Effects of Chemical and Biological Fungicide Applications on Sexual Sporulation of Rhizoctonia solani AG-3 TB on Tobacco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Fungicides Induce Sexual Sporulation in R. solani AG-3 TB
2.2. Circadian Rhythm and Conditions of Sexual Sporulation
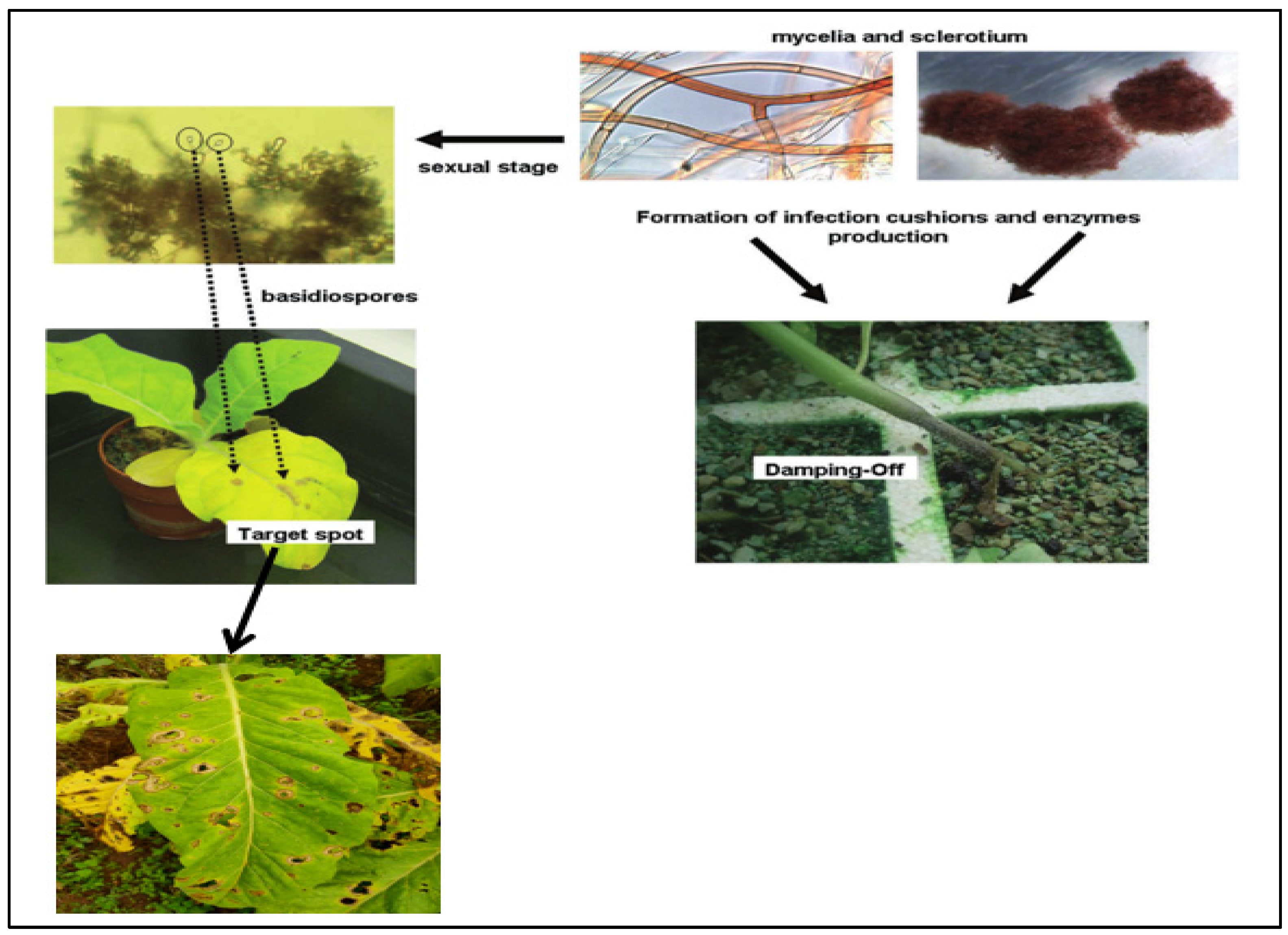
2.3. The Infection Process of Sexual Spores
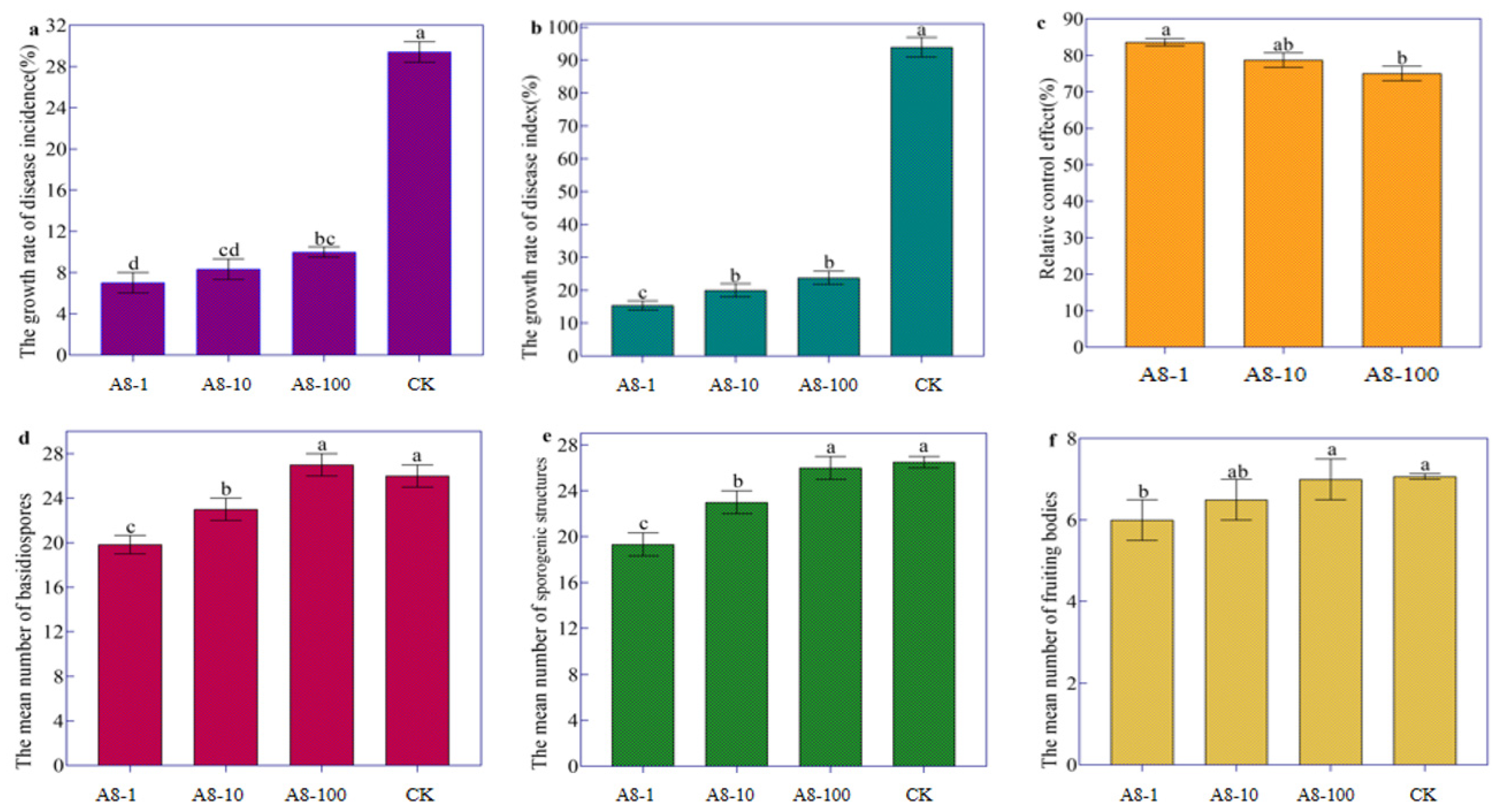
2.4. Biocontrol of Streptomyces rectiolaceus A8 against Tobacco Target Spot Disease
2.5. Determination of Antibacterial Substances in Streptomyces rectiolaceus A8
2.6. Statistical Analyses
3. Results
3.1. Induction of Chemical Fungicides on the Sex Spores of R. solani AG-3 TB
3.2. Circadian Rhythm and Conditions of Sexual Sporulation
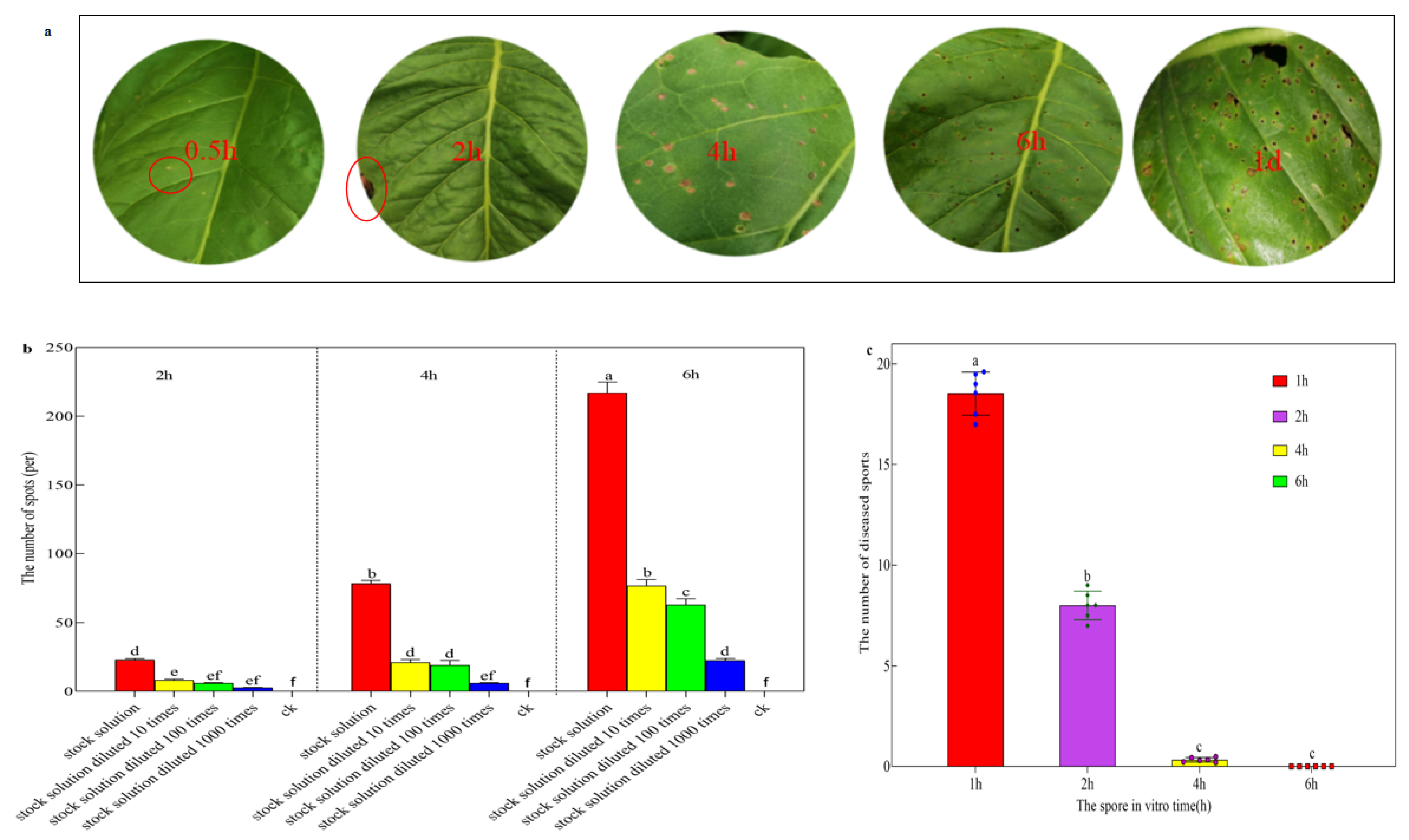
3.3. The Infection Process of Sex Spores of R. solani AG-3 TB
3.4. Streptomyces rectiolaceus A8 Suppressed Tobacco Target Spot Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, G.B. Diseases of Tobacco, 3rd ed.; Biological Consulting Associates: Raleigh, NC, USA, 1975. [Google Scholar] [CrossRef]
- Sneh, B.; Jajabi-Hare, S.; Neate, S.; Dijst, G. Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Gonzalez, D.; Carling, D.E.; Kuninaga, S.; Vilgalys, R.; Cubeta, M.A. Ribosomal DNA systematics of Ceratobasidium and Thanatephorus with Rhizoctonia anamorphs. Mycologia 2001, 93, 1138–1150. [Google Scholar] [CrossRef]
- Gonzalez, M.; Pujol, M.; Metraux, J.P.; Vicente, G.G.; Bolton, M.D.; Orlando, B.H. Tobacco leaf spot and root rot caused by Rhizoctonia solani Kühn. Mol. Plant Pathol. 2011, 12, 209–216. [Google Scholar] [CrossRef]
- Zachow, C.; Grosch, R.; Berg, G. Impact of biotic and a-biotic parameters on structure and function of microbial communities living on sclerotia of the soil-borne pathogenic fungus Rhizoctonia solani. Appl. Soil Ecol. 2011, 48, 193–200. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, D.T.; Wilson, D.M.; Nelsen, T.C. Survival of Aspergillus flavus sclerotia and conidia buried in soil in Illinois or Georgia. Phytopathology 1993, 83, 1141–1147. [Google Scholar] [CrossRef]
