1166. [Google Scholar] [CrossRef]
- Sopková, D.; Hertelyová, Z.; Andrejčáková, Z.; Vlčková, R.; Gancarčíková, S.; Petrilla, V.; Ondrašovičová, S.; Krešáková, L. The application of probiotics and flaxseed promotes metabolism of n-3 polyunsaturated fatty acids in pigs. J. Appl. Anim. Res. 2017, 45, 93–98. [Google Scholar] [CrossRef]
- Andrejčáková, Z.; Vlčková, R.; Sopková, D.; Kozioł, K.; Koziorowski, M.; Fabián, D.; Šefčíková, Z.; Holovská, K.; Almášiová, V.; Sirotkin, A.V. Dietary flaxseed’s protective effects on body tissues of mice after oral exposure to xylene. Saudi J. Biol. Sci. 2021, 28, 3789–3798. [Google Scholar] [CrossRef]
- Rizvi, Q.; Shams, R.; Pandey, V.K.; Dar, A.H.; Tripathi, A.; Singh, R. A descriptive review on nutraceutical constituents, detoxification methods and potential health benefits of flaxseed. Appl. Food Res. 2022, 2, 100239. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Influence of Flaxseed (Linum usitatissimum) on Female Reproduction. Planta Med. 2023, 89, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C.; Chen, J.; Thompson, L.U. Dose, timing, and duration of flaxseed exposure affect reproductive indices and sex hormone levels in rats. Toxicol. Environ. Health A 1999, 56, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Haran, W.H.; Al-Saeed, M.H.; Al-Masoudi, E.A. Study the effect of flax lignan extract of Linum Usitatissimum and conjugated estrogen on physiological parameters in female rats. Bras. J. Vet. Res. 2017, 16, 20–49. [Google Scholar] [CrossRef]
- Vlčková, R.; Andrejčáková, Z.; Sopková, D.; Kozioł, K.; Hertelyová, Z.; Koziorowska, A.; Gancarčíková, S. Effects of supplemental flaxseed on the ovarian and uterine functions of adult cycling mice. Gen. Physiol. Biophys. 2022, 41, 205–219. [Google Scholar] [CrossRef]
- Vlčková, R.; Sopková, D.; Andrejčáková, Z.; Lecová, M.; Fabian, D.; Šefčíková, Z.; Seidavi, A.; Sirotkin, A.V. Dietary supplementation of flaxseed (Linum usitatissimum L.) alters ovarian functions of xylene-exposed mice. Life 2022, 12, 1152. [Google Scholar] [CrossRef]
- Vlčková, R.; Andrejčáková, Z.; Sopková, D.; Hertelyová, Z.; Kozioł, K.; Koziorowski, M.; Gancarčíková, S. Supplemental flaxseed modulates ovarian functions of weanling gilts via the action of selected fatty acids. Anim. Reprod. Sci. 2018, 193, 171–181. [Google Scholar] [CrossRef]
- Nugroho, P.; Wiryawan, K.G.; Astuti, D.A.; Manalu, W. Stimulation of follicle growth and development during estrus in Ettawa Grade does fed a flushing supplement of different polyunsaturated fatty acids. Vet. World 2021, 14, 11–22. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Tarko, A.; Fabova, Z.; Valocky, I.; Alwasel, S.; Kotwica, J.; Harrath, A.H. Can flaxseed, chia or puncture vine affect mare ovarian cell functions and prevent the toxic effect of the environmental contaminant toluene? Theriogenology 2023, 208, 178–184. [Google Scholar] [CrossRef]
