Influence of Intraoperative Active and Passive Breaks in Simulated Minimally Invasive Procedures on Surgeons’ Perceived Discomfort, Performance, and Workload
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Sample
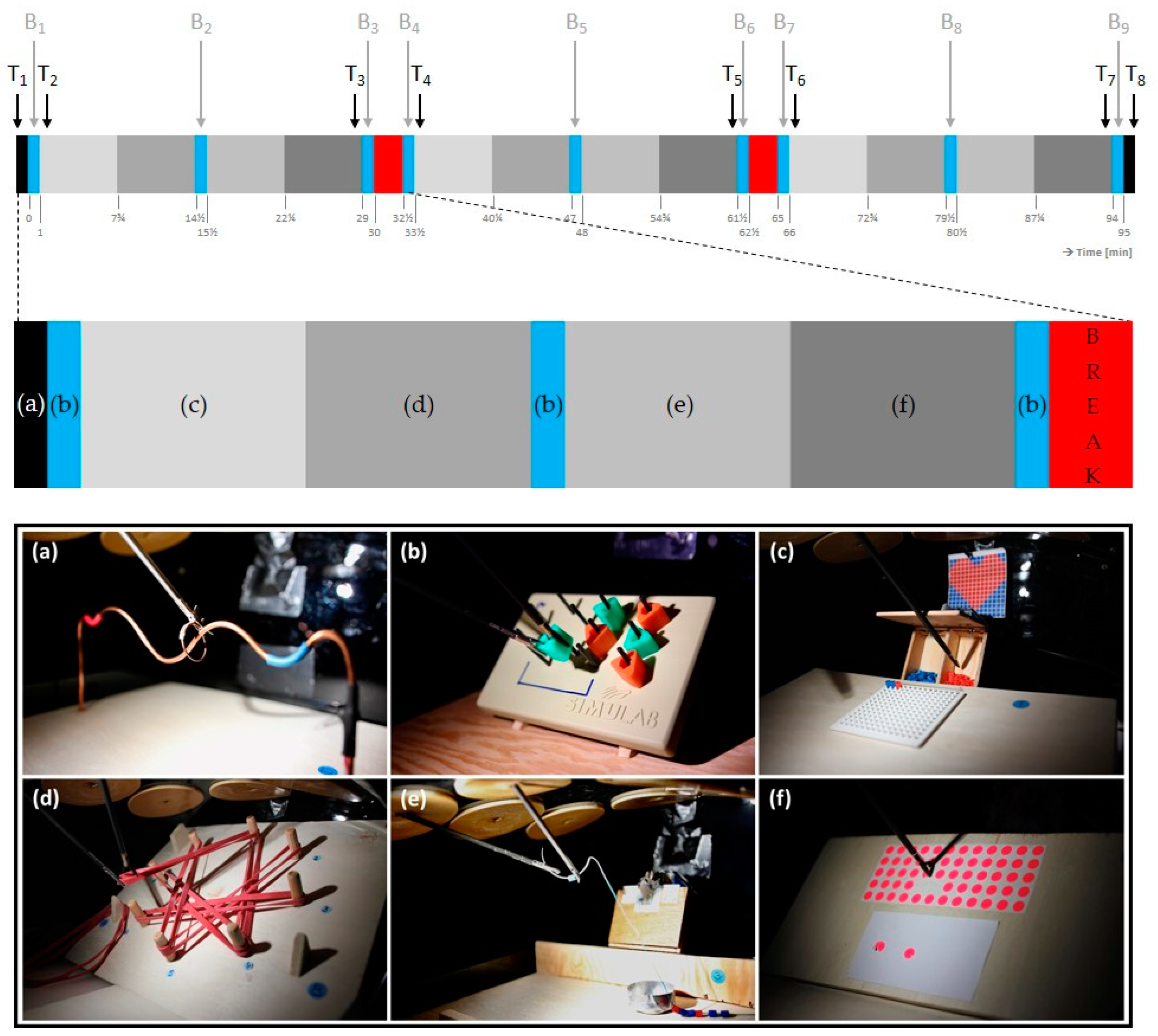
2.3. Intervention
2.4. Experimental Procedure
2.5. Data Collection and Data Analysis
2.5.1. Rating of Perceived Discomfort
2.5.2. Performance
2.5.3. Perceived Workload
2.5.4. Subjective Evaluation of the Implemented Breaks
2.6. Statistical Analysis
Exploratory Subgroup Analyses
3. Results
3.1. Study Sample
3.2. Rating of Perceived Discomfort
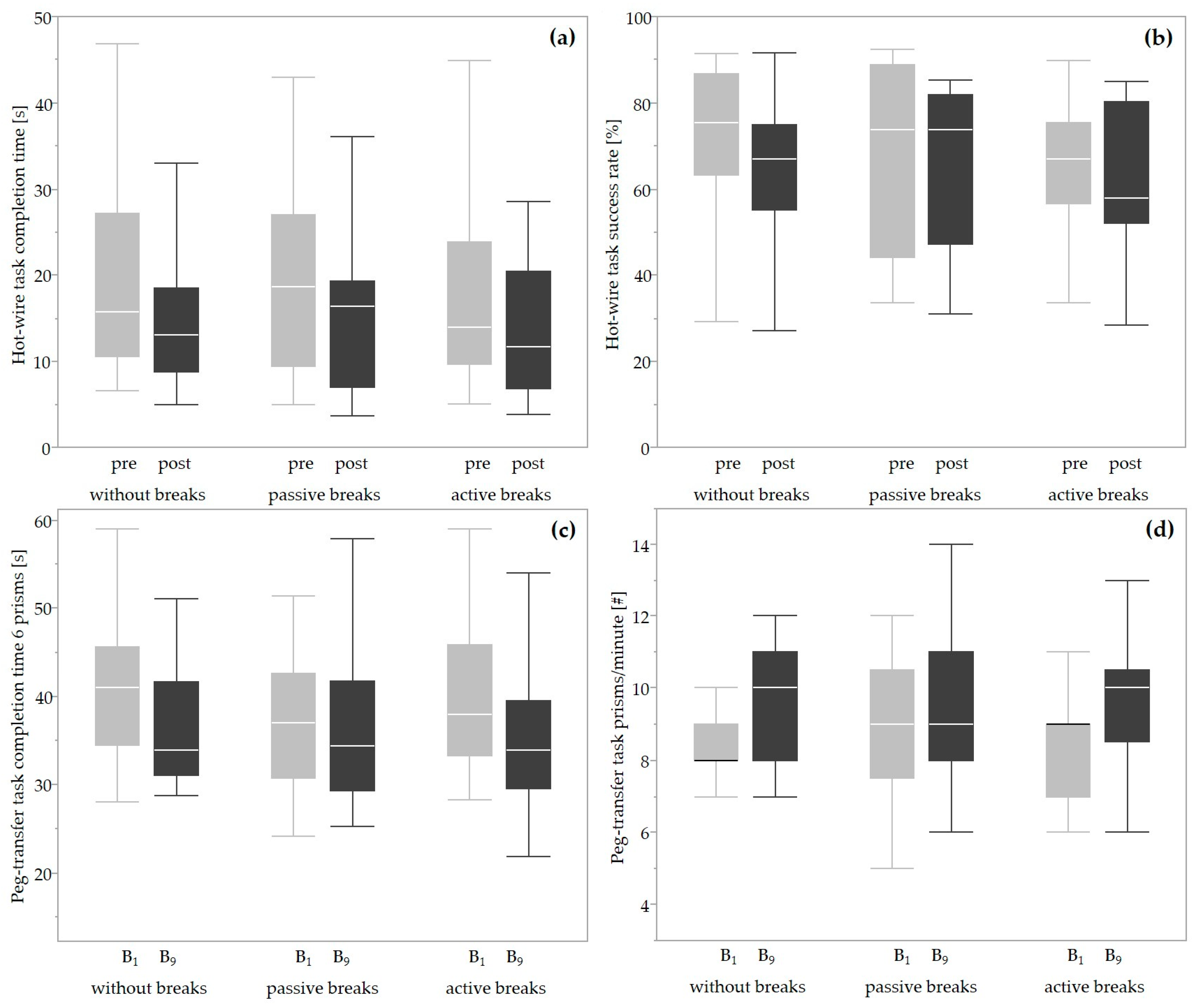
3.3. Performance
3.4. Perceived Workload
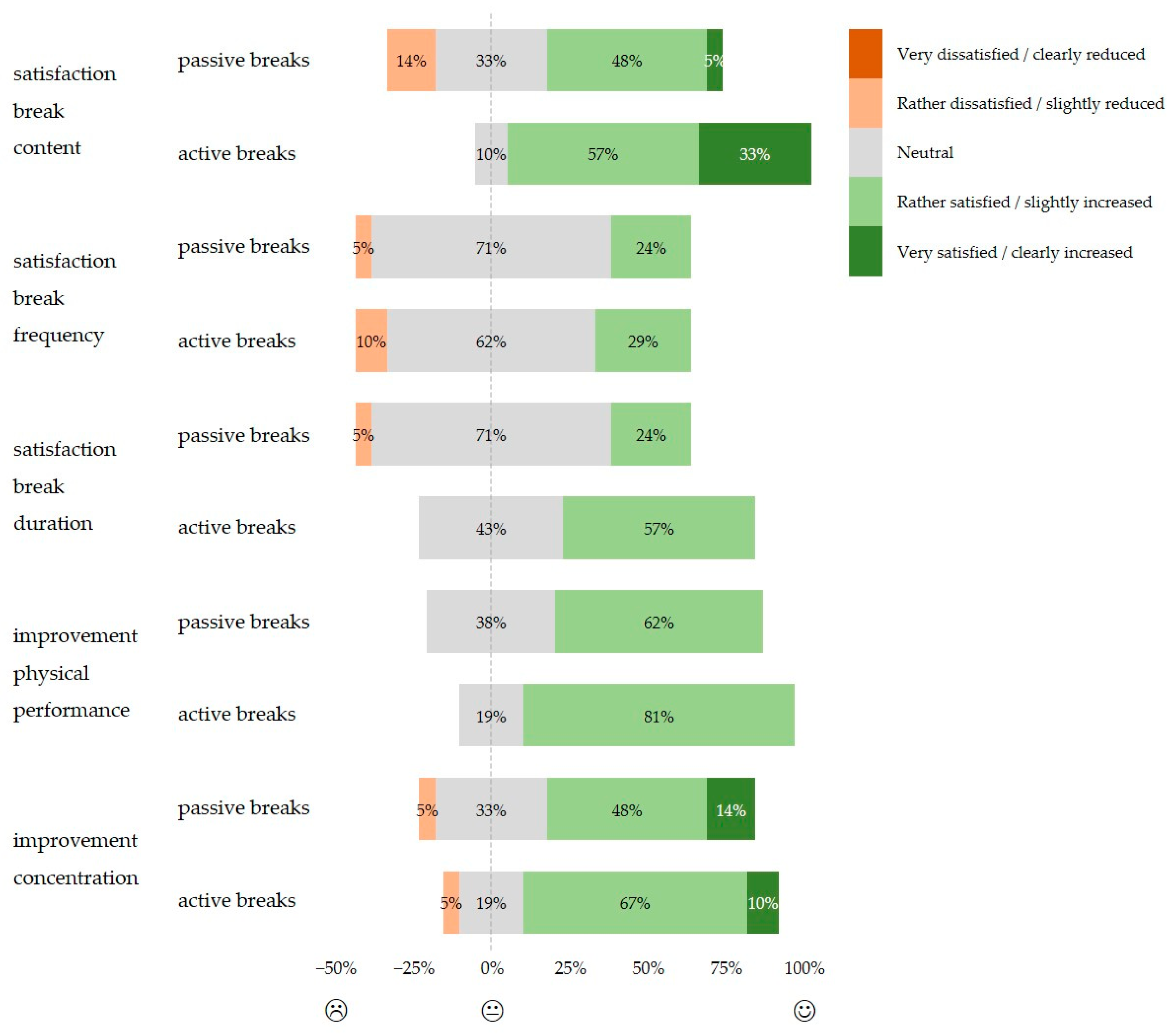
3.5. Subjective Evaluation of the Implemented Breaks
4. Discussion
4.1. Rating of Perceived Discomfort
4.2. Performance
4.3. Perceived Workload
4.4. Subjective Assessment of the Implemented Breaks
4.5. Study Implications, Strengths, and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Medical Association. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Committee on Patient Safety and Quality Improvement. Patient Safety in the Surgical Environment; The American College of Obstetricians and Gynecologists: Washington, DC, USA, 2010. [Google Scholar]
- Berguer, R.; Rab, G.T.; Abu-Ghaida, H.; Alarcon, A.; Chung, J. A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg. Endosc. 1997, 11, 139–142. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Ho, H.S.; Smith, W.D.; Philipps, C.; Lewis, C.; De Vera, R.M.; Berguer, R. An ergonomic evaluation of surgeons’ axial skeletal and upper extremity movements during laparoscopic and open surgery. Am. J. Surg. 2001, 182, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Allen, M.S.; Wigle, D.A.; Shen, K.R.; Cassivi, S.D.; Nichols, F.C., III; Deschamps, C. Does surgeon workload per day affect outcomes after pulmonary lobectomies? Ann. Thorac. Surg. 2012, 94, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Kc, D.S.; Terwiesch, C. Impact of workload on service time and patient safety: An econometric analysis of hospital operations. Manag. Sci. 2009, 55, 1486–1498. [Google Scholar] [CrossRef]
- West, M.; Coia, D. Caring for Doctors, Caring for Patients; General Medical Council: London, UK, 2019. [Google Scholar]