- Senapati, M.; Tiwari, A.; Sharma, N.; Chandra, P.; Bashyal, B.M.; Ellur, R.K.; Bhowmick, P.K.; Bollinedi, H.; Vinod, K.K.; Singh, A.K.; et al. Rhizoctonia solani Kühn Pathophysiology: Status and Prospects of Sheath Blight Disease Management in Rice. Front. Plant Sci. 2022, 13, 881116. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.B.; Chopperla, R.; Prathi, N.B.; Balakrishnan, M.; Prakasam, V.; Laha, G.S.; Balachandran, S.M.; Mangrauthia, S.K. A Comprehensive Gene Expression Profile of Pectin Degradation Enzymes Reveals the Molecular Events during Cell Wall Degradation and Pathogenesis of Rice Sheath Blight Pathogen Rhizoctonia solani AG1-IA. J. Fungi 2020, 6, 71. [Google Scholar] [CrossRef]
- Su, Y.N.; Dong, X.; Zhao, Y.Q.; Sun, H.W.; Sun, J.P.; Wu, Y.H. Study on the Fusion Group, Pathogenicity Differentiation and Variety Disease Resistance of Tobacco Target Spot Pathogen (Rhizoctonia solani) in Northeast China. Plant Prot. 2016, 42, 170–174. [Google Scholar] [CrossRef]
- Zhuang, Z.X.; Fan, J.Y.; Xu, Y.; Hu, Z.J.; Han, Y.X.; Zuo, W.J. Combined Effect of Thifluzamide and Pyraclostrobin on Potato Black Scurf and Its Field Effect. Mod. Agrochem. 2017, 16, 48–51. [Google Scholar]
- Zheng, L.; Shu, C.; Zhang, M.; Yang, M.; Zhou, E. Molecular Characterization of a Novel Endornavirus Conferring Hypovirulence in Rice Sheath Blight Fungus Rhizoctonia solani AG-1 IA Strain GD-2. Viruses 2019, 11, 178. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Yu, D. Induction of Sexual Reproduction of Rhizoctonia solani Anastomosis Groups 2-1 and 4 Using Soil Treatment. Plant Dis. 2016, 100, 115–120. [Google Scholar]
- Jurick, W.M.; Vico, I.; Gaskins, V.L.; Janisiewicz, W.J. Medium-Induced Production of Sexual and Asexual Spores by Rhizoctonia solani AG-1 IA, AG-3, AG-5, AG-6, and AG-8. Mycologia 2013, 105, 164–174. [Google Scholar]