- Fabová, Z.; Tarko, A.; Mlynček, M.; Kotwica, J.; Sirotkin, A.V. Flaxseed can prevent adverse effects of toluene on human ovarian cell functions. Curr. Bioact. Comp. 2023, 19, 29–38. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Bender, K.; Walsh, S.; Evans, A.C.O.; Fair, T.; Brennan, L. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction 2010, 139, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, S.; Liu, L.; Cai, S.; Ye, Q.; Xue, B.; Wang, X.; Zhang, S.; Chen, F.; Cai, C.; et al. Role of functional fatty acids in modulation of reproductive potential in livestock. J. Anim. Sci. Biotechnol. 2023, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, L.L.; Chura, L.R.; Liang, X.; Lane, M.; Norman, R.J.; Robker, R.L. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 2012, 97, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Liu, F.C.; Hsieh, J.S.; Chen, C.H.; Hsiao, S.Y.; Lin, C.S. Matrix metalloproteinase 2 level in human follicular fluid is a reliable marker of human oocyte maturation in in vitro fertilization and intracytoplasmic sperm injection cycles. Reprod. Biol. Endocrin. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baddela, V.S.; Michaelis, M.; Sharma, A.; Plinski, C.H.; Viergutz, T.; Venselow, J. Estradiol production of granulosa cells is unaffected by the physiological mix of nonesterified fatty acids in follicular fluid. JBC 2022, 298, 102477. [Google Scholar] [CrossRef]
- Bartkovský, M.; Sopková, D.; Andrejčáková, Z.; Vlčková, R.; Semjon, B.; Marcinčák, S.; Bujňák, L.; Pospiech, M.; Nagy, J.; Popelka, P.; et al. Effect of concentration of flaxseed (Linum usitatissimum) and duration of administration on fatty acid profile, and oxidative stability of pork meat. Animals 2022, 12, 1087. [Google Scholar] [CrossRef] [PubMed]
- Vlčková, R.; Sopková, D.; Andrejčáková, Z.; Valocký, I.; Kádasi, A.; Harrath, A.H.; Petrilla, V.; Sirotkin, A.V. Dietary supplementation of yucca (Yucca schidigera) affects ovine ovarian functions. Theriogenology 2017, 15, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane, S.G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Jelodar, G.; Masoomi, S.; Rahmanifar, F. Hydroalcoholic extract of flaxseed improves polycystic ovary syndrome in a rat model. Iran J. Basic Med. Sci. 2018, 21, 645–650. [Google Scholar] [CrossRef]
- Komal, F.; Khan, M.K.; Imran, M.; Ahmad, M.H.; Anwar, H.; Ashfaq, U.A.; Ahmad, N.; Masroor, A.; Ahmad, R.S.; Nadeem, M.; et al. Impact of different omega-3 fatty acid sources on lipid, hormonal, blood glucose, weight gain and histopathological damages profile in PCOS rat model. J. Transl. Med. 2020, 18, 349. [Google Scholar] [CrossRef]
- Mehraban, M.; Jelodar, G.; Rahmanifar, F. A combination of spearmint and flaxseed extract improved endocrine and histomorphology of ovary in experimental PCOS. J. Ovarian Res. 2020, 13, 32. [Google Scholar] [CrossRef]