- Epstein, S.; Sparer, E.H.; Tran, B.N.; Ruan, Q.Z.; Dennerlein, J.T.; Singhal, D.; Lee, B.T. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists—A systematic review and meta-analysis. JAMA Surg. 2018, 153, e174947. [Google Scholar] [CrossRef] [PubMed]
- Plerhoples, T.A.; Hernandez-Boussard, T.; Wren, S.M. The aching surgeon: A survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J. Robot. Surg. 2012, 6, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Dabholkar, T.; Yardi, S.; Dabholkar, Y. Prevalence of work-related musculoskeletal symptoms in surgeons performing minimally invasive surgery: A review of literature. Int. Surg. J. 2016, 34, 1028–1034. [Google Scholar] [CrossRef]
- Alleblas, C.C.J.; de Man, A.M.; van den Haak, L.; Vierhout, M.E.; Jansen, F.W.; Nieboer, T.E. Prevalence of musculoskeletal disorders among surgeons performing minimally invasive surgery. Ann. Surg. 2017, 266, 905–920. [Google Scholar] [CrossRef]
- Park, A.; Lee, G.; Seagull, F.J.; Meenaghan, N.; Dexter, D. Patients benefit while surgeons suffer: An impending epidemic. J. Am. Coll. Surg. 2010, 210, 306–313. [Google Scholar] [CrossRef]
- Wendsche, J.; Lohmann-Haislah, A.; Wegge, J. The impact of supplementary short rest breaks on task performance—A meta-analysis. Sozialpolitik. CH 2016, 2, 1–24. [Google Scholar] [CrossRef]
- Kinnunen, U.; Natti, J. Work ability score and future work ability as predictors of register-based disability pension and long-term sickness absence: A three-year follow-up study. Scand. J. Public Health 2018, 46, 321–330. [Google Scholar] [CrossRef]
- Gómez, M.A.; Jiménez, S.; Navarro, R.; Lago-Penas, C.; Sampaio, J. Effects of coaches’ timeouts on basketball teams’ offensive and defensive performances according to momentary differences in score and game period. Eur. J. Sport Sci. 2011, 11, 303–308. [Google Scholar] [CrossRef]
- Park, A.E.; Zahiri, H.R.; Hallbeck, M.S.; Augenstein, V.; Sutton, E.; Yu, D.; Lowndes, B.R.; Bingener, J. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: A multicenter cohort study. Ann. Surg. 2017, 265, 340–346. [Google Scholar] [CrossRef]
- Dorion, D.; Darveau, S. Do micropauses prevent surgeon’s fatigue and loss of accuracy associated with prolonged surgery? An experimental prospective study. Ann. Surg. 2013, 257, 256–259. [Google Scholar] [CrossRef]