- Shew, H.D.; Lucas, G.B. Induction and Spread of Basidiospores of Rhizoctonia solani from Tobacco Stems. Phytopathology 1980, 70, 656–660. [Google Scholar]
- Jurick, W.M.; Rollins, J.A.; Liu, Y. Induction of Sexual Reproduction and Genetic Diversity in the Plant Pathogen Rhizoctonia solani AG-3 through Parasexual Recombination Using Hyphal Anastomosis. Phytopathology 2012, 102, 441–449. [Google Scholar]
- Kangatharalingam, N.; Carson, M.L. Technique to Induce Sporulation in Thanatephorus cucumeris. Plant Dis. 1988, 72, 146–148. [Google Scholar] [CrossRef]
- Shew, H.D.; Lucas, G.B.; Goley, M. Basidiospore Production by Rhizoctonia solani on Tobacco. Phytopathology 1983, 73, 1532–1536. [Google Scholar]
- De, C.A.; Pascual, S.; Melgarejo, P. Effect of Temperature, pH, and Water Activity on Mycelial Growth, Colony Morphology, and Conidial Germination of Rosellinia necatrix. Mycologia 2000, 92, 116–126. [Google Scholar]
- Xu, X.M.; Jeffries, P. Effects of Temperature and Relative Humidity on Conidial Germination, Germ Tube Growth, and Lesion Development of Sclerotinia sclerotiorum on Detached Leaves of Soybean and Dry Bean. Plant Dis. 2007, 91, 1658–1663. [Google Scholar]
- Zhang, Z.; Li, J.; Pan, H.; Qiu, D. Effects of Temperature, Light, and pH on Mycelial Growth, Sporulation, and Pathogenicity of Fusarium oxysporum f. sp. Niveum. Eur. J. Plant Pathol. 2014, 139, 195–204. [Google Scholar]
- Deng, M.X.; Tang, B.; Huang, L.P.; Jiang, L. Study on the Factors Affecting the Germination of AM Fungal Spores. J. Guizhou Univ. 2014, 31, 38–41+54. [Google Scholar] [CrossRef]
- Shew, H.D. Rhizoctonia Leaf Spot of Flue-Cured Tobacco in North Carolina. Plant Dis. 1985, 69, 901–903. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, F.; Wang, B.; Qu, P.; Liu, J.; Zhang, Y.; Liu, W.; Tong, Z.; Deng, G. Influences of Serendipita indica and Dictyophorae echinovolvata on the Growth and Fusarium Wilt Disease Resistance of Banana. Biology 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Huang, L.; Li, Y.; Zhao, C.; Tao, B.; Zhang, W. Characteristics of Soil Fungal Communities in Soybean Rotations. Front. Plant Sci. 2022, 13, 926731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Hu, L.; Jia, R.; Ma, Q.; Tang, J.; Wang, Y. Antagonistic Action of Streptomyces pratensis S10 on Fusarium graminearum and Its Complete Genome Sequence. Environ. Microbiol. 