Lower-Limb Perfusion and Cardiovascular Physiology Are Significantly Improved in Non-Healthy Aged Adults by Regular Home-Based Physical Activities—An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setting
2.3. Statistical Analysis
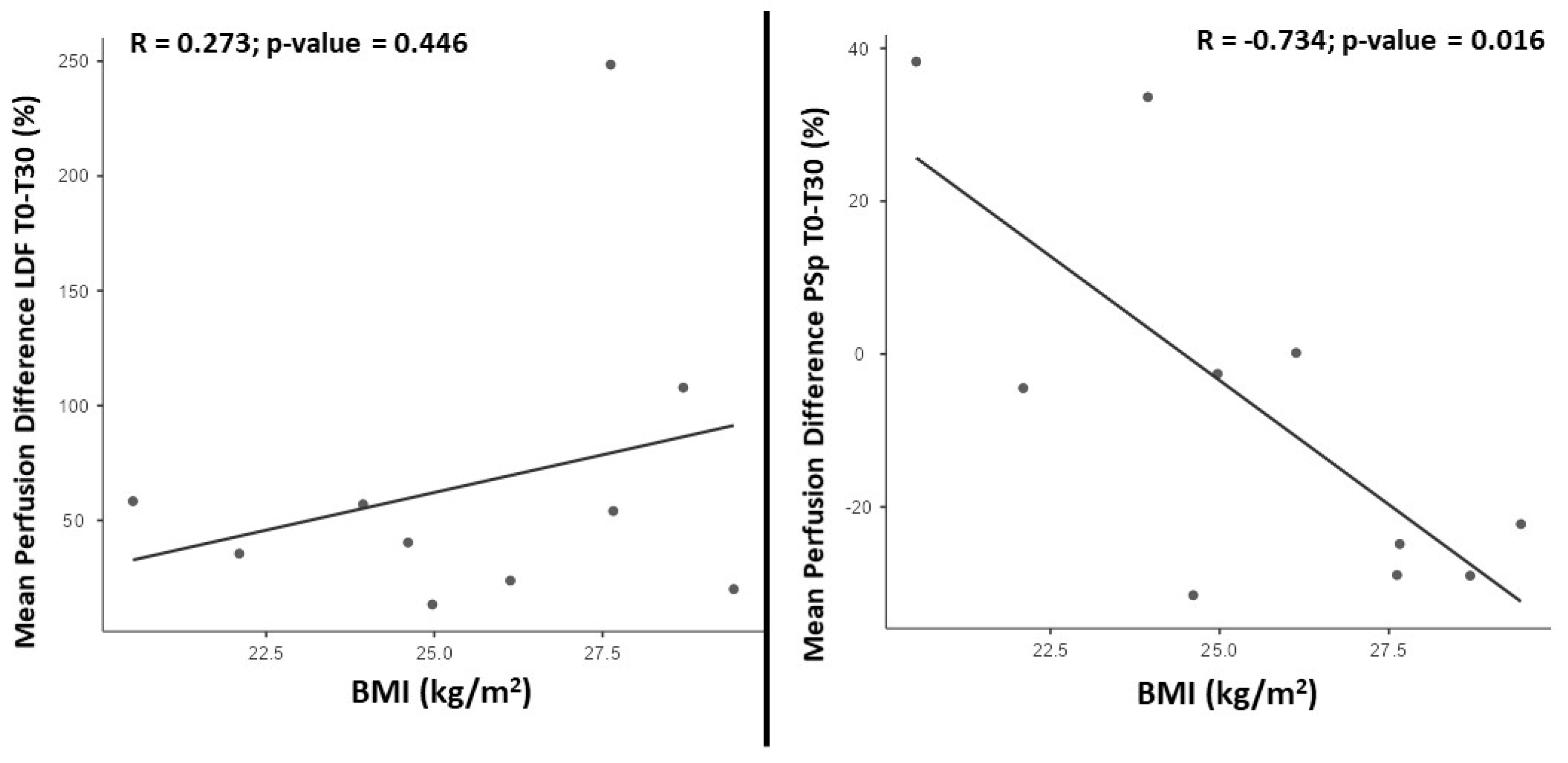
3. Results
4. Discussion
- -
- This set of common activities, previously studied in healthy cohorts, might be used in a home-health program to improve the cardiovascular status and the general health conditions of older, non-healthy, sedentary patients.