- Riaz, S.; Zahid, M.; Rehman, R.U.; Aftab, B.; Imran, M.; Shahrukh, S.I. Effect of Phytoestrogens in the Treatment of Polycystic Ovary Syndrome in Rat model. J. Food Nutr. Res. 2022, 10, 518–525. [Google Scholar] [CrossRef]
- Jahani-Moghadam, M.; Mahjoubi, E.; Dirandeh, E. Effect of linseed feeding on blood metabolites, incidence of cystic follicles, and productive and reproductive performance in fresh Holstein dairy cows. J. Dairy Sci. 2015, 98, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Ulfina, G.G.; Kimothi, S.P.; Oberoi, P.S.; Baithalu, R.K.; Kumaresan, A.; Mohanty, T.K.; Imtiwati, P.; Dang, A.K. Modulation of post-partum reproductive performance in dairy cows through supplementation of long- or short-chain fatty acids during transition period. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1056–1064. [Google Scholar] [CrossRef]
- Hutchinson, I.A.; Hennessy, A.A.; Waters, S.M.; Dewhurst, R.J.; Evans, A.C.; Lonergan, P.; Butler, S.T. Effect of supplementation with different fat sources on the mechanisms involved in reproductive performance in lactating dairy cattle. Theriogenology 2012, 78, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Macejková, M.; Tarko, A.; Fabová, Z.; Alwasel, S.; Kotwica, J.; Harrath, A.H. Ginkgo, fennel, and flaxseed can affect hormone release by porcine ovarian cells and modulate the effect of toluene. Reprod. Biol. 2023, 23, 100736. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Irving-Rodgers, H.F. Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 2010, 82, 1021–1029. [Google Scholar] [CrossRef]
- Baddela, V.S.; Sharma, A.; Vanselow, J. Non-esterified fatty acids in the ovary: Friends or foes? Reprod. Biol. Endocrinol. 2020, 18, 60. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Regulators of Ovarian Functions; Nova Publishers, Inc.: New York, NY, USA, 2014; p. 1194. [Google Scholar]
- Orisaka, M.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Tsuyoshi, H.; Tsang, B.K.; Yoshida, Y. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini-review. Reprod. Med. Biol. 2021, 20, 169–175. [Google Scholar] [CrossRef]
- Khalid, M.; Haresign, W.; Luck, M.R. Secretion of IGF-I by bovine granulosa cells: Effects of growth hormone and follicle-stimulating hormone. Anim. Reprod. Sci. 2000, 58, 261–272. [Google Scholar] [CrossRef]
- Aardema, H.; Vos, P.L.A.M.; Lolicato, F.; Roelen, B.A.J.; Knijn, H.M.; Vaandrager, A.B.; Helms, J.B.; Gadella, B.M. Oleic Acid Prevents Detrimental Effects of Saturated Fatty Acids on Bovine Oocyte Developmental Competence. Biol. Reprod. 2011, 85, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Valckx, S.D.M.; Hoeck, V.V.; Alvarez, M.A.; Maillo, V.; Lopez-Cordona, A.P.; Gutierrez-Adan, A.; Berth, M.; Cortvrindt, R.; Bols, P.E.J.; Leroy, J.L.M.R. Elevated non-esterified fatty acid concentrations during in vitro murine follicle growth alter follicular physiology and reduce oocyte developmental competence. Fertil. Steril. 2014, 102, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Genicot, G.; Leroy, J.; van Soom, A.; Donnay, I. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology 2005, 63, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, H.; Ishiguro, A.; Inoue, Y.; Koumei, S.; Kuwayama, T.; Iwata, H. Mechanism of palmitic acid-induced deterioration of in vitro development of porcine oocytes and granulosa cells. Theriogenology 2020, 141, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Catandi, G.D.; Cheng, M.H.; Chicco, A.J.; Chen, T.; Carnevale, E.M. L-carnitine enhances developmental potential of bovine oocytes matured under high lipid concentrations in vitro. Anim. Reprod. Sci. 2023, 252, 107249. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Wu, L.L.Y.; Purdey, M.; Abel, A.D.; Goldys, E.M.; Macmillan, K.L.; Thompson, J.G.; Robker, R.L. Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence. Biol. Reprod. 2016, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Warzych, E.; Cieslak, A.; Pawlak, P.; Renska, N.; Pers-Kamczyc, E.; Lechniak, D. Maternal nutrition affects the composition of follicular fluid and transcript content in gilt oocytes. Vet. Med. 2011, 56, 156–167. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, B.; Cai, S.; Zeng, X.; Ye, Q.; Mao, X.; Zhang, S.; Zeng, X.; Ye, C.; Qiao, S. Metabolic disorder of amino acids, fatty acids and purines reflects the decreases in oocyte quality and potential in sows. J. Proteom. 2019, 200, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, P.; Warzych, E.; Cieslak, A.; Malyszka, N.; Maciejewska, E.; Madeja, Z.E.; Lechniak, D. The consequences of porcine IVM medium supplementation with follicular fluid become reflected in embryo quality, yield and gene expression patterns. Sci. Rep. 2018, 8, 15306. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanz, J.I.; Pérez-Ruiz, I.; Meijide, S.; Ferrando, M.; Larreategui, Z.; Ruiz-Larrea, M.-B. Lower follicular n-3 polyunsaturated fatty acid levels are associated with a better response to ovarian stimulation. J. Assist. Reprod. Genet. 2019, 36, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Renaville, B.; Bacciu, N.; Comin, A.; Motta, M.; Poli, I.; Vanini, G. Plasma and follicular fluid fatty acid profiles in dairy cows. Reprod. Domest. Anim. 2010, 45, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Veshkini, A.; Khadem, A.A.; Mohammadi-Sangcheshmeh, A.; Alamouti, A.A.; Soleimani, M.; Gastal, E.L. Linolenic acid improves oocyte developmental competence and decreases apoptosis of in vitro-produced blastocysts in goat. Zygote 2016, 24, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Viafara, J.A.S.; Portilho, R.V.; Maculan, R.; DeSouza, J.F.; Silva, C.M.; Rodrigues, P.G.; El Azzi, M.S.; de Souza, J.C. Fatty acid profiles of the plasma and follicular fluid mares fed a combination of linseed and salmon oil. An. Acad. Bras. Ciênc. 2021, 93, e20190443. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.A.; Bie, J.D.; Mohey-Elsaeed, O.; Wydooghe, E.; Bols, P.E.J.; Leroy, J.L.M.R. Alpha-linolenic acid protects the developmental capacity of bovine cumulus-oocyte complexes matured under lipotoxic conditions in vitro. Biol. Reprod. 2017, 96, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Baddela, V.S.; Roettgen, V.; Vernunft, A.; Viergutz, T.; Dannenberger, D.; Hammon, H.M.; Schoen, J.; Vanselow, J. Effects of dietary fatty acids on bovine oocyte competence and granulosa cells. Front. Endocrinol. 2020, 11, 87. [Google Scholar] [CrossRef]
- Gunstone, F.D. Fatty Acids and Lipid Chemistry; Springer: London, UK, 2012; p. 268. [Google Scholar] [CrossRef]
- Uemura, H. Synthesis and production of unsaturated and polyunsaturated fatty acids in yeast: Current state and perspectives. Appl. Microbiol. Biotechnol. 2012, 95, 1–12. [Google Scholar] [CrossRef]
- Brouwers, H.; Jónasdóttir, H.S.; Kuipers, M.E.; Kwekkeboom, J.C.; Auger, J.L.; Gonzalez-Torres, M.; López-Vicario, C.; Clària, J.; Freysdottir, J.; Hardardottir, I.; et al. Anti-Inflammatory and proresolving effects of the omega-6 polyunsaturated fatty acid adrenic acid. J. Immunol. 2020, 205, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Ko, C.M.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels with Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Kermack, A.J.; Wellstead, S.J.; Fisk, H.L.; Cheong, Y.; Houghton, F.D.; Macklon, N.S.; Calder, P.C. The fatty acid composition of human follicular fluid is altered by a 6-week dietary intervention that includes marine omega-3 fatty acids. Lipids 2021, 56, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Karmon, A.E.; Gaskins, A.J.; Arvizu, M.; Williams, P.L.; Souter, I.; Rueda, B.R.; Hauser, R.; Chavarro, J.E. for the Earth Study Team. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum. Reprod. 2018, 33, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Lowen, P.; Wellstead, S.J.; Fisk, H.L.; Montag, M.; Cheong, Y.; Osmond, C.; Houghton, F.D.; Calder, P.C.; Macklon, N.S. Effect of a 6-week “Mediterranean” dietary intervention on in vitro human embryo development: The preconception dietary supplements in assisted reproduction double-blinded randomized controlled trial. Fertil. Steril. 2020, 113, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T.; Fukasaku, Y.; Miyazaki, N.; Kawato, H.; Minoura, H. Usefulness of expanding the indications of early rescue intracytoplasmic sperm injection. Reprod. Med. Biol. 2021, 13, e12432. [Google Scholar] [CrossRef]
- Moallem, U.; Shafran, A.; Zachut, M.; Dekel, I.; Portnick, Y.; Arieli, A. Dietary α-linolenic acid from flaxseed oil improved folliculogenesis and IVF performance in dairy cows, similar to eicosapentaenoic and docosahexaenoic acids from fish oil. Reproduction 2013, 146, 603–614. [Google Scholar] [CrossRef]
- Rajaram, S. Health benefits of plant-derived α-linolenic acid. AJCN 2014, 100, 4435–4485. [Google Scholar] [CrossRef] [PubMed]