2021, 23, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Saxena, R.K.; Chaturvedi, P.; Virdi, J.S. Chitinase Production by Streptomyces viridificans: Its Potential in Fungal Cell Wall Lysis. J. Appl. Bacteriol. 1995, 78, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, G.A.; Liu, J.; Tahlan, K.; Bignell, D.R.D. Nigericin and Geldanamycin Are Phytotoxic Specialized Metabolites Produced by the Plant Pathogen Streptomyces sp. 11-1-2. Microbiol. Spectr. 2022, 10, e0231421. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, J. Correction for Han et al., Identifcation and Predictions Regarding the Biosynthesis Pathway of Polyene Macrolides Produced by Streptomyces roseofavus Men-Myco-93-63. Appl. Environ. Microbiol. 2021, 87, e00802-21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Zhang, D.H.; Duan, X.Y.; Lu, X.Y. Studies on the Fusion Classification and Sexual Generation of Rhizoctonia solani Hyphae. Acta Phytopathol. Sin. 1985, 15, 140–143. [Google Scholar] [CrossRef]
- Jurick, W.M.I.; Vico, I.; Gaskins, V.L.; Garrett, W.M.; Conway, W.S. Purification and Biochemical Characterization of Polygalacturonase Produced by Penicillium expansum during Postharvest Decay of “Anjou” Pear. Phytopathology 2010, 100, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ii, W.M.J.; Vico, I.; Gaskins, V.L.; Peter, K.A.; Park, E.; Janisiewicz, W.J.; Conway, W.S. Carbon, Nitrogen and pH Regulate the Production and Activity of a Polygalacturonase isozyme Produced by Penicillium expansum. Arch. Phytopathol. Plant Prot. 2012, 45, 1101–1114. [Google Scholar] [CrossRef]
- Shew, H.D. Infection and Development of Target Spot of Flue-Cured Tobacco Caused by Thanatephorus cucumeris. Plant Dis. 1991, 74, 1009–1013. [Google Scholar] [CrossRef]