- -
- This FITT-inspired sequence favoured the lower-limb distal perfusion and systemic haemodynamics of all patients, as detected by the consistent improvement in LDF perfusion and the reduction in systolic blood pressure and MAP.
- -
- These impacts might be attributed to the program intervention since (a) intra-individual related features (such as skin temperature, body mass, and the fact that the patients’ medication(s) did not change from D0 to D30), (b) interindividual differences involving the fulfilment of the program were not anticipated, considering the low intensity level of activities involved, and (c) data reliability was fully ensured by the experience of the operator with the measurement technologies and methods applied here.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Gibbs, B.B.; Hivert, M.-F.; Jerome, G.J.; Kraus, W.E.; Rosenkranz, S.K.; Schorr, E.N.; Spartano, N.L.; Lobelo, F.; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; et al. Physical Activity as a Critical Component of First-Line Treatment for Elevated Blood Pressure or Cholesterol: Who, What, and How?: A Scientific Statement From the American Heart Association. Hypertension 2021, 78, e26–e37. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/physical activity and health outcomes: An overview of Cochrane systematic reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; on behalf of the American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Pearce, M.; Abbas, A.; Mok, A.; Strain, T.; Ali, S.; Crippa, A.; Dempsey, P.C.; Golubic, R.; Kelly, P.; et al. Non-occupational physical activity and risk of cardiovascular disease, cancer and mortality outcomes: A dose-response meta-analysis of large prospective studies. Br. J. Sports Med. 2023, 57, 979–989. [Google Scholar] [CrossRef] [PubMed]
- NCD Countdown 2030 Collaborators. NCD Countdown 2030: Pathways to achieving Sustainable Development Goal target 3. Lancet 2020, 396, 918. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, N.M.; Creager, M.A. Pathophysiology of Intermittent Claudication in Peripheral Artery Disease. Circ. J. Off. J. Jpn. Circ. Soc. 2017, 81, 281–289. [Google Scholar] [CrossRef]
- Florindo, F.; Nuno, S.; Gregório, J.; Rodrigues, L.M. Regular walking significantly improves foot perfusion independently of age. Physiology 2021, 2021. Available online: https://static.physoc.org/app/uploads/2021/04/14105215/Future-Physiology-2021-programme-and-abstracts.pdf (accessed on 21 January 2024).
- Monteiro Rodrigues, L.; Rocha, C.; Ferreira, H.T.; Silva, H.N. Lower limb massage in humans increases local perfusion and impacts systemic hemodynamics. J. Appl. Physiol. 2020, 128, 1217–1226. [Google Scholar] [CrossRef]
- Florindo, M.; Silva, H.; Rodrigues, M. Impact of the isometric contraction of the calf on the local microcirculation. Biomed. Biopharm. Res. 2017, 2, 179–186. [Google Scholar] [CrossRef]
- Florindo, M.; Gregório, J.; Rodrigues, L.M. Short duration—Low intensity isometric plantar flexion increases distal perfusion: Observations from a healthy cohort. Biomed. Biopharm. Res. 2022, 19, 58–71. [Google Scholar] [CrossRef]
- Florindo, M.; Nuno, S.; Rodrigues, L.M. Studying the human lower limb perfusion dynamics with the step-in place model. Biomed. Biopharm. Res. 2019, 2, 195–201. [Google Scholar] [CrossRef]
- Nuno, S.L.; Florindo, M.; Silva, H.; Rodrigues, L.M. Studying the impact of different body positioning, squatting, and unipodal flexion on perfusion in the lower limb—An exploratory approach complemented with optical spectroscopy (TiVi). Biomed. Biopharm. Res. 2020, 17, 187–196. [Google Scholar] [CrossRef]
- Florindo, M.; Nuno, S.L.; Rodrigues, L.M. Lower Limb Dynamic Activity Significantly Reduces Foot Skin Perfusion: Exploring Data with Different Optical Sensors in Age-Grouped Healthy Adults. Ski. Pharmacol. Physiol. 2022, 35, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Argent, R.; Daly, A.; Caulfield, B. Patient Involvement with Home-Based Exercise Programs: Can Connected Health Interventions Influence Adherence? JMIR mHealth and uHealth 2018, 6, e47. [Google Scholar] [CrossRef] [PubMed]