- Engelmann, C.; Schneider, M.; Kirschbaum, C.; Grote, G.; Dingemann, J.; Schoof, S.; Ure, B.M. Effects of intraoperative breaks on mental and somatic operator fatigue: A randomized clinical trial. Surg. Endosc. 2011, 25, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Hallbeck, M.S.; Lowndes, B.R.; Bingener, J.; Abdelrahman, A.M.; Yu, D.; Bartley, A.; Park, A.E. The impact of intraoperative microbreaks with exercises on surgeons: A multi-center cohort study. Appl. Ergon. 2017, 60, 334–341. [Google Scholar] [CrossRef]
- Kromberg, L.S.; Kildebro, N.V.; Mortensen, L.Q.; Amirian, I.; Rosenberg, J. Microbreaks in laparoscopic appendectomy have no effect on surgeons’ performance and well-being. J. Surg. Res. 2020, 251, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, A.; Usero, D.; Rodil, J.; Topor-Mądry, R. The influence of micropauses on surgeons’ precision after short laparoscopy procedures. Pol. Prz. Chir. 2015, 87, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Bonsch, R.; Seibt, R.; Kramer, B.; Rieger, M.A.; Steinhilber, B. Intraoperative active and passive breaks during minimally invasive surgery influence upper extremity physical strain and physical stress response-a controlled, randomized cross-over, laboratory trial. Surg. Endosc. 2023, 37, 5975–5988. [Google Scholar] [CrossRef] [PubMed]
- ClinialTrials.gov [Internet]. Work breaks during simulated minimally invasive surgery. In ClinicalTrials.gov Identifier NCT03715816; National Library of Medicine: Bethesda, MD, USA, 2018. [Google Scholar]
- Kadam, P.; Bhalerao, S. Sample size calculation. Int. J. Ayurveda Res. 2010, 1, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Wenger, L.; Richardson, C.; Tsuda, S. Retention of fundamentals of laparoscopic surgery (fls) proficiency with a biannual mandatory training session. Surg. Endosc. 2015, 29, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Seibt, R.; Kraemer, B.; Bonsch, R.; Rieger, M.A.; Steinhilber, B. Implementation of short passive and active breaks during simulared laparoscopic work. In Proceedings of the 10th International Scientific Conference on the Prevention of Work-Related Musculoskeletal Disorders (PREMUS), Bologna, Italy, 2–5 September 2019; p. 362. [Google Scholar]