- Mahla, A.S.; Bunkar, S.K.; Kumawat, B.L.; Saxena, V.K.; Selvaraju, S.; Bhatt, R.S.; Singh, R.; Kumar, A. Dietary n-3 PUFA augments pre-ovulatory follicle turnover and prolificacy in well-fed ewes. Anim. Reprod. Sci. 2023, 252, 107231. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chiu, W.C.; Hsu, Y.P.; Lo, Y.L.; Wang, Y.H. Effects of omega-3 fatty acids on muscle mass, muscle strength and muscle performance among the elderly: A Meta-Analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef]
- Rada, P.; González-Rodriguez, Á.; García-Monzón, C.; Valverde, A.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Mo, Z.; Li, Y.; Huang, L.; Yu, S.; Ge, L.; Hu, Y.; Shi, S.; Zhang, L.; Wang, L.; et al. Oleic acid reduces steroidogenesis by changing the lipid type stored in lipid droplets of ovarian granulosa cells. J. Anim. Sci. Biotechnol. 2022, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Yenuganti, V.R.; Viergutz, T.; Vanselow, J. Oleic acid induces specific alterations in the morphology, gene expression and steroid hormone production of cultured bovine granulosa cells. Gen. Comp. Endocrinol. 2016, 232, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Luo, G.; Tang, X.; Ma, L.; Zheng, Y.; Liu, S.A.; Price, C.; Jiang, Z. Arachidonic acid regulation of intracellular signaling pathways and target gene expression in bovine granulosa cells. Animals 2019, 9, 374. [Google Scholar] [CrossRef]
- Maillard, V.; Desmarchais, A.; Durcin, M.; Uzbekova, S.; Elis, S. Docosahexaenoic acid (DHA) effects on proliferation and steroidogenesis of bovine granulosa cells. Reprod. Biol. Endocrinol. 2018, 16, 40. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | % |
|---|---|
| Maize | 13 |
| Wheat | 36.5 |
| Barley | 32 |
| Soybean meal | 8.5 |
| Wheat bran | 7 |
| CaHPO4 | 0.78 |
| CaCO3 | 1.29 |
| NaCl | 0.45 |
| Mineral premix | 0.48 |
| Item | Basal Diet | Basal Diet + 10% Flaxseed |
|---|---|---|
| Analytical and nutrient composition * | ||
| Crude protein (g/kg) | 140.42 | 150.51 |
| Crude fat (g/kg) | 18.07 | 42.23 |
| Crude fiber (g/kg) | 35.69 | 68.29 |
| Neutral detergent fiber (g/kg) | 176.11 | 189.04 |
| Acid detergent fiber (g/kg) | 42.27 | 74.47 |
| Ash (g/kg) | 60.12 | 48.30 |
| Starch (g/kg) | 574.62 | 510.28 |
| Ca (g/kg) | 8.12 | 12.80 |
| Mg (g/kg) | 3.35 | 3.59 |
| Na (g/kg) | 1.45 | 1.52 |
| K (g/kg) | 5.58 | 6.29 |
| P (g/kg) | 4.80 | 5.84 |
| Cu (mg/kg) | 40.82 | 57.40 |
| Zn (mg/kg) | 131.83 | 103.45 |
| Mn (mg/kg) | 150.57 | 125.80 |
| ME (MJ/kg) | 13.26 | 13.36 |
| Fatty acid composition (%) | ||
| C14:0, Myristic acid | 0.101 | 0.050 |
| C16:00, Palmitic acid | 14.281 | 4.496 |
| C18:00, Stearic acid | 1.899 | 2.547 |
| C18:2n-6, Linoleic acid | 55.730 | 8.547 |
| C18:3n-6, Gamma-linolenic acid | 0.090 | 0.018 |
| C18:3n-3, Alpha-linoleic acid | 7.109 | 72.546 |
| C20:4n-6, Arachidonic acid | 0.691 | 0.052 |
| C20:5n-3, Eicosapentaenoic acid | 0.125 | 0.0003 |
| C22:5n-6, Docosapentanoic acid | 0.245 | 0.022 |