- Sabetsarvestani, P.; Mohammadi, F.; Tehranineshat, B.; Bijani, M.; Fereidouni, Z. Barriers to efficient management of in-home care: A qualitative content analysis. Nurs. Open 2022, 9, 1200–1209. [Google Scholar] [CrossRef]
- Wringe, A.; Cataldo, F.; Stevenson, N.; Fakoya, A. Delivering comprehensive home-based care programmes for HIV: A review of lessons learned and challenges ahead in the era of antiretroviral therapy. Health Policy Plan. 2010, 25, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Shier, V.; Trieu, E.; Ganz, D.A. Implementing exercise programs to prevent falls: Systematic descriptive review. Inj. Epidemiol. 2016, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Harwood, A.E.; Pymer, S.; Ingle, L.; Doherty, P.; Chetter, I.C.; Parmenter, B.; Askew, C.D.; Tew, G.A. Exercise training for intermittent claudication: A narrative review and summary of guidelines for practitioners. BMJ Open Sport Exerc. Med. 2020, 6, e000897. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, W.; Li, J.; Shi, H.; Shen, M.; Zhang, X.; Pang, Y.; Ni, Y.; Zhang, W.; Li, Y.; et al. Role of the intelligent exercise rehabilitation management system on adherence of cardiac rehabilitation in patients with coronary heart disease: A randomised controlled crossover study protocol. BMJ Open 2020, 10, e036720. [Google Scholar] [CrossRef]
- Baroudi, L.; Newman, M.W.; Jackson, E.A.; Barton, K.; Shorter, K.A.; Cain, S.M. Estimating Walking Speed in the Wild. Front. Sports Act. Living 2020, 2, 583848. [Google Scholar] [CrossRef]
- Dunford, E.C.; Valentino, S.E.; Dubberley, J.; Oikawa, S.Y.; McGlory, C.; Lonn, E.; Jung, M.E.; Gibala, M.J.; Phillips, S.M.; MacDonald, M.J. Brief Vigorous Stair Climbing Effectively Improves Cardiorespiratory Fitness in Patients with Coronary Artery Disease: A Randomized Trial. Front. Sports Act. Living 2021, 3, 630912. [Google Scholar] [CrossRef] [PubMed]
- Teques, P.; Calmeiro, L.; Silva, C.; Borrego, C. Validation and adaptation of the Physical Activity Enjoyment Scale (PACES) in fitness group exercisers. J. Sport Health Sci. 2020, 9, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Forte, P.; Teixeira, D.S.; Cid, L.; Monteiro, D. The Physical Activity Enjoyment Scale (Paces) as a Two-Dimensional Scale: Exploratory and Invariance Analysis. Montenegrin J. Sports Sci. Med. 2021, 10, 61–66. [Google Scholar] [CrossRef]
- Zierle-Ghosh, A.; Jan, A. Physiology, Body Mass Index. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535456/ (accessed on 21 January 2024).
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Naylor, A.R.; Roffi, M.; Tendera, M.; Vlachopoulos, C.; et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, S.; Lindberg, L.G.; Ek, A.C.; Linde, M.; Lindgren, M. Blood flow measurements at different depths using photoplethysmography and laser Doppler techniques. Ski. Res. Technol. 2009, 15, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, I.; Larsson, M.; Strömberg, T. Measurement depth and volume in laser Doppler flowmetry. Microvasc. Res. 2009, 78, 4–13. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Zhai, H.; Chan, H.P.; Farahmand, S.; Maibach, H.I. Cutaneous bioengineering instrumentation standardization: The Tissue Viability Imager. Skin Res. Technol. 2009, 15, 6–13. [Google Scholar] [CrossRef]
- Pedersen, B.L.; Bækgaard, N.; Quistorff, B. A near infrared spectroscopy-based test of calf muscle function in patients with peripheral arterial disease. Int. J. Angiol. 2015, 24, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, M.J. The efects of training on heart rate: A longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar] [PubMed]
- Jamovi Project. Jamovi (Version 2.3) [Computer Software]. 2022. Available online: https://www.jamovi.org (accessed on 21 January 2024).