- Luger, T.; Rieger, M.A.; Bonsch, R.; Krämer, B.; Seibt, R.; Steinhilber, B. Active and passive work breaks during simulated laparoscopy among laparoscopic surgeons: Study protocol for a controlled, randomised cross-over laboratory trial. BMJ Open 2020, 10, e038952. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Maher, C.G.; Rieger, M.A.; Steinhilber, B. Work-break schedules for preventing musculoskeletal symptoms and disorders in healthy workers. Cochrane Database Syst. Rev. 2019, 7, CD012886. [Google Scholar] [CrossRef] [PubMed]
- Coleman Wood, K.A.; Lowndes, B.R.; Buus, R.J.; Hallbeck, M.S. Evidence-based intraoperative microbreak activities for reducing musculoskeletal injuries in the operating room. Work 2018, 60, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Rothmund, R.; Michaelis, M.; Rieger, M.A.; Steinhilber, B. Welche muskuloskelettalen beschwerden und arbeitsorganisatorische maßnahmen zu ihrer reduktion berichtet das chirurgische personal in der gynäkologie? Studiendesign einer standardisierten befragung mit fokus auf laparoskopische eingriffe. In Proceedings of the 63. Frühjahrskongress 2017 der Gesellschaft für Arbeitswissenschaft e.V., Dortmund, Germany, 15–17 February 2017. [Google Scholar]
- Steinhilber, B.; Karle, E.; Schmidt, J.; Rothmund, R.; Michaelis, M.; Rieger, M.A.; Krämer, B. Prevalence of musculoskeletal complaints in minimal invasive surgery. In Proceedings of the 10th International Conference on the Prevention of Work-Related Musculoskeletal Disorders (PREMUS), Bologna, Italy, 2–5 September 2019. [Google Scholar]
- Caffier, G.; Steinberg, U.; Liebers, F. Praxisorientiertes Methodeninventar zur Belastungs- und Beanspruchungsbeurteilung im Zusammenhang mit Arbeitsbedingten Muskel-Skelett-Erkrankunen; Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAUA): Dortmund/Berlin, Germany, 1999. [Google Scholar]
- van Det, M.J.; Meijerink, W.J.; Hoff, C.; Totte, E.R.; Pierie, J.P. Optimal ergonomics for laparoscopic surgery in minimally invasive surgery suites: A review and guidelines. Surg. Endosc. 2009, 23, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Hamberg-van Reenen, H.H.; van der Beek, A.J.; Blatter, B.M.; van der Grinten, M.P.; van Mechelen, W.; Bongers, P.M. Does musculoskeletal discomfort at work predict future musculoskeletal pain? Ergonomics 2008, 51, 637–648. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998; p. 104. [Google Scholar]
- Corlett, E.N. The evaluation of posture and its effects. In Evaluation of Human Work: A Practical Ergonomics Methodology; Wilson, J.R., Corlett, E.N., Eds.; Taylor & Francis: London, UK, 1995. [Google Scholar]
- Hart, S.G.; Staveland, L.E. Development of nasa-tlx (task load index): Results of emprical and theoretical research. Adv. Psychol. 1988, 52, 139–183. [Google Scholar] [CrossRef]
- Hoonakker, P.; Carayon, P.; Gurses, A.; Brown, R.; McGuire, K.; Khunlertkit, A.; Walker, J.M. Measuring workload of icu nurses with a questionnaire survey: The nasa task load index (tlx). IIE Trans. Healthc. Syst. Eng. 2011, 1, 131–143. [Google Scholar] [CrossRef]
- Kim, H.Y. Statistical notes for clinical researchers: Assessing normal distribution (1). Restor. Dent. Endod. 2012, 37, 245–248. [Google Scholar] [CrossRef]
- Ballinger, G.A. Using generalized estimating equations for longitudinal data analysis. Organ. Res. Methods 2016, 7, 127–150. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and anovas. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Koshy, K.; Syed, H.; Luckiewicz, A.; Alsoof, D.; Koshy, G.; Harry, L. Interventions to improve ergonomics in the operating theatre: A systematic review of ergonomics training and intra-operative microbreaks. Ann. Med. Surg. 2020, 55, 135–142. [Google Scholar] [CrossRef]
- Lin, E.; Young, R.; Shields, J.; Smith, K.; Chao, L. Growing pains: Strategies for improving ergonomics in minimally invasive gynecologic surgery. Curr. Opin. Obstet. Gynecol. 2023, 35, 361–367. [Google Scholar] [CrossRef]
- Tijam, I.M.; Goossens, R.H.; Schout, B.M.; Koldewijn, E.L.; Hendrikx, A.J.; Muijtjens, A.M.; Scherpbier, A.J.; Witjes, J.A. Ergonomics in endourology and laparoscopy: An overview of musculoskeletal problems in urology. J. Endourol. 2014, 28, 605–611. [Google Scholar] [CrossRef]
- Stomberg, M.W.; Tronstad, S.E.; Hedberg, K.; Bengtsson, J.; Jonsson, P.; Johansen, L.; Lindvall, B. Work-related musculoskeletal disorders when performing laparoscopic surgery. Surg. Laparosc. Endosc. Percutaneous Tech. 2010, 20, 49–53. [Google Scholar] [CrossRef]
- de Kok, J.; Vroonhof, P.; Snijders, J.; Roullis, G.; Clarke, M.; Peereboom, K.; van Dorst, P.; Isusi, I. Work-Related Musculoskeletal Disorders: Prevalence, Costs and Demographics in the EU: European Risk Observatory Report; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Macpherson, R.A.; Lane, T.J.; Collie, A.; McLeod, C.B. Age, sex, and the changing disability burden of compensated work-related musculoskeletal disorders in canada and australia. BMC Public Health 2018, 18, 758. [Google Scholar] [CrossRef] [PubMed]