- Bai, A.; Chen, A.; Chen, W.; Luo, X.; Liu, S.; Zhang, M.; Liu, Y.; Zhang, D. Study on Degradation Behaviour, Residue Distribution, and Dietary Risk Assessment of Propiconazole in Celery and Onion under Field Application. J. Sci. Food Agric. 2021, 101, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society (APS Press): St. Paul, MN, USA, 1996; Volume 90, pp. 1092–1093. [Google Scholar]
- Tan, Q.; Schnabel, G.; Chaisiri, C.; Yin, L.-F.; Yin, W.-X.; Luo, C.-X. Colletotrichum Species Associated with Peaches in China. J. Fungi 2022, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Chen, X.J.; Cai, L.T.; Cao, Y.; Lu, N.; Xia, H.Q.; Wang, M.S.; Shang, S.H. Race Distribution and Distribution of Sensitivities to Mefenoxam among Isolates of Phytophthora parasitica Var. Nicotianae in Guizhou Province of China. Crop Prot. 2013, 52, 136–140. [Google Scholar] [CrossRef]
- Han, X.B.; Zhao, J.; Cao, J.M.; Zhang, C.S. Essential Oil of Chrysanthemum indicum L.: Potential Biocontrol Agent against Plant Pathogen Phytophthora nicotianae. Environ. Sci. Pollut. Res. Int. 2019, 26, 7013–7023. [Google Scholar] [CrossRef]
- Yang, Y.M.; Lu, C.H.; Luo, C.W.; Su, J.E.; Wu, D.X.; Xia, Z.Y. Screening of Biocontrol Agents against Tobacco Black Shank and Its Effect on Rhizosphere Soil Microbial Community. Southwest Agric. J. 2022, 35, 790–796. [Google Scholar] [CrossRef]
- Pál, K.; van Diepeningen, A.D.; Varga, J.; Hoekstra, R.F.; Dyer, P.S.; Debets, A.J.M. Sexual and Vegetative Compatibility Genes in the Aspergilli. Stud. Mycol. 2007, 59, 19–30. [Google Scholar] [CrossRef]
- Clarkson, J.P.; Staveley, J.; Phelps, K.; Young, C.S.; Whipps, J.M. Ascospore Release and Survival in Sclerotinia sclerotiorum. Mycol. Res. 2003, 107, 213–222. [Google Scholar] [CrossRef]
- Pearson, R.C.; Siegfried, W.; Bodmer, M.; Schüepp, H. Ascospore Discharge and Survival in Pseudopezicula tracheiphila, Causal Agent of Rotbrenner of Grape. J. Phytopathol. 1991, 132, 177–185. [Google Scholar] [CrossRef]
