Longitudinal Cognitive Trajectories in Older Adults with Restless Legs Syndrome or Willis–Ekbom Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, and Settings
2.2. Neuropsychological Assessments and Diagnostic Approach
2.3. Statistical Analysis
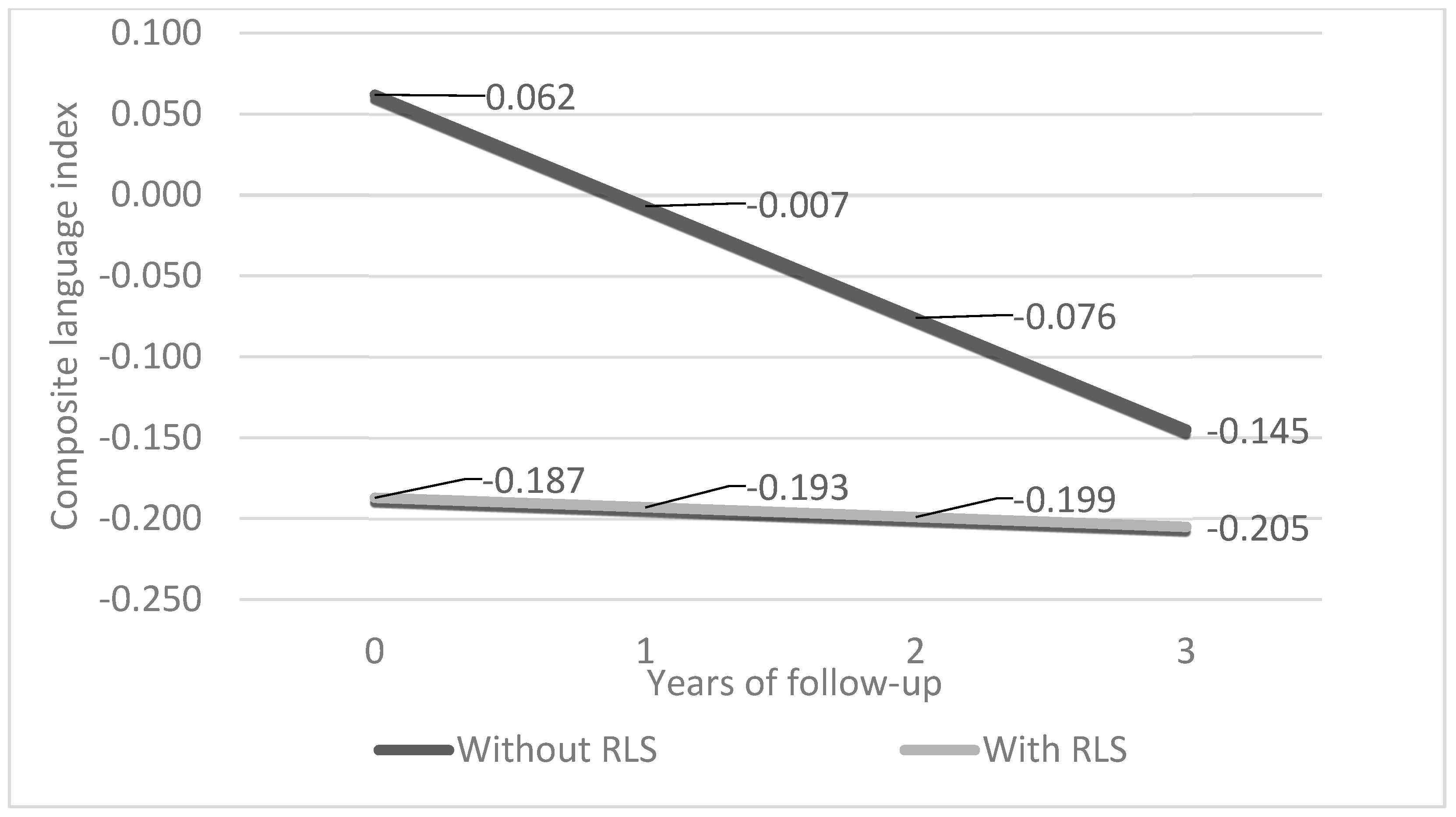
3. Results
3.1. Baseline Characteristics and Missing Data
3.2. Differences in Cognition and Cognitive Trajectories in Participants with and without RLS/WED
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amir, A.; Masterson, R.M.; Halim, A.; Nava, A. Restless Leg Syndrome: Pathophysiology, Diagnostic Criteria, and Treatment. Pain. Med. 2022, 23, 1032–1035. [Google Scholar] [CrossRef]
- Guo, S.; Huang, J.; Jiang, H.; Han, C.; Li, J.; Xu, X.; Zhang, G.; Lin, Z.; Xiong, N.; Wang, T. Restless Legs Syndrome: From Pathophysiology to Clinical Diagnosis and Management. Front. Aging Neurosci. 2017, 9, 171. [Google Scholar] [CrossRef]
- Nagandla, K.; De, S. Restless legs syndrome: Pathophysiology and modern management. Postgrad. Med. J. 2013, 89, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Picchietti, D.; Winkelman, J.W. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep 2005, 28, 891–898. [Google Scholar] [PubMed]
- Mackie, S.; Winkelman, J.W. Restless Legs Syndrome and Psychiatric Disorders. Sleep Med. Clin. 2015, 10, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Didato, G.; Di Giacomo, R.; Rosa, G.J.; Dominese, A.; de Curtis, M.; Lanteri, P. Restless Legs Syndrome across the Lifespan: Symptoms, Pathophysiology, Management and Daily Life Impact of the Different Patterns of Disease Presentation. Int. J. Environ. Res. Public Health 2020, 17, 3658. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, J.; Prager, M.; Lieb, R.; Pfister, H.; Spiegel, B.; Wittchen, H.U.; Holsboer, F.; Trenkwalder, C.; Ströhle, A. ‘Anxietas tibiarum’. Depression and anxiety disorders in patients with restless legs syndrome. J. Neurol. 2005, 252, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y. Cognition in Restless Legs Syndrome. J. Sleep Med. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Pearson, V.; Allen, R.; Dean, T.; Gamaldo, C.; Lesage, S.; Earley, C. Cognitive deficits associated with restless legs syndrome (RLS). Sleep Med. 2006, 7, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Celle, S.; Roche, F.; Kerleroux, J.; Thomas-Anterion, C.; Laurent, B.; Rouch, I.; Pichot, V.; Barthélémy, J.C.; Sforza, E. Prevalence and Clinical Correlates of Restless Legs Syndrome in an Elderly French Population: The Synapse Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65A, 167–173. [Google Scholar] [CrossRef]