- Yizengaw, M.A.; Mustofa, S.Y.; Ashagrie, H.E.; Zeleke, T.G. Prevalence and factors associated with work-related musculoskeletal disorder among health care providers working in the operation room. Ann. Med. Surg. 2021, 72, 102989. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Galizzi, M.; Cifuentes, M.; d’Errico, A.; Gore, R.; Punnett, L.; Slatin, C. Promoting Healthy Safe Employment in Healthcare, T. Ergonomic and socioeconomic risk factors for hospital workers’ compensation injury claims. Am. J. Ind. Med. 2009, 52, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, H.; Cvitkovich, Y.; Yu, S.; Yassi, A. Work-related injury among direct care occupations in british columbia, canada. Occup. Environ. Med. 2007, 64, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Oranye, N.O.; Bennett, J. Prevalence of work-related musculoskeletal and non-musculoskeletal injuries in health care workers: The implications for work disability management. Ergonomics 2018, 61, 355–366. [Google Scholar] [CrossRef]
- Häkkänen, M.; Viikari-Juntura, E.; Martikainen, R. Job experience, work load, and risk of musculoskeletal disorders. Occup. Environ. Med. 2001, 58, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Abdelall, E.S.; Lowndes, B.R.; Abdelrahman, A.M.; Hawthorne, H.J.; Hallbeck, M.S. Mini breaks, many benefits: Development and pilot testing of an intraoperative microbreak stretch web-application for surgeons. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2018, 62, 1042–1046. [Google Scholar] [CrossRef]
- Krämer, B.; Neis, F.; Reisenauer, C.; Walter, C.; Brucker, S.; Wallwiener, D.; Seibt, R.; Gabriel, J.; Rieger, M.A.; Steinhilber, B. Save our surgeons (sos)—An explorative comparison of surgeons’ muscular and cardiovascular demands, posture, perceived workload and discomfort during robotic vs. Laparoscopic surgery. Arch. Gynecol. Obstet. 2023, 307, 849–862. [Google Scholar] [CrossRef]
- Thurston, T.; Dolan, J.P.; Husain, F.; Stroud, A.; Funk, K.; Borzy, C.; Zhu, X. Assessment of muscle activity and fatigue during laparoscopic surgery. Surg. Endosc. 2022, 36, 6672–6678. [Google Scholar] [CrossRef]