- Wang, C.Z.; Huang, H.; Dong, X.; Su, Y.N. Screening of Fungicides for Controlling Tobacco Target Spot. Henan Agric. Sci. 2016, 45, 98–101. [Google Scholar] [CrossRef]
- Parlevliet, J.E. What Is Durable Resistance, A General Outline; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar] [CrossRef]
- LI, N. Effects of Nutrient Elements on the Growth of Phytophthora nicotianae and Gene Differential Transcription in the Sporangiogenesis of P. nicotianae by Boron. Master’s Thesis, Southwest University, Chongqing, China, 2014; p. 78. [Google Scholar]
- Sara, M.P.; Squarzoni, A.; Guzmán, C.H.; Alejandra, J.P.A.; Santiago, G.; Pedro, A.C. Organic and Conventional Bean Pesticides in Development of Autochthonous trichoderma Strains. J. Fungi 2022, 8, 603. [Google Scholar] [CrossRef]
- Cannon, S.; Kay, W.; Kilaru, S.; Schuster, M.; Gurr, S.J.; Steinberg, G. Multi-site fungicides suppress banana Panama disease, caused by Fusarium oxysporum f. sp. cubense Tropical Race 4. PLoS Pathog. 2022, 18, e1010860. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Mandal, A.; Kundu, A.; Malik, M.; Chaudhary, A.; Khan, M.R.; Shanmugam, V.; Rao, U.; Saha, S. Deciphering the Behavioral Response of Meloidogyne incognita and Fusarium oxysporum Toward Mustard Essential Oil. Front. Plant Sci. 2021, 12, 714730. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.; Atkins, S.; Hanley, S.; Macdonald, A.; Lucas, J. The Multi-Fungicide Resistance Status of Aspergillus fumigatus Populations in Arable Soils and the Wider European Environment. Front. Microbiol. 2020, 11, 599233. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, F.; Cheng, C.; Chen, L.N.; Shi, X.J.; Gao, X.D.; Li, J. Synergistic Antifungal Activity of Graphene Oxide and Fungicides against Fusarium Head Blight In Vitro and In Vivo. Nanomaterials 2021, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.M.; Undabeytia, T.; Jaworski, M.; Morillo, E.; Sanchez, R.M.T. Organo-montmorillonites as adsorbent materials for thiophanate-methyl removal: Adsorption-desorption studies and technological applications. J. Environ. Chem. Eng. 2020, 8, 103806. [Google Scholar] [CrossRef]