| C22:5n-3, Docosapentaenoic acid | 0.043 | 0.140 |
| C22:6n-3, Docosahexaenoic acid | 0.073 | 0.055 |
| Σ n-3 | 7.349 | 72.741 |
| Σ n-6 | 57.227 | 8.720 |
| n-6/n-3 | 7.787 | 0.120 |
| EPA/AA | 0.181 | 0.005 |
| Fatty Acids (mol%) | Fatty Acid Trivial Name | None (Control) 3 mm F | Flaxseed 3 w/3 mm F | Flaxseed 6 w/3 mm F | None (Control) 5 mm F | Flaxseed 3 w/5 mm F | Flaxseed 6 w/5 mm F |
|---|---|---|---|---|---|---|---|
| C12:0 | Lauric | 0.09 ± 0.01 | 0.02 ± 0.00 a | 0.34 ± 0.03 bz | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.44 ± 0.03 bz |
| C14:0 | Myristic | 0.60 ± 0.03 | 0.42 ± 0.02 b | 0.94 ± 0.02 bz | 0.60 ± 0.03 | 0.70 ± 0.04 | 0.82 ± 0.04 bx |
| C14:1n5 | Myristoleic | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.10 ± 0.01 bz | 0.06 ± 0.00 | 0.03 ± 0.00 b | 0.11 ± 0.01 bz |
| C16:0 | Palmitic | 23.47 ± 0.17 | 22.61 ± 0.16 b | 28.20 ± 0.17 bz | 23.48 ± 0.24 | 27.05 ± 0.21 b | 26.43 ± 0.24 b |
| C16:1n7t | Palmitelaidic | 0.11 ± 0.02 | 0.08 ± 0.00 | 0.28 ± 0.01 bz | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.19 ± 0.01 bz |
| C16:1n7 | Palmitoleic | 1.44 ± 0.02 | 0.65 ± 0.02 b | 0.73 ± 0.02 bx | 1.44 ± 0.02 | 0.71 ± 0.00 b | 0.98 ± 0.05 bz |
| C16:1n-9 | 7-hexadecenoic | 1.25 ± 0.00 | 0.81 ± 0.02 b | 0.72 ± 0.05 b | 1.25 ± 0.020 | 0.86 ± 0.02 b | 0.58 ± 0.02 bz |
| C18:0 | Stearic | 14.72 ± 0.31 | 16.77 ± 0.26 b | 18.21 ± 0.17 by | 14.72 ± 0.20 | 15.77 ± 0.19 b | 16.85 ± 0.25 by |
| C18:1n7 | Vaccenic | 2.91 ± 0.26 | 1.95 ± 0.23 b | 1.29 ± 0.12 b | 2.91 ± 0.22 | 1.46 ± 0.26 b | 1.11 ± 0.13 b |
| C18:1n9 | Oleic | 25.84 ± 0.38 | 14.09 ± 0.24 b | 11.18 ± 0.19 bz | 25.84 ± 0.26 | 13.73 ± 0.30 b | 10.75 ± 0.24 bz |
| C18:2n6 | Linoleic | 11.99 ± 0.28 | 24.15 ± 0.24 b | 21.95 ± 0.20 bz | 11.99 ± 0.38 | 20.43 ± 0.23 b | 24.91 ± 0.22 bz |
| C18:3n6 | γ-linoleic | 0.03 ± 0.01 | 0.13 ± 0.02 b | 0.11 ± 0.02 b | 0.03 ± 0.01 | 0.08 ± 0.01 a | 0.08 ± 0.01 a |
| C18:3n3 | α-linolenic | 1.19 ± 0.12 | 2.04 ± 0.01 b | 2.36 ± 0.01 b | 1.19 ± 0.03 | 1.53 ± 0.16 | 1.32 ± 0.10 |
| C20:0 | Arachidic | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.01 a | 0.04 ± 0.01 | 0.11 ± 0.00 b | 0.09 ± 0.01 b |
| C20:1n9 | Gondoic | 0.11 ± 0.01 | 0.07 ± 0.01 b | 0.01 ± 0.00 bz | 0.11 ± 0.00 | 0.03 ± 0.01 b | 0.01 ± 0.00 b |
| C20:2n6 | Eicosadienoic | 0.45 ± 0.06 | 0.27 ± 0.02 b | 0.16 ± 0.00 bx | 0.45 ± 0.01 | 0.25 ± 0.01 b | 0.15 ± 0.01 bz |
| C20:3n6 | Dihomo-γ-linoleic | 0.50 ± 0.01 | 0.61 ± 0.01 b | 0.41 ± 0.01 bz | 0.50 ± 0.10 | 0.58 ± 0.03 | 0.44 ± 0.03 |
| C20:3n9 | Mead | 0.26 ± 0.01 | 0.31 ± 0.03 | 0.39 ± 0.02 bx | 0.26 ± 0.02 | 0.36 ± 0.01 b | 0.38 ± 0.01 b |
| C20:4n6 | Arachidonic (AA) | 11.20 ± 0.03 | 11.02 ± 0.03 | 8.87 ± 0.15 bz | 11.20 ± 0.27 | 11.67 ± 0.20 | 9.81 ± 0.24 bz |
| C20:5n3 | Timnodonic (EPA) | 0.27 ± 0.08 | 0.29 ± 0.09 | 0.23 ± 0.07 | 0.27 ± 0.10 | 0.31 ± 0.10 | 0.77 ± 0.10 bx |
| C22:4n6 | Adrenic | 0.06 ± 0.02 | 0.54 ± 0.12 b | 0.30 ± 0.07 | 0.06 ± 0.01 | 0.33 ± 0.10 | 0.26 ± 0.09 |