- Haney, C.M.; Kowalewski, K.F.; Schmidt, M.W.; Lang, F.; Bintintan, V.; Fan, C.; Wehrtmann, F.; Studier-Fischer, A.; Felinska, E.A.; Muller-Stich, B.P.; et al. Robotic-assisted versus laparoscopic bowel anastomoses: Randomized crossover in vivo experimental study. Surg. Endosc. 2023, 37, 5894–5901. [Google Scholar] [CrossRef] [PubMed]
- Carayon, P.; Schoofs Hundt, A.; Karsh, B.T.; Gurses, A.P.; Alvarado, C.J.; Smith, M.; Flatley Brennan, P. Work system design for patient safety: The seips model. Qual. Saf. Health Care 2006, 15, i50–i58. [Google Scholar] [CrossRef]
- Giagio, S.; Volpe, G.; Pillastrini, P.; Gasparre, G.; Frizziero, A.; Squizzato, F. A preventive program for work-related musculoskeletal disorders among surgeons: Outcomes of a randomized controlled clinical trial. Ann. Surg. 2019, 270, 969–975. [Google Scholar] [CrossRef]
- Bretonnier, M.; Michinov, E.; Morandi, X.; Riffaud, L. Interruptions in surgery: A comprehensive review. J. Surg. Res. 2020, 247, 190–196. [Google Scholar] [CrossRef]

| Task | Parameter | Without Breaks | Passive Breaks | Active Breaks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre/B1/T2/WB1 | WB2 | T7/B9/WB3/Post | Pre/B1/T2/WB1 | WB2 | T7/B9/WB3/Post | Pre/B1/T2/WB1 | WB2 | T7/B9/WB3/Post | ||
| RPD | Median (IQR) | 0.00 (0.00) | - | 0.00 (2.00) | 0.00 (0.00) | - | 0.00 (2.00) | 0.00 (0.00) | - | 0.00 (2.50) |
| Mean (SD) | 0.00 (0.00) | - | 0.90 (1.26) | 0.10 (0.44) | - | 0.71 (1.10) | 0.24 (0.77) | - | 1.10 (1.61) | |
| Hot wire | CT (s) | 15.72 (16.67) | - | 13.10 (9.78) | 18.66 (17.71) | - | 16.41 (12.75) | 13.93 (14.21) | - | 11.66 (13.48) |
| SR (%) | 75.48 (23.59) | - | 67.08 (19.79) | 73.91 (44.65) | - | 73.89 (34.73) | 67.08 (18.90) | - | 57.98 (28.19) | |
| Peg transfer | CT/6 prisms (s) | 41.00 (11.12) | - | 33.93 (10.53) | 37.01 (11.74) | - | 34.38 (12.44) | 37.93 (12.61) | - | 33.93 (9.98) |
| Prisms/60 s (#) | 8.0 (1.0) | - | 10.0 (3.0) | 9.0 (3.0) | - | 9.0 (3.0) | 9.0 (2.0) | - | 10.0 (2.0) | |
| Pick-and-place | Beads/6.75 min (#) | 22.0 (7.0) | 24.0 (6.5) | 23.0 (7.5) | 20.0 (7.5) | 23.0 (6.5) | 23.0 (8.5) | 22.0 (10.5) | 22.0 (5.5) | 21.0 (7.0) |
| Pick-and-tighten | Gummies/6.75 min (#) | 22.0 (4.0) | 22.0 (3.5) | 23.0 (4.0) | 22.0 (6.0) | 22.0 (6.5) | 23.0 (7.0) | 22.0 (6.5) | 26.0 (7.5) | 25.0 (6.5) |