- Cha, K.S.; Sunwoo, J.; Byun, J.; Kim, T.; Shin, J.; Kim, K.H.; Jung, K.Y. Working memory deficits in patients with idiopathic restless legs syndrome are associated with abnormal theta-band neural synchrony. J. Sleep Res. 2021, 30, e13287. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Beitinger, M.E.; Reppermund, S.; Winkelmann, J.; Wetter, T.C. Short-term attention and verbal fluency is decreased in restless legs syndrome patients: Cognitive Functioning in RLS. Mov. Disord. 2010, 25, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Choi, J.W.; Lee, C.; Lee, B.U.; Koo, Y.S.; Kim, K.H.; Jung, K.Y. Working memory deficit in patients with restless legs syndrome: An event-related potential study. Sleep Med. 2014, 15, 808–815. [Google Scholar] [CrossRef]
- Li, G.; Tang, H.; Chen, J.; Qi, X.; Chen, S.; Ma, J. Executive and Visuospatial Dysfunction in Patients with Primary Restless Legs Syndrome/Willis-Ekbom Disease: Study of a Chinese Population. J. Clin. Sleep Med. 2018, 14, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, A.; Marelli, S.; Giora, E.; Zucconi, M.; Oldani, A.; Ferini-Strambi, L. Neurocognitive function in patients with idiopathic Restless Legs Syndrome before and after treatment with dopamine-agonist. Int. J. Psychophysiol. 2015, 95, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Cederberg, K.L.; Brinkley, E.B.; Belotserkovkaya, N.; Memon, R.A.; Motl, R.W.; Amara, A.W. Does restless legs syndrome impact cognitive function via sleep quality in adults with Parkinson’s disease? Int. J. Neurosci. 2020, 130, 322–329. [Google Scholar] [CrossRef]
- Cederberg, K.L.; Jeng, B.; Sasaki, J.E.; Motl, R.W. Restless legs syndrome, sleep quality, and perceived cognitive impairment in adults with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 43, 102176. [Google Scholar] [CrossRef]
- Driver-Dunckley, E.; Connor, D.; Hentz, J.; Sabbagh, M.; Silverberg, N.; Hernandez, J.; Vedders, L.; Evidente, V.G.; Shill, H.; Caviness, J.; et al. No evidence for cognitive dysfunction or depression in patients with mild restless legs syndrome: No Cognitive Dysfunction or Depression in RLS. Mov. Disord. 2009, 24, 1843–1847. [Google Scholar] [CrossRef]
- Lee, H.B.; Ramsey, C.M.; Spira, A.P.; Vachon, J.; Allen, R.; Munro, C.A. Comparison of Cognitive Functioning among Individuals with Treated Restless Legs Syndrome (RLS), Untreated RLS, and No RLS. JNP 2014, 26, 87–91. [Google Scholar] [CrossRef]
- Rist, P.M.; Elbaz, A.; Dufouil, C.; Tzourio, C.; Kurth, T. Restless Legs Syndrome and Cognitive Function: A Population-Based Cross-Sectional Study. Am. J. Med. 2015, 128, 1023.e33–1023.e39. [Google Scholar] [CrossRef]
- Moon, Y.J.; Song, J.Y.; Lee, B.U.; Koo, Y.S.; Lee, S.K.; Jung, K.Y. Comparