The Circadian Rhythm of the Behavior and Gut Microbiota in Dybowski’s Frogs (Rana dybowskii) during the Autumn Migration Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. DNA Extraction
2.4. 16S rRNA Gene Amplicon Sequencing
2.5. Bioinformatics and Statistical Analysis
3. Results
3.1. Basic Metrics of R. dybowskii
3.2. Circadian Rhythms in the Behavior of R. dybowskii
3.3. Bacterial Sequencing of the Gut Microbiota
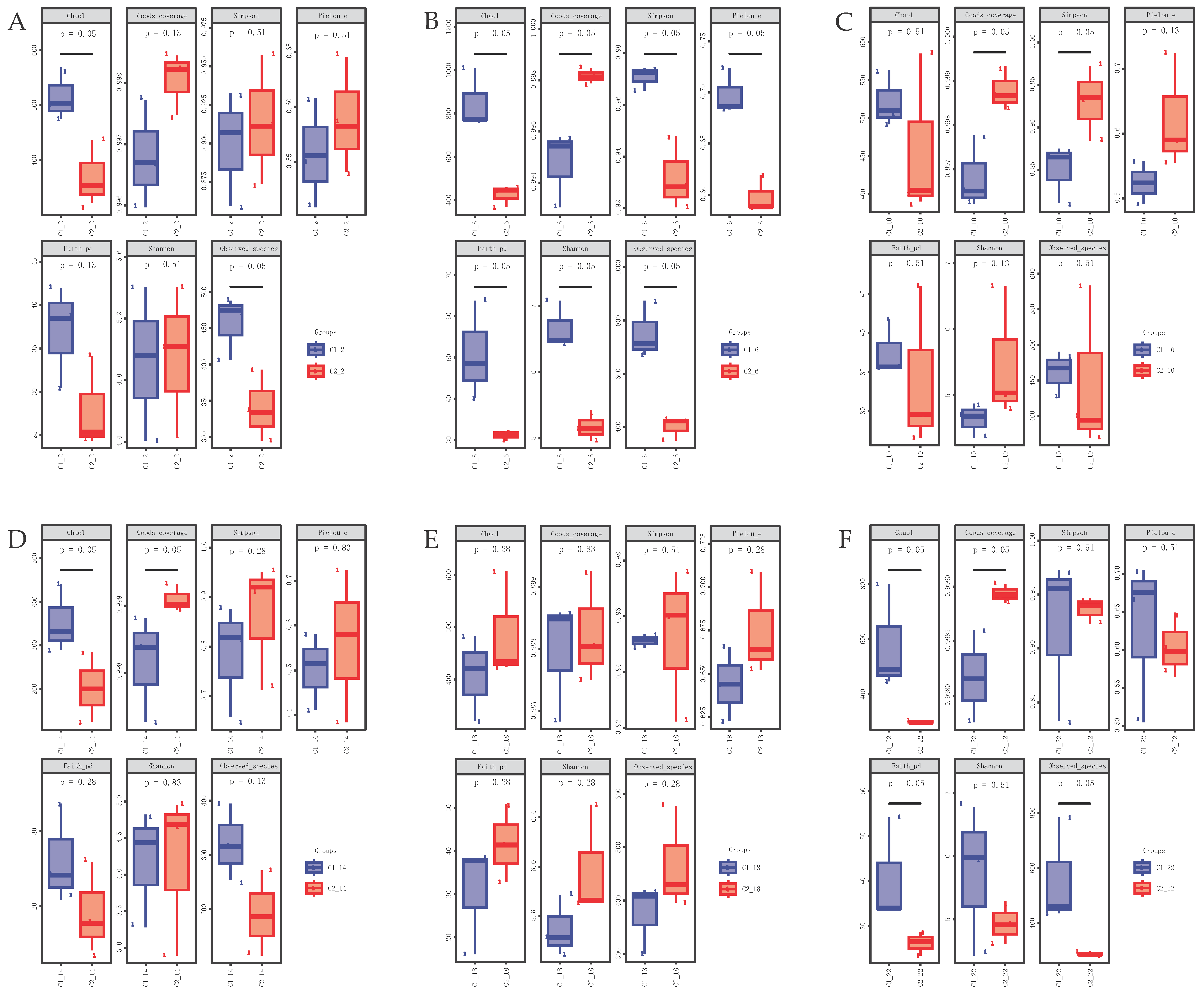
3.4. Alpha Diversity of the Gut Microbial Community
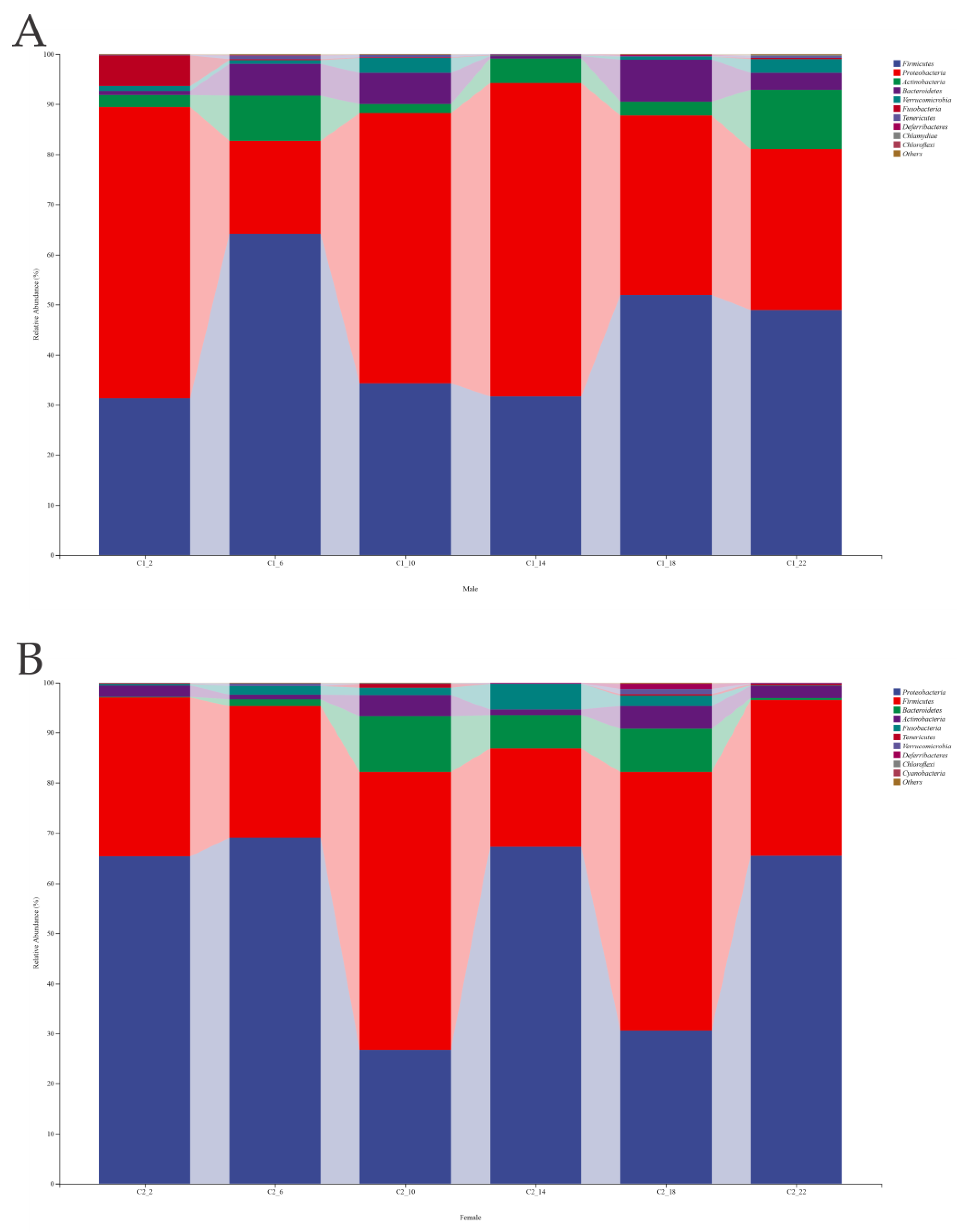
3.5. Analysis of Microbial Community Structure
3.6. Beta Diversity and Sexual Dimorphism in R. dybowskii
3.7. Analysis of Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamblen-Coyle, M.J.; Wheeler, D.A.; Rutila, J.E.; Rosbash, M.; Hall, J.C. Behavior of period-altered circadian rhythm mutants of Drosophila in light: Dark cycles (Diptera: Drosophilidae). J. Insect Behav. 1992, 5, 417–446. [Google Scholar] [CrossRef]
- Yang, S.C.; Shieh, K.R. Implications of Circadian Rhythms on Metabolic Disorders. Tzu Chi Med. J. 2009, 21, 285–288. [Google Scholar] [CrossRef]
- Li, R.W.; Cheng, S.T.; Wang, Z.R. Clock gene and mammal reproduction. J. Biol. 2013, 30, 67–70. [Google Scholar]
- Guchhait, P.; Haldar, C. Circadian Rhythms of Melatonin and Sex Steroids in a Nocturnal Bird, Indian Spotted Owlet Athene brama During Reproductively Active and Inactive Phases. Biol. Rhythm Res. 1999, 30, 508–516. [Google Scholar] [CrossRef]
- Sura, P. Circadian rhythm in the subcommissural organ of the frog Rana kl. esculenta under constant darkness. Acta Biol. Cracoviensia Ser. Zool. 2000, 42, 95–98. [Google Scholar]
- Pancak, M.K.; Taylor, D.H. Seasonal and daily plasma corticosterone rhythms in American toads, Bufo americanus. Gen. Comp. Endocrinol. 1983, 50, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Sanjita Devi, H.; Rajiv, C.; Mondal, G.; Khan, Z.A.; Devi, S.D.; Bharali, R.; Chattoraj, A. Influence of photoperiod variations on the mRNA expression pattern of melatonin bio-synthesizing enzyme genes in the pineal organ and retina: A study in relation to the serum melatonin profile in the tropical carp Catla catla. J. Fish Biol. 2022, 101, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Vera, L.M.; de Alba, G.; Santos, S.; Szewczyk, T.M.; Mackenzie, S.A.; Sánchez-Vázquez, F.J.; Rey Planellas, S. Circadian rhythm of preferred temperature in fish: Behavioural thermoregulation linked to daily photocycles in zebrafish and Nile tilapia. J. Therm. Biol. 2023, 113, 103544. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wang, J.; Zhao, Y.; Wei, H.; Li, Y. Daily cycle of melatonin in different tissues of dybowski’s frog ( Rana dybowskii ). Biol. Rhythm Res. 2021, 53, 1364–1372. [Google Scholar] [CrossRef]
- Hu, N.; Zhao, X.; Jin, J.; Zhao, Y.; Wei, H.; Li, X.; Li, Y. Effects of photoperiod on the melatonin cycle of Dybowski’s frog (Rana dybowskii). Biol. Rhythm Res. 2022, 53, 1539–1549. [Google Scholar] [CrossRef]
- Edwards, D.H. The social regulation of competition and aggression in animals. Am. J. Hum. Biol. 2000, 12, 569–571. [Google Scholar] [CrossRef]
- Kroodsma, D.E.; Byers, B.E. The Function(s) of Bird Song. Am. Zool. 1991, 31, 318–328. [Google Scholar] [CrossRef]
- Lamoureux, V.S. Ecology and Seasonal Behavior of the Green Frog (Rana clamitans); State University of New York at Binghamton: Binghamton, NY, USA, 2000. [Google Scholar]
- Ohmer, M.E.B.; Hammond, T.T.; Switzer, S.; Wantman, T.; Bednark, J.G.; Paciotta, E.; Coscia, J.; Richards-Zawacki, C.L. Developmental environment has lasting effects on amphibian post-metamorphic behavior and thermal physiology. J. Exp. Biol. 2023, 226, jeb244883. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, A.; Balestrieri, A.; Scribano, G.; Fontana, A.; Pellitteri-Rosa, D. Contextual behavioural plasticity in Italian agile frog (Rana latastei) tadpoles exposed to native and alien predator cues. J. Exp. Biol. 2021, 224, jeb240465. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.X.; Xu, Z.M.; Narins, P.M. Male antiphonal calls and phonotaxis evoked by female courtship calls in the large odorous frog (Odorrana graminea). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2023, 209, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lin, Z.H.; Ma, X.M.; Zhao, L.H.; Ma, X.H. Acoustic characteristics of the tiger frog, Hoplobatrachus rugulosus, during the breeding season. Dong Wu Xue Yan Jiu = Zool. Res. 2011, 32, 456–460. [Google Scholar] [CrossRef]
- Matesz, K.; Kecskes, S.; Bácskai, T.; Rácz, É.; Birinyi, A. Brainstem circuits underlying the prey-catching behavior of the frog. Brain Behav. Evol. 2014, 83, 104–111. [Google Scholar] [CrossRef]
- Woolley, S.C.; Sakata, J.T.; Crews, D. Evolutionary insights into the regulation of courtship behavior in male amphibians and reptiles. Physiol. Behav. 2004, 83, 347–360. [Google Scholar] [CrossRef]
- Woodley, S. Chemosignals, hormones, and amphibian reproduction. Horm. Behav. 2015, 68, 3–13. [Google Scholar] [CrossRef]
- Niu, Z.; Xue, H.; Jiang, Z.; Chai, L.; Wang, H. Effects of temperature on intestinal microbiota and lipid metabolism in Rana chensinensis tadpoles. Environ. Sci. Pollut. Res. Int. 2023, 30, 35398–35412. [Google Scholar] [CrossRef]
- Colombo, B.M.; Scalvenzi, T.; Benlamara, S.; Pollet, N. Microbiota and mucosal immunity in amphibians. Front. Immunol. 2015, 6, 111. [Google Scholar] [CrossRef]
- Niu, Y.; Li, X.; Zhang, H.; Xu, T.; Wei, D.; An, Z.; Storey, K.B. Hepatic transcriptome and gut microbiome provide insights into freeze tolerance in the high-altitude frog, Nanorana parkeri. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101147. [Google Scholar] [CrossRef]
- Rice, J.A. Does Gut Microbiota Affect the Diet Preference in Anurans? Bachelor’s Thesis, University of Southern Mississippi, Hattiesburg, MS, USA, 2015; p. 344. [Google Scholar]
- Emerson, K.J.; Fontaine, S.S.; Kohl, K.D.; Woodley, S.K. Temperature and the microbial environment alter brain morphology in a larval amphibian. J. Exp. Biol. 2023, 226, jeb.245333. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhai, Y.; Song, J.; Zhang, J.; Li, X. The structural discrepancy between the small and large gut microbiota of Asiatic toad (Bufo gargarizans) during hibernation. Folia Microbiol. 2023, 68, 537–546. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Jiang, Y.; Wang, P.; Xiang, J.; Pan, W. Rotten-skin disease significantly changed giant spiny frog(Paa spinosa) gut microbiota. bioRxiv. 2020. [Google Scholar] [CrossRef]
- Kohl, K.D.; Cary, T.L.; Karasov, W.H.; Dearing, M.D. Restructuring of the amphibian gut microbiota through metamorphosis. Environ. Microbiol. Rep. 2013, 5, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yu, C.; Jin, J.; Zhao, X.; Zhao, Y.; Wei, H.; Li, Y. Impact of photoperiods on the specific activities of immune and antioxidant enzymes in different tissues of Dybowski’s frog (Rana dybowskii). Biol. Rhythm Res. 2022, 53, 1790–1799. [Google Scholar] [CrossRef]
- Xu, Y.G.; Chai, L.H.; Shi, W.; Wang, D.D.; Zhang, J.Y.; Xiao, X.H. Transcriptome profiling and digital gene expression analysis of the skin of Dybowski’s frog (Rana dybowskii) exposed to Aeromonas hydrophila. Appl. Microbiol. Biotechnol. 2017, 101, 5799–5808. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Ramette, A. Multivariate analyses in microbial ecology. Fems Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 1–12. [Google Scholar] [CrossRef]
- Grassie, O.C. Environmental Stress Shapes the Neurobiology and Behavior of Fish. Doctor’s Thesis, The Pennsylvania State University, State College, PA, USA, 2013. [Google Scholar]