| Pick-and-thread | Beads/6.75 min (#) | 11.0 (8.0) | 11.0 (7.5) | 14.0 (6.0) | 11.0 (6.5) | 13.0 (6.0) | 13.0 (8.5) | 9.0 (7.5) | 12.0 (9.5) | 13.0 (5.0) |
| Pull-and-stick | Stick-on points/6.75 min (#) | 23.0 (11.5) | 26.0 (11.5) | 26.0 (13.5) | 24.0 (10.0) | 27.0 (13.0) | 30.0 (11.5) | 28.0 (11.5) | 30.0 (16.5) | 32.0 (10.0) |
| Task | Parameter | Condition | Time | Condition × Time | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 (df) | p | w | χ2 (df) | p | w | χ2 (df) | p | w | ||
| - | RPD | 1.646 (2) | 0.439 | 0.114 | 12.198 (1) | 0.000 * | 0.311 † | 4.594 (2) | 0.101 | 0.191 |
| Hot wire | CT (s) | 1.139 (2) | 0.566 | 0.095 | 18.867 (1) | 0.000 * | 0.387 † | 2.196 (2) | 0.334 | 0.132 |
| SR (%) | 4.295 (2) | 0.117 | 0.185 | 3.602 (1) | 0.058 | 0.170 | 0.707 (2) | 0.702 | 0.075 | |
| Peg transfer | CT/6 prisms (s) | 3.521 (2) | 0.172 | 0.168 | 21.078 (1) | 0.000 * | 0.411 † | 3.489 (2) | 0.175 | 0.167 |
| Prisms/60 s (#) | 1.312 (2) | 0.519 | 0.102 | 31.203 (1) | 0.000 * | 0.498 † | 0.885 (2) | 0.642 | 0.084 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonsch, R.; Seibt, R.; Krämer, B.; Rieger, M.A.; Steinhilber, B.; Luger, T. Influence of Intraoperative Active and Passive Breaks in Simulated Minimally Invasive Procedures on Surgeons’ Perceived Discomfort, Performance, and Workload. Life 2024, 14, 426. https://doi.org/10.3390/life14040426
Bonsch R, Seibt R, Krämer B, Rieger MA, Steinhilber B, Luger T. Influence of Intraoperative Active and Passive Breaks in Simulated Minimally Invasive Procedures on Surgeons’ Perceived Discomfort, Performance, and Workload. Life. 2024; 14(4):426. https://doi.org/10.3390/life14040426
Chicago/Turabian StyleBonsch, Rosina, Robert Seibt, Bernhard Krämer, Monika A. Rieger, Benjamin Steinhilber, and Tessy Luger. 2024. "Influence of Intraoperative Active and Passive Breaks in Simulated Minimally Invasive Procedures on Surgeons’ Perceived Discomfort, Performance, and Workload" Life 14, no. 4: 426. https://doi.org/10.3390/life14040426
APA StyleBonsch, R., Seibt, R., Krämer, B., Rieger, M. A., Steinhilber, B., & Luger, T. (2024). Influence of Intraoperative Active and Passive Breaks in Simulated Minimally Invasive Procedures on Surgeons’ Perceived Discomfort, Performance, and Workload. Life, 14(4), 426. https://doi.org/10.3390/life14040426