| C22:5n6 | Osbond | 1.26 ± 0.07 | 1.14 ± 0.05 | 1.09 ± 0.01 | 1.26 ± 0.09 | 1.31 ± 0.10 | 1.00 ± 0.00 x |
| C22:5n3 | Clupanodonic | 1.20 ± 0.04 | 1.42 ± 0.11 | 1.57 ± 0.13 a | 1.20 ± 0.06 | 1.79 ± 0.10 b | 1.73 ± 0.15 b |
| C22:6n3 | Cervonic | 0.95 ± 0.13 | 0.45 ± 0.09 b | 0.55 ± 0.08 a | 0.95 ± 0.08 | 0.70 ± 0.08 | 0.73 ± 0.09 |
| ΣSFA | 38.86 ± 0.34 | 39.55 ± 0.49 | 47.50 ± 0.29 bz | 38.81 ± 0.36 | 43.66 ± 0.18 b | 44.58 ± 0.18 b | |
| ΣUSFA | 61.05 ± 0.06 | 60.04 ± 0.07 b | 52.20 ± 0.06 bz | 61.05 ± 0.05 | 56.24 ± 0.07 b | 55.52 ± 0.23 by | |
| ΣMUFA | 32.03 ± 0.26 | 18.00 ± 0.26 b | 14.76 ± 0.12 bz | 31.94 ± 0.06 | 17.09 ± 0.13 b | 14.08 ± 0.14 bz | |
| ΣPUFA | 29.95 ± 0.13 | 42.72 ± 0.12 b | 37.94 ± 0.13 bz | 29.82 ± 0.20 | 39.59 ± 0.14 b | 41.69 ± 0.18 bz | |
| ΣTRANS | 0.11 ± 0.01 | 0.06 ± 0.01 | 0.26 ± 0.02 bz | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.19 ± 0.01 bz | |
| Σn-3 | 3.60 ± 0.11 | 4.20 ± 0.18 a | 4.71 ± 0.17 b | 3.60 ± 0.14 | 4.33 ± 0.13 b | 4.55 ± 0.17 b | |
| Σn-6 | 25.75 ± 0.64 | 38.17 ± 0.67 b | 33.23 ± 0.50 bz | 25.75 ± 0.67 | 35.01 ± 0.35 b | 37.03 ± 0.62 b | |
| n-6: n-3 | 7.17 ± 0.16 | 9.14 ± 0.23 b | 7.11 ± 0.32 z | 7.18 ± 0.12 | 8.09 ± 0.27 a | 8.17 ± 0.22 b | |
| EPA: AA | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.01 by | |
| Σn-7 | 4.46 ± 0.23 | 2.67 ± 0.10 b | 2.30 ± 0.12 b | 4.46 ± 0.11 | 2.27 ± 0.06 b | 2.28 ± 0.12 b | |
| Σn-9 | 27.20 ± 0.54 | 14.97 ± 0.51 b | 11.91 ± 0.17 bz | 27.20 ± 0.34 | 14.62 ± 0.28 b | 11.35 ± 0.19 bz |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecová, M.; Babjáková, D.; Sopková, D.; Andrejčáková, Z.; Hertelyová, Z.; Petrilla, V.; Polláková, M.; Vlčková, R. Different Lengths of Diet Supplementation with 10% Flaxseed Alter the Hormonal Profile and the Follicular Fluid Fatty Acid Content of Fattening Gilts. Life 2024, 14, 240. https://doi.org/10.3390/life14020240
Lecová M, Babjáková D, Sopková D, Andrejčáková Z, Hertelyová Z, Petrilla V, Polláková M, Vlčková R. Different Lengths of Diet Supplementation with 10% Flaxseed Alter the Hormonal Profile and the Follicular Fluid Fatty Acid Content of Fattening Gilts. Life. 2024; 14(2):240. https://doi.org/10.3390/life14020240
Chicago/Turabian StyleLecová, Martina, Diana Babjáková, Drahomíra Sopková, Zuzana Andrejčáková, Zdenka Hertelyová, Vladimír Petrilla, Magdaléna Polláková, and Radoslava Vlčková. 2024. "Different Lengths of Diet Supplementation with 10% Flaxseed Alter the Hormonal Profile and the Follicular Fluid Fatty Acid Content of Fattening Gilts" Life 14, no. 2: 240. https://doi.org/10.3390/life14020240
APA StyleLecová, M., Babjáková, D., Sopková, D., Andrejčáková, Z., Hertelyová, Z., Petrilla, V., Polláková, M., & Vlčková, R. (2024). Different Lengths of Diet Supplementation with 10% Flaxseed Alter the Hormonal Profile and the Follicular Fluid Fatty Acid Content of Fattening Gilts. Life, 14(2), 240. https://doi.org/10.3390/life14020240