- Plumb, J.M.; Perry, R.W.; Adams, N.S.; Rondorf, D.W. The Effects of River Impoundment and Hatchery Rearing on the Migration Behavior of Juvenile Steelhead in the Lower Snake River, Washington. N. Am. J. Fish. Manag. 2006, 26. [Google Scholar] [CrossRef]
- Richardson, W.J.; Finley, K.J.; Miller, G.W.; Davis, R.A.; Koski, W.R. Feeding, social and migration behavior of bowhead whales, balaena mysticetus, in baffin bay vs. the beaufort sea—Regions with different amounts of human activity. Mar. Mammal Sci. 1995, 11, 1–45. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, C.; Guo, Y. Satellite tracking reveals a new migration route of black-necked cranes (Grus nigricollis) in Qinghai-Tibet Plateau. PeerJ 2020, 8, e9715. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A. The Ecology and Behavior of Amphibians. Austral Ecol. 2009, 34, 116. [Google Scholar] [CrossRef]
- Miwa, T. Conditions controlling the timing of the autumn migration to hibernation sites in a Japanese headwater frog, Rana sakuraii. J. Zool. 2018, 304, 45–54. [Google Scholar] [CrossRef]
- Shakhparonov, V.V.; Golovlev, A.P.; Grytsyshina, E.E.; Bolshakova, A.A. Orientation in the European common frog Rana temporaria during the first wintering migration. J. Exp. Biol. 2022, 225, jeb.243761. [Google Scholar] [CrossRef]
- Tong, Q.; Hu, Z.F.; Du, X.P.; Bie, J.; Wang, H.B. Effects of Seasonal Hibernation on the Similarities between the Skin Microbiota and Gut Microbiota of an Amphibian (Rana dybowskii). Microb. Ecol. 2020, 79, 898–909. [Google Scholar] [CrossRef]
- Costanzo, J.P.; do Amaral, M.C.; Rosendale, A.J.; Lee, R.E., Jr. Hibernation physiology, freezing adaptation and extreme freeze tolerance in a northern population of the wood frog. J. Exp. Biol. 2013, 216, 3461–3473. [Google Scholar] [CrossRef]
- Navarrete, P.; Espejo, R.T.; Romero, J. Molecular analysis of microbiota along the digestive tract of juvenile Atlantic salmon (Salmo salar L.). Microb. Ecol. 2009, 57, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Song, D.; Ho, C.T.; Zhang, X.; Zhang, C.; Cao, J.; Wu, Z. Omics Analyses of Gut Microbiota in a Circadian Rhythm Disorder Mouse Model Fed with Oolong Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 8847–8854. [Google Scholar] [CrossRef] [PubMed]
- Nikouli, E.; Meziti, A.; Smeti, E.; Antonopoulou, E.; Mente, E.; Kormas, K.A. Gut Microbiota of Five Sympatrically Farmed Marine Fish Species in the Aegean Sea. Microb. Ecol. 2021, 81, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Yahn, J. Effects of environmental temperature on the gut microbial communities of tadpoles. Environ. Microbiol. 2016, 18, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.H.; Chang, C.W.; Huang, C.W.; Gao, J.; Liao, P.C. Composition and Functional Specialists of the Gut Microbiota of Frogs Reflect Habitat Differences and Agricultural Activity. Front. Microbiol. 2017, 8, 2670. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.C.; Yang, Y.J.; Wang, D. Functional analysis for gut microbes of the brown tree frog (Polypedates megacephalus) in artificial hibernation. BMC Genom. 2016, 17, 1024. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Hong, P.; Tang, D.; Qing, H.; Omondi Donde, O.; Wang, H.; Xiao, B.; Wu, H. Comparison of intestinal microbes in female and male Chinese concave-eared frogs (Odorrana tormota) and effect of nematode infection on gut bacterial communities. MicrobiologyOpen 2019, 8, e00749. [Google Scholar] [CrossRef]
- Li, J.; Rui, J.; Li, Y.; Tang, N.; Zhan, S.; Jiang, J.; Li, X. Ambient temperature alters body size and gut microbiota of Xenopus tropicalis. Sci. China Life Sci. 2020, 63, 915–925. [Google Scholar] [CrossRef]
- Bletz, M.C.; Goedbloed, D.J.; Sanchez, E.; Reinhardt, T.; Tebbe, C.C.; Bhuju, S.; Geffers, R.; Jarek, M.; Vences, M.; Steinfartz, S. Amphibian gut microbiota shifts differentially in community structure but converges on habitat-specific predicted functions. Nat. Commun. 2016, 7, 13699. [Google Scholar] [CrossRef]
- Chang, C.W.; Huang, B.H.; Lin, S.M.; Huang, C.L.; Liao, P.C. Changes of diet and dominant intestinal microbes in farmland frogs. BMC Microbiol. 2016, 16, 33. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.S. Microbes in Gastrointestinal Health and Disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Titon, S.C.M.; Titon Junior, B.; Assis, V.R.; Vasconcelos-Teixeira, R.; Garcia Neto, P.G.; Lima, A.S.; Ferreira, L.d.F.; Fernandes, P.A.; Gomes, F.R.; Markus, R.P. Hormonal daily variation co-varies with immunity in captive male bullfrogs (Lithobates catesbeianus). Gen. Comp. Endocrinol. 2021, 303, 113702. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhang, B.; Liu, Q.; Pan, T.; Gou, J. Sexual dimorphism in the Chinese endemic species Pachyhynobius shangchengensis Fei, Qu and Wu, 1983 (Urodela: Hynobiidae). PeerJ 2019, 7, e6408. [Google Scholar] [CrossRef] [PubMed]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus gnavus: Friend or foe for human health. FEMS Microbiol. Rev. 2023, 47, fuad014. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Yan, W.; Mai, C.; Duan, Z.; Zheng, J.; Sun, C.; Yang, N. Joint contributions of the gut microbiota and host genetics to feed efficiency in chickens. Microbiome 2021, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Huang, C.; Liao, W. Seasonal Variation in Gut Microbiota Related to Diet in Fejervarya limnocharis. Animals 2021, 11, 1393. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Song, J.; Zhai, Y. Decisive Effects of Life Stage on the Gut Microbiota Discrepancy Between Two Wild Populations of Hibernating Asiatic Toads (Bufo gargarizans). Front. Microbiol. 2021, 12, 665849. [Google Scholar] [CrossRef]
- Dursun, C.; Gül, S.; Özdemir, N. Sexual size and shape dimorphism in Turkish common toads (Bufo bufo Linnaeus 1758). Anat. Rec. 2022, 305, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T. Ontogenetic change in the diet of the pond frog, Rana nigromaculata. Ecol. Res. 2002, 17, 639–644. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Hayakawa, T.; Kurihara, Y.; Lee, W.; Hanya, G. Seasonal responses and host uniqueness of gut microbiome of Japanese macaques in lowland Yakushima. Anim. Microbiome 2022, 4, 54. [Google Scholar] [CrossRef]



| Age | Sex | Weight (g) | Length (mm) | Sex Ratio |
|---|---|---|---|---|
| froglets | ♀ | 5.41 | 39.44 | 87:138 |
| ♂ | 4.81 | 36.75 | ||
| adult frogs | ♀ | 24.24 | 62.41 | 64:112 |
| ♂ | 17.68 | 56.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, N.; Li, Y.; Wang, M.; Ji, H.; Zhang, X.; San, B.; Shi, H. The Circadian Rhythm of the Behavior and Gut Microbiota in Dybowski’s Frogs (Rana dybowskii) during the Autumn Migration Period. Life 2024, 14, 322. https://doi.org/10.3390/life14030322
Hu N, Li Y, Wang M, Ji H, Zhang X, San B, Shi H. The Circadian Rhythm of the Behavior and Gut Microbiota in Dybowski’s Frogs (Rana dybowskii) during the Autumn Migration Period. Life. 2024; 14(3):322. https://doi.org/10.3390/life14030322
Chicago/Turabian StyleHu, Nan, Yingdong Li, Meizhang Wang, Haoyu Ji, Xian Zhang, Baolong San, and Hongyue Shi. 2024. "The Circadian Rhythm of the Behavior and Gut Microbiota in Dybowski’s Frogs (Rana dybowskii) during the Autumn Migration Period" Life 14, no. 3: 322. https://doi.org/10.3390/life14030322
APA StyleHu, N., Li, Y., Wang, M., Ji, H., Zhang, X., San, B., & Shi, H. (2024). The Circadian Rhythm of the Behavior and Gut Microbiota in Dybowski’s Frogs (Rana dybowskii) during the Autumn Migration Period. Life, 14(3), 322. https://doi.org/10.3390/life14030322







