Vulnerability of Store-Operated Calcium Entry to Inhibitors and Microenvironment in Cells of Different Breast Cancer Subtypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Fluorescence Measurements
2.4. Statistics
3. Results
3.1. Effect of Selective SOCE Inhibitors on Breast Cancer Cells
3.2. Effect of Nonspecific SOCE Blockers Leflunomide and Teriflunomide
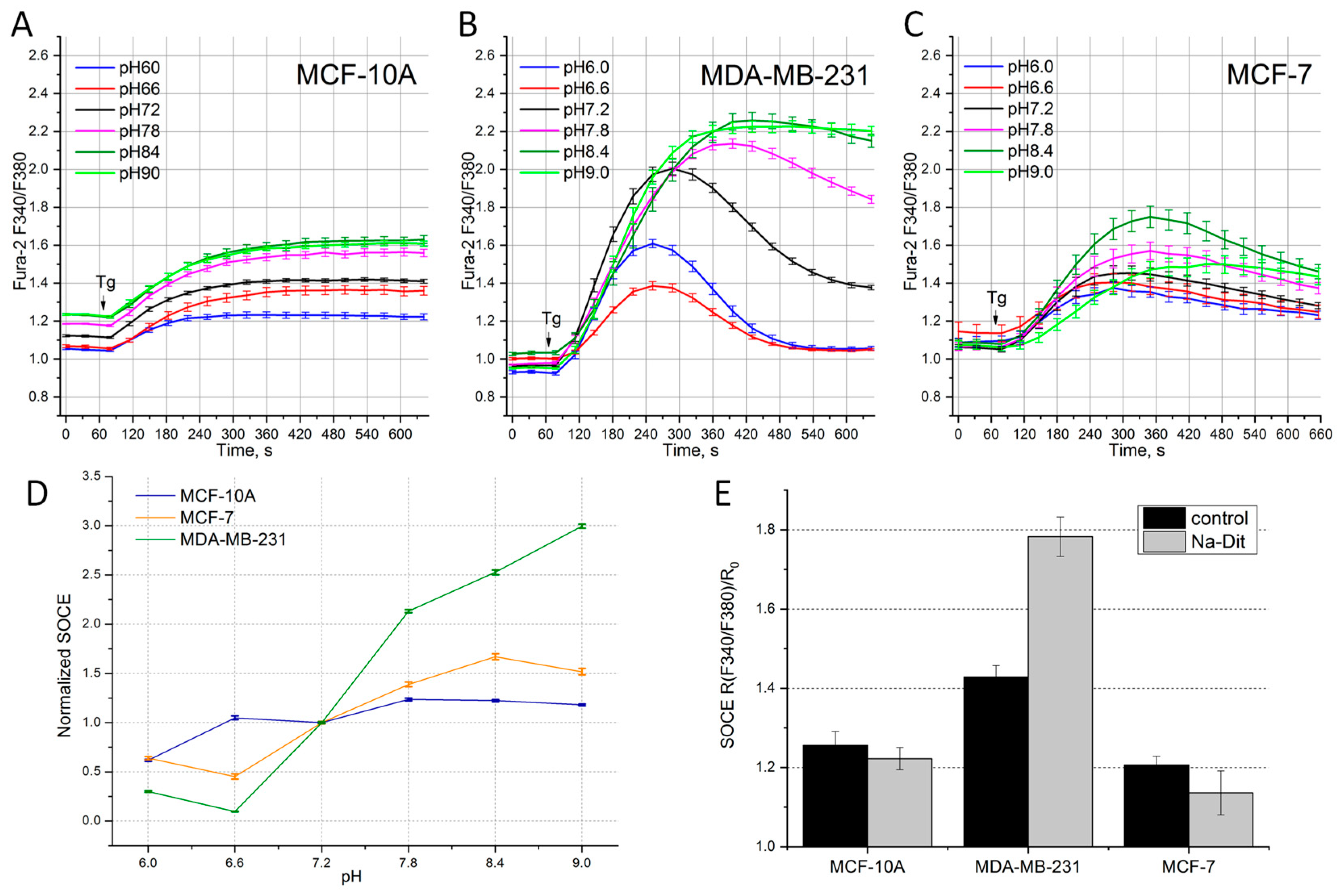
3.3. Sensitivity of SOCE in Breast Cancer Cells to the Extracellular pH
3.4. Sensitivity of SOCE in Breast Cancer Cells to Na-Dithionite
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frisch, J.; Angenendt, A.; Hoth, M.; Roma, L.P.; Lis, A. STIM-Orai Channels and Reactive Oxygen Species in the Tumor Microenvironment. Cancers 2019, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.T.; Hwang, S.Y.; Tomita, T.; DeHaven, W.I.; Mercer, J.C.; Putney, J.W. Activation and Regulation of Store-Operated Calcium Entry. J. Cell. Mol. Med. 2010, 14, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Krause, E.; Pfeiffer, F.; Schmid, A.; Schulz, I. Depletion of Intracellular Calcium Stores Activates a Calcium Conducting Nonselective Cation Current in Mouse Pancreatic Acinar Cells. J. Biol. Chem. 1996, 271, 32523–32528. [Google Scholar] [CrossRef] [PubMed]
- Skopin, A.; Shalygin, A.; Vigont, V.; Zimina, O.; Glushankova, L.; Mozhayeva, G.N.; Kaznacheyeva, E. TRPC1 Protein Forms Only One Type of Native Store-Operated Channels in HEK293 Cells. Biochimie 2013, 95, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Kaznacheyeva, E.; Glushankova, L.; Bugaj, V.; Zimina, O.; Skopin, A.; Alexeenko, V.; Tsiokas, L.; Bezprozvanny, I.; Mozhayeva, G.N. Suppression of TRPC3 Leads to Disappearance of Store-Operated Channels and Formation of a New Type of Store-Independent Channels in A431 Cells. J. Biol. Chem. 2007, 282, 23655–23662. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E.; Meyer, T. STIM Is a Ca2+ Sensor Essential for Ca2+-Store-Depletion-Triggered Ca2+ Influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 Is an Essential Pore Subunit of the CRAC Channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef]
- Sanchez-Collado, J.; Lopez, J.J.; Cantonero, C.; Jardin, I.; Regodón, S.; Redondo, P.C.; Gordillo, J.; Smani, T.; Salido, G.M.; Rosado, J.A. Orai2 Modulates Store-Operated Ca2+ Entry and Cell Cycle Progression in Breast Cancer Cells. Cancers 2022, 14, 114. [Google Scholar] [CrossRef]
- Anderson, M.; Kim, E.Y.; Hagmann, H.; Benzing, T.; Dryer, S.E. Opposing Effects of Podocin on the Gating of Podocyte TRPC6 Channels Evoked by Membrane Stretch or Diacylglycerol. Am. J. Physiol.-Cell Physiol. 2013, 305, C276–C289. [Google Scholar] [CrossRef] [PubMed]