- Domingues, L.; Cruz, E. Cultural Adaptation and Contribute to Validate the Patient Global Impression of Change Scale. Ifisionline 2011, 2, 31–37. Available online: https://comum.rcaap.pt/bitstream/10400.26/8856/1/artigo4_vol2n1.pdf (accessed on 5 February 2024).
- McDermott, M.M.; Kibbe, M.R.; Guralnik, J.M.; Ferrucci, L.; Criqui, M.H.; Domanchuk, K.; Tian, L.; Zhao, L.; Li, L.; Patel, K.; et al. Durability of Benefits from Supervised Treadmill Exercise in People with Peripheral Artery Disease. J. Am. Heart Assoc. 2019, 8, e009380. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Shimoda, M.; Tatsumi, F.; Kohara, K.; Obata, A.; Katakura, Y.; Sanada, J.; Fushimi, Y.; Iwamoto, Y.; Onishi, M.; et al. Effects of sedentary behavior and daily walking steps on body mass index and body composition: Prospective observational study using outpatient clinical data of Japanese patients with type 2 diabetes. J. Diabetes Investig. 2021, 12, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Smith-Ryan, A.E.; Weaver, M.A.; Viera, A.J.; Weinberger, M.; Blue, M.; Hirsch, K.R. Promoting Exercise and Healthy Diet Among Primary Care Patients: Feasibility, Preliminary Outcomes, and Lessons Learned from a Pilot Trial with High Intensity Interval Exercise. Front. Sports Act. Living 2021, 3, 690243. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, F.; Divsalar, D.N.; Fadil, R.; Tavakolian, K.; Blaber, A.P. Canadian aging and inactivity study: Spaceflight-inspired exercises during head-down tilt bedrest blunted reductions in muscle-pump but not cardiac baroreflex in older persons. Front. Physiol. 2022, 13, 943630. [Google Scholar] [CrossRef]
- Hearon, C.M., Jr.; Dinenno, F.A. Regulation of skeletal muscle blood flow during exercise in ageing humans. J. Physiol. 2016, 594, 2261–2273. [Google Scholar] [CrossRef]
- Verma, A.K.; Xu, D.; Garg, A.; Blaber, A.P.; Tavakolian, K. Effect of Aging on Muscle-Pump Baroreflex of Individual Leg Muscles During Standing. Front. Physiol. 2019, 10, 845. [Google Scholar] [CrossRef]
- Craig, J.C.; Broxterman, R.M.; Cerbie, J.F.; La Salle, D.T.; Roundy, C.S.; Jarrett, C.L.; Richardson, R.S.; Trinity, J.D. The dynamic adjustment of mean arterial pressure during exercise: A potential tool for discerning cardiovascular health status. J. Appl. Physiol. 2021, 130, 1544–1554. [Google Scholar] [CrossRef]
- Magder, S. The meaning of blood pressure. Crit. Care 2018, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Low, D.A.; Jones, H.; Cable, N.T.; Alexander, L.M.; Kenney, W.L. Historical reviews of the assessment of human cardiovascular function: Interrogation and understanding of the control of skin blood flow. Eur. J. Appl. Physiol. 2020, 120, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.; Salvi, P.; Furlanis, G.; Baldi, C.; Rovina, M.; Salvi, L.; Faini, A.; Bilo, G.; Fabris, B.; Carretta, R.; et al. Mean arterial pressure estimated by brachial pulse wave analysis and comparison with currently used algorithms. J. Hypertens. 2020, 38, 2161–2168. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/ PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Di Federico, S.; Filippini, T.; Whelton, P.K.; Cecchini, M.; Iamandii, I.; Boriani, G.; Vinceti, M. Alcohol Intake and Blood Pressure Levels: A Dose-Response Meta-Analysis of Nonexperimental Cohort Studies. Hypertension 2023, 80, 1961–1969. [Google Scholar] [CrossRef]
- Cracowski, J.L.; Roustit, M. Human Skin Microcirculation. Compr. Physiol. 2020, 10, 1105–1154. [Google Scholar] [CrossRef]
- Monteiro Rodrigues, L.; Granja, T.F.; de Andrade, S.F. Optoacoustic Imaging Offers New Insights into In Vivo Human Skin Vascular Physiology. Life 2022, 12, 1628. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Rocha, C.G.; Florindo, M.E.; Gregório, J. Lower Limb Perfusion Asymmetries in Humans at Rest and Following Activity—A Collective View. Symmetry 2021, 13, 2348. [Google Scholar] [CrossRef]
| D0 | Participants | |
|---|---|---|
| N | 10 | |
| Age, years | 62.4 ± 5.6 | |
| BMI, kg/m2 | 25.6 ± 2.9 | |
| ABI | 1.1 ± 0.1 | |
| MAP, mmHg | 95.7 ± 6.5 | |
| Steps/day (number) | 3400.5 ± 826.7 | |
| Activity(h)/week | 87.0 ± 12.3 | |
| Lower-limb pain (VAS) | 1.7 ± 1.1 | |
| Identified Comorbidities | Identified comedication | |
| Pre-diabetes n (%) | 3 (30) |
|
| Hypertension n (%) | 5 (50) |
|
| Dyslipidaemia n (%) | 6(60) |
|
| Overweight n (%) | 7 (60) |
|
| Two or more in the same individual n (%) | 9 (90) | not applicable |
| Baseline Variables (D0) | Phase 1 | Phase 2 | Phase 1–Phase 2 p-Value | Phase 3 | Phase 1–Phase 3 p-Value | |
|---|---|---|---|---|---|---|
| LDF PU (AU) | med (Q1–Q3) | 7.7 (6.2–10.4) | 12.9 (9.0–19.9) | 0.005 * | 8.3 (7.4–11.0) | 0.093 |
| PSp CRBC (AU) | med (Q1–Q3) | 117.8 (103.4–127.7) | 111.0 (104.1–122.3) | 0.169 | 113.4 (99.0–122.3) | 0.415 |
| SYS_P (mmHg) | med (Q1–Q3) | 127.5 (118.8–130.8) | 136.0 (124.5–138.0) | 0.005 * | 121.0 (111.5–130.3) | 0.038 * |
| DIAS_P (mmHg) | med (Q1–Q3) | 80.5 (76.0–82.5) | 82.5 (77.5–85.0) | 0.011 * | 77.0 (73.5–82.3) | 0.049 * |
| MAP (mmHg) | med (Q1–Q3) | 95.8 (89.8–98.8) | 99.5 (95.4–103.2) | 0.005 * | 90.0 (87.1–95.3) | 0.007 * |
| PR (bpm) | med (Q1–Q3) | 73.0 (72.3–76.3) | 83.0 (77.5–91.3) | 0.005 * | 73.5 (69.0–79.8) | 0.266 |
| Day Thirty (D30) | Phase 1 | Phase 2 | Phase 1–Phase 2 p-Value | Phase 3 | Phase 1–Phase 3 p-Value | |
| LDF PU (AU) | med (Q1–Q3) | 13.2 (11.6–15.7) | 19.0 (15.7–19.5) | 0.022 * | 13.0 (9.9–14.7) | 0.475 |
| PSp CRBC (AU) | med (Q1–Q3) | 97.7 (91.8–121.4) | 119.9 (99.2–127.6) | 0.037 * | 101.6 (91.0–119.9) | 0.721 |
| SYS_P (mmHg) | med (Q1–Q3) | 120.0 (114.8–124.8) | 127.5 (125.5–133.0) | 0.012 * | 121.0 (116.0–126.3) | 0.282 |