- Dong, B.Z.; Hu, J.Y. Residue dissipation and dietary intake risk assessment of thiophanate-methyl and its metabolite carbendazim in watercress under Chinese field conditions. Int. J. Environ. Anal. Chem. 2021, 103, 561–574. [Google Scholar] [CrossRef]
- Xie, X. The Reduction Effect of Sulfur Form and Complex Factor on Nitrate Accumulation Pollution of Chinese Chive; Hebei Agricultural University: Baoding, China, 2014. [Google Scholar]
- Jain, S.; Wiemann, P.; Thill, E.; Williams, B.; Keller, N.P.; Kabbage, M. A Bcl-2 Associated Athanogene (bagA) Modulates Sexual Development and Secondary Metabolism in the Filamentous Fungus Aspergillus nidulans. Front. Microbiol. 2018, 9, 1316. [Google Scholar] [CrossRef]
- Naito, S.; Mochida, H.; Nakajima, T.; Ohto, Y. Infection with Basidiospores of Thanatephorus cucumeris (AG-2-3 of Rhizoctonia solani) and Development of Soybean Foliar Blight Lesions. Jpn. J. Phytopathol. 1995, 61, 362–368. [Google Scholar] [CrossRef]
- Windels, C.E.; Kuznia, R.A.; Call, J. Characterization and Pathogenicity of Thanatephorus cucumeris from Sugar Beet in Minnesota. Plant Dis. 1997, 81, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.C.; Butler, E.E. Environmental Factors Influencing the Formation of Basidia and Basidiospores in Thanatephorus cucumeris. Phytopathology 1983, 73, 152–155. [Google Scholar] [CrossRef]
- Liu, X.R.; Zhang, J.; Wang, X.J.; Sun, Y.; Yang, J.; Yang, M.; Dong, W.H. Field Observation of Environmental Conditions and Pathogenicity of Sexual Spores of Tobacco Target Spot Disease. J. Yunnan Agric. Univ. 2020, 35, 221–226. [Google Scholar] [CrossRef]
- Araujo, R. Advances in Soil Engineering: Sustainable Strategies for Rhizosphere and Bulk Soil Microbiome Enrichment. Front. Biosci. Landmark 2022, 27, 195. [Google Scholar] [CrossRef]
- Newitt, J.T.; Prudence, S.M.M.; Hutchings, M.I.; Worsley, S.F. Biocontrol of Cereal Crop Diseases Using Streptomycetes. Pathogens 2019, 8, 78. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and Identification of Bioactive Compounds (Eicosane and Dibutyl Phthalate) Produced by Streptomyces Strain KX852460 for the Biological Control of Rhizoctonia solani AG-3 Strain KX852461 to Control Target Spot Disease in Tobacco Leaf. AMB Express 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.M.; Xiao, K.Y.; Yu, L.G.; Chen, J.; Hu, L.F.; Wang, Y. A Potential Biocontrol Agent Streptomyces tauricus XF for Managing Wheat Stripe Rust. Phytopathol. Res. 2023, 5, 14. [Google Scholar] [CrossRef]



| Treatments | Disease Incidence (%) | Disease Index (%) | ||
|---|---|---|---|---|
| Before the Application of A8 | After the Application of A8 | Before the Application of A8 | After the Application of A8 | |
| A8-1 | 57.00 | 61.00 | 2.60 | 3.00 |
| A8-10 | 60.00 | 65.00 | 3.50 | 4.20 |
| A8-100 | 70.00 | 77.00 | 4.20 | 5.20 |
| CK | 52.00 | 67.30 | 3.30 | 6.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, J.; Yan, J.; Zhao, L.; Luo, L.; Li, C.; Yang, G. Effects of Chemical and Biological Fungicide Applications on Sexual Sporulation of Rhizoctonia solani AG-3 TB on Tobacco. Life 2024, 14, 404. https://doi.org/10.3390/life14030404
Yang Y, Zhang J, Yan J, Zhao L, Luo L, Li C, Yang G. Effects of Chemical and Biological Fungicide Applications on Sexual Sporulation of Rhizoctonia solani AG-3 TB on Tobacco. Life. 2024; 14(3):404. https://doi.org/10.3390/life14030404
Chicago/Turabian StyleYang, Yingmei, Jie Zhang, Jiduo Yan, Lianjin Zhao, Li Luo, Chengyun Li, and Genhua Yang. 2024. "Effects of Chemical and Biological Fungicide Applications on Sexual Sporulation of Rhizoctonia solani AG-3 TB on Tobacco" Life 14, no. 3: 404. https://doi.org/10.3390/life14030404
APA StyleYang, Y., Zhang, J., Yan, J., Zhao, L., Luo, L., Li, C., & Yang, G. (2024). Effects of Chemical and Biological Fungicide Applications on Sexual Sporulation of Rhizoctonia solani AG-3 TB on Tobacco. Life, 14(3), 404. https://doi.org/10.3390/life14030404