- Dubois, C.; Vanden Abeele, F.; Lehen’kyi, V.; Gkika, D.; Guarmit, B.; Lepage, G.; Slomianny, C.; Borowiec, A.S.; Bidaux, G.; Benahmed, M.; et al. Remodeling of Channel-Forming ORAI Proteins Determines an Oncogenic Switch in Prostate Cancer. Cancer Cell 2014, 26, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Milevskiy, M.J.G.; Chalmers, S.B.; Yapa, K.T.D.S.; Robitaille, M.; Henry, C.; Baillie, G.J.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. ORAI1 and ORAI3 in Breast Cancer Molecular Subtypes and the Identification of ORAI3 as a Hypoxia Sensitive Gene and a Regulator of Hypoxia Responses. Cancers 2019, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Chamlali, M.; Kouba, S.; Rodat-Despoix, L.; Todesca, L.M.; Pethö, Z.; Schwab, A.; Ouadid-Ahidouch, H. Orai3 Calcium Channel Regulates Breast Cancer Cell Migration through Calcium-Dependent and-Independent Mechanisms. Cells 2021, 10, 3487. [Google Scholar] [CrossRef]
- Sanchez-Collado, J.; Lopez, J.J.; Gonzalez-Gutierrez, L.; Cantonero, C.; Jardin, I.; Salido, G.M.; Rosado, J.A. Functional Role of TRPC6 and STIM2 in Cytosolic and Endoplasmic Reticulum Ca2+ Content in Resting Estrogen Receptor-Positive Breast Cancer Cells. Biochem. J. 2020, 477, 3183–3197. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Mallu, P.; Joshi, H.; Schultz, B.; Go, C.; Soboloff, J. Chapter Seven—Ca2+ as a Therapeutic Target in Cancer. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 148, pp. 233–317. [Google Scholar] [CrossRef]
- Tanwar, J.; Arora, S.; Motiani, R.K. Orai3: Oncochannel with Therapeutic Potential. Cell Calcium 2020, 90, 102247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, P.; Yoast, R.E.; Emrich, S.M.; Johnson, M.T.; Pathak, T.; Benson, J.C.; Azimi, I.; Gill, D.L.; Monteith, G.R.; et al. Distinct Pharmacological Profiles of ORAI1, ORAI2, and ORAI3 Channels. Cell Calcium 2020, 91, 102281. [Google Scholar] [CrossRef]
- Skopin, A.Y.; Grigoryev, A.D.; Glushankova, L.N.; Shalygin, A.V.; Wang, G.; Kartzev, V.G.; Kaznacheyeva, E.V. A Novel Modulator of STIM2-Dependent Store-Operated Ca2+ Channel Activity. Acta Naturae 2021, 13, 140–146. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, T. Unveiling Some FDA-Approved Drugs as Inhibitors of the Store-Operated Ca2+ Entry Pathway. Sci. Rep. 2017, 7, 12881. [Google Scholar] [CrossRef]
- Zhang, C.; Chu, M. Leflunomide: A Promising Drug with Good Antitumor Potential. Biochem. Biophys. Res. Commun. 2018, 496, 726–730. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Wang, J.; Ren, C.; Tang, P.; Ouyang, L.; Wang, Y. Recent Advances of Human Dihydroorotate Dehydrogenase Inhibitors for Cancer Therapy: Current Development and Future Perspectives. Eur. J. Med. Chem. 2022, 232, 114176. [Google Scholar] [CrossRef]