| DIAS_P (mmHg) | med (Q1–Q3) | 77.5 (76–81.3) | 82.0 (79.3–84.5) | 0.011 * | 76.5 (75.0–79.3) | 0.673 |
| MAP (mmHg) | med (Q1–Q3) | 92.5 (88.9–95.6) | 97.3 (96.0–99.1) | 0.005 * | 90.8 (87.7–93.5) | 0.677 |
| PR (bpm) | med (Q1–Q3) | 73.5 (68.3–76.8) | 80.5 (75.0–93.5) | 0.005 * | 74.5 (69.0–77.8) | 0.256 |
| D0 | D30 | % (Mean) | p-Value | |||
|---|---|---|---|---|---|---|
| Phase 1 | LDF PU (AU) | med (Q1–Q3) | 7.7 (6.2–10.4) | 13.2 (11.6–15.7) | 57.3 | 0.005 * |
| PSp CRBC (AU) | med (Q1–Q3) | 117.8 (103.4–127.7) | 97.7 (91.8–121.4) | (-) 7.9 | 0.445 | |
| SYS_P (mmHg) | med (Q1–Q3) | 127.5 (118.8–130.8) | 120.0 (114.8–124.8) | (-) 5.7 | 0.008 * | |
| DIAS_P (mmHg) | med (Q1–Q3) | 80.5 (76.0–82.5) | 77.5 (76–81.3) | (-) 3.0 | 0.137 | |
| MAP (mmHg) | med (Q1–Q3) | 95.8 (89.8–98.8) | 92.5 (88.9–95.6) | (-) 4.2 | 0.037 * | |
| PR (bpm) | med (Q1–Q3) | 73.0 (72.3–76.3) | 73.5 (68.3–76.8) | (-) 1.0 | 0.888 | |
| Phase 2 | LDF PU (AU) | med (Q1–Q3) | 12.9 (9.0–19.9) | 19.0 (15.7–19.5) | 25.7 | 0.169 |
| PSp CRBC (AU) | med (Q1–Q3) | 111.0 (104.1–122.3) | 119.9 (99.2–127.6) | 8.0 | 0.646 | |
| SYS_P (mmHg) | med (Q1–Q3) | 136.0 (124.5–138.0) | 127.5 (125.5–133.0) | (-) 3.9 | 0.139 | |
| DIAS_P (mmHg) | med (Q1–Q3) | 82.5 (77.5–85.0) | 82.0 (79.3–84.5) | 0.5 | 0.905 | |
| MAP (mmHg) | med (Q1–Q3) | 99.5 (95.4–103.2) | 97.3 (96.0–99.1) | (-) 1.5 | 0.333 | |
| PR (bpm) | med (Q1–Q3) | 83.0 (77.5–91.3) | 80.5 (75.0–93.5) | (-) 1.9 | 0.798 | |
| Phase 3 | LDF PU (AU) | med (Q1–Q3) | 8.3 (7.4–11) | 13.0 (9.9–14.7) | 37.6 | 0.025 * |
| PSp CRBC (AU) | med (Q1–Q3) | 113.4 (99.0–122.3) | 101.6 (91.0–119.9) | (-) 6.8 | 0.386 | |
| SYS_P (mmHg) | med (Q1–Q3) | 121.0 (111.5–130.3) | 121.0 (116.0–126.3) | 2.6 | 0.513 | |
| DIAS_P (mmHg) | med (Q1–Q3) | 77.0 (73.5–82.3) | 76.5 (75.0–79.3) | 0.8 | 0.888 | |
| MAP (mmHg) | med (Q1–Q3) | 90.0 (87.1–95.3) | 90.8 (87.7–93.0) | (-) 0.6 | 0.677 | |
| PR (bpm) | med (Q1–Q3) | 73.5 (69.0–79.8) | 74.5 (69.0–77.8) | 1.3 | 0.593 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florindo, M.; Gregório, J.; Rodrigues, L.M. Lower-Limb Perfusion and Cardiovascular Physiology Are Significantly Improved in Non-Healthy Aged Adults by Regular Home-Based Physical Activities—An Exploratory Study. Life 2024, 14, 241. https://doi.org/10.3390/life14020241
Florindo M, Gregório J, Rodrigues LM. Lower-Limb Perfusion and Cardiovascular Physiology Are Significantly Improved in Non-Healthy Aged Adults by Regular Home-Based Physical Activities—An Exploratory Study. Life. 2024; 14(2):241. https://doi.org/10.3390/life14020241
Chicago/Turabian StyleFlorindo, Margarida, João Gregório, and Luís Monteiro Rodrigues. 2024. "Lower-Limb Perfusion and Cardiovascular Physiology Are Significantly Improved in Non-Healthy Aged Adults by Regular Home-Based Physical Activities—An Exploratory Study" Life 14, no. 2: 241. https://doi.org/10.3390/life14020241