- Gehlot, P.; Vyas, V.K. A Patent Review of Human Dihydroorotate Dehydrogenase (HDHODH) Inhibitors as Anticancer Agents and Their Other Therapeutic Applications (1999–2022). Recent Pat. Anti-Cancer Drug Discov. 2024, 19, 280–297. [Google Scholar] [CrossRef]
- Rubaiy, H.N. ORAI Calcium Channels: Regulation, Function, Pharmacology, and Therapeutic Targets. Pharmaceuticals 2023, 16, 162. [Google Scholar] [CrossRef]
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Íñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.J.; Huang, X.Y. Orai1 and STIM1 Are Critical for Breast Tumor Cell Migration and Metastasis. Cancer Cell 2009, 15, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Motiani, R.K.; Zhang, X.; Harmon, K.E.; Keller, R.S.; Matrougui, K.; Bennett, J.A.; Trebak, M. Orai3 Is an Estrogen Receptor α-Regulated Ca2+ Channel That Promotes Tumorigenesis. FASEB J. 2013, 27, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Motiani, R.K.; Abdullaev, I.F.; Trebak, M. A Novel Native Store-Operated Calcium Channel Encoded by Orai3: Selective Requirement of Orai3 versus Orai1 in Estrogen Receptor-Positive versus Estrogen Receptor-Negative Breast Cancer Cells. J. Biol. Chem. 2010, 285, 19173–19183. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Stasevich, O.V.; Salnikova, D.I.; Andreeva, O.E.; Mikhaevich, E.I. Antiestrogenic and Antiproliferative Potency of Secoisolariciresinol Diglucoside Derivatives on MCF-7 Breast Cancer Cells. Nat. Prod. Res. 2020, 35, 6099–6105. [Google Scholar] [CrossRef]
- Audero, M.M.; Prevarskaya, N.; Pla, A.F. Ca2+ Signalling and Hypoxia/Acidic Tumour Microenvironment Interplay in Tumour Progression. Int. J. Mol. Sci. 2022, 23, 7377. [Google Scholar] [CrossRef]
- Pethő, Z.; Najder, K.; Carvalho, T.; McMorrow, R.; Todesca, L.M.; Rugi, M.; Bulk, E.; Chan, A.; Löwik, C.W.G.M.; Reshkin, S.J.; et al. PH-Channeling in Cancer: How PH-Dependence of Cation Channels Shapes Cancer Pathophysiology. Cancers 2020, 12, 2484. [Google Scholar] [CrossRef]
- Stauderman, K.A. CRAC Channels as Targets for Drug Discovery and Development. Cell Calcium 2018, 74, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Zitt, C.; Strauss, B.; Schwarz, E.C.; Spaeth, N.; Rast, G.; Hatzelmann, A.; Hoth, M. Potent Inhibition of Ca2+ Release-Activated Ca2+ Channels and T-Lymphocyte Activation by the Pyrazole Derivative BTP2. J. Biol. Chem. 2004, 279, 12427–12437. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, S.; Wang, Y.; Deng, X.; Landesberg, G.; Scalia, R.; Panettieri, R.A.; Mallilankaraman, K.; Tang, X.D.; Madesh, M.; Gill, D.L. Hypoxia-Induced Acidosis Uncouples the STIM-Orai Calcium Signaling Complex. J. Biol. Chem. 2011, 286, 44788–44798. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Goldman, W.F.; Tod, M.L.; Rubin, L.J.; Blaustein, M.P. Hypoxia Reduces Potassium Currents in Cultured Rat Pulmonary but Not Mesenteric Arterial Myocytes. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1993, 264, L116–L123. [Google Scholar] [CrossRef]
- Ishikawa, J.; Ohga, K.; Yoshino, T.; Takezawa, R.; Ichikawa, A.; Kubota, H.; Yamada, T. A Pyrazole Derivative, YM-58483, Potently Inhibits Store-Operated Sustained Ca2+ Influx and IL-2 Production in T Lymphocytes. J. Immunol. 2003, 170, 4441–4449. [Google Scholar] [CrossRef] [PubMed]
- Nikotina, A.D.; Vladimirova, S.A.; Komarova, E.Y.; Alexeev, D.; Efremov, S.; Leonova, E.; Pavlov, R.; Kartsev, V.G.; Polonik, S.G.; Margulis, B.A.; et al. Prevention of High Glucose-Mediated EMT by Inhibition of Hsp70 Chaperone. Int. J. Mol. Sci. 2021, 22, 6902. [Google Scholar] [CrossRef] [PubMed]
- Shuvalov, O.; Daks, A.; Fedorova, O.; Petukhov, A.; Barlev, N. Linking Metabolic Reprogramming, Plasticity and Tumor Progression. Cancers 2021, 13762. [Google Scholar] [CrossRef] [PubMed]
- Nikotina, A.D.; Vladimirova, S.A.; Kokoreva, N.E.; Komarova, E.Y.; Aksenov, N.D.; Efremov, S.; Leonova, E.; Pavlov, R.; Kartsev, V.G.; Zhang, Z.; et al. Combined Cytotoxic Effect of Inhibitors of Proteostasis on Human Colon Cancer Cells. Pharmaceuticals 2022, 15, 923. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The Tumor Microenvironment at a Glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Hempel, N.; Trebak, M. Crosstalk between Calcium and Reactive Oxygen Species Signaling in Cancer. Cell Calcium 2017, 63, 70–96. [Google Scholar] [CrossRef]
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L. Cancer Cell Behaviors Mediated by Dysregulated PH Dynamics at a Glance. J. Cell Sci. 2017, 130, 663–669. [Google Scholar] [CrossRef]
- Beck, A.; Fleig, A.; Penner, R.; Peinelt, C. Regulation of Endogenous and Heterologous Ca2+ Release-Activated Ca2+ Currents by PH. Cell Calcium 2014, 56, 235–243. [Google Scholar] [CrossRef]
- Thorne, G.D.; Ishida, Y.; Paul, R.J. Hypoxic Vasorelaxation: Ca2+-Dependent and Ca2+-Independent Mechanisms. Cell Calcium 2004, 36, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kandilci, H.B.; Richards, M.A.; Fournier, M.; Şimşek, G.; Chung, Y.J.; Lakhal-Littleton, S.; Swietach, P. Cardiomyocyte Na+/H+ Exchanger-1 Activity Is Reduced in Hypoxia. Front. Cardiovasc. Med. 2021, 7, 617038. [Google Scholar] [CrossRef] [PubMed]

| DMSO | CM4620 | BTP2 | Lef | Ter | |
|---|---|---|---|---|---|
| MCF-10A | 100 ± 6.9% | 11 ± 3% (p < 0.001) | 8 ± 1% (p < 0.001) | 66 ± 1% (p < 0.001) | 88 ± 5% (p = 0.149) |
| MCF-7 | 100 ± 8.1% | 32 ± 13% (p < 0.01) | 35 ± 5% (p < 0.01) | 104 ± 3% (p = 0.817) | 102 ± 2% (p = 0.796) |
| MDA-MB-231 | 100 ± 3.7% | 16 ± 1% (p < 0.001) | 14 ± 1% (p < 0.001) | 79 ± 2% (p < 0.001) | 63 ± 1% (p < 0.001) |
| pH 6 | pH 6.6 | pH 7.2 | pH 7.8 | pH 8.4 | pH 9 | Na-Dit | |
|---|---|---|---|---|---|---|---|
| MCF-10A | 62 ± 6% (p < 0.001) | 107 ± 7% (p = 0.712) | 100 ± 3% | 123 ± 7% (p < 0.01) | 125 ± 7% (p < 0.01) | 118 ± 4% (p < 0.001) | 87 ± 2% (p < 0.01) |
| MCF-7 | 65 ± 3% (p < 0.001) | 46 ± 8% (p < 0.001) | 100 ± 2% | 140 ± 3% (p < 0.001) | 167 ± 7% (p < 0.001) | 162 ± 7% (p < 0.001) | 66 ± 5% (p < 0.001) |
| MDA-MB-231 | 32 ± 4% (p < 0.001) | 11 ± 2% (p < 0.001) | 100 ± 2% | 209 ± 4% (p < 0.001) | 255 ± 6% (p < 0.001) | 307 ± 5% (p < 0.001) | 183 ± 3% (p < 0.001) |
| CM4620 | BTP2 | Lef | Ter | pH 6/6,6 | Na-Dit | |
|---|---|---|---|---|---|---|
| MCF-10A | 89 ± 3% (p < 0.001) | 92 ± 1% (p < 0.001) | 34 ± 1% (p < 0.001) | 12 ± 5% (p = 0.149) | 38 ± 6% (p < 0.001) | 13 ± 2% (p = 0.003) |
| MCF-7 | 66 ± 17% (p = 0.019) | 57 ± 6% (p = 0.008) | - | - | 54 ± 8% (p < 0.001) | 34 ± 5% (p < 0.001) |
| MDA-MB-231 | 84 ± 1% (p < 0.001) | 86 ± 1% (p < 0.001) | 21 ± 2% (p < 0.001) | 37 ± 1% (p < 0.001) | 89 ± 2% (p < 0.001) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skopin, A.Y.; Glushankova, L.N.; Gusev, K.O.; Kaznacheyeva, E.V. Vulnerability of Store-Operated Calcium Entry to Inhibitors and Microenvironment in Cells of Different Breast Cancer Subtypes. Life 2024, 14, 357. https://doi.org/10.3390/life14030357
Skopin AY, Glushankova LN, Gusev KO, Kaznacheyeva EV. Vulnerability of Store-Operated Calcium Entry to Inhibitors and Microenvironment in Cells of Different Breast Cancer Subtypes. Life. 2024; 14(3):357. https://doi.org/10.3390/life14030357
Chicago/Turabian StyleSkopin, Anton Y., Lubov N. Glushankova, Konstantin O. Gusev, and Elena V. Kaznacheyeva. 2024. "Vulnerability of Store-Operated Calcium Entry to Inhibitors and Microenvironment in Cells of Different Breast Cancer Subtypes" Life 14, no. 3: 357. https://doi.org/10.3390/life14030357
APA StyleSkopin, A. Y., Glushankova, L. N., Gusev, K. O., & Kaznacheyeva, E. V. (2024). Vulnerability of Store-Operated Calcium Entry to Inhibitors and Microenvironment in Cells of Different Breast Cancer Subtypes. Life, 14(3), 357. https://doi.org/10.3390/life14030357






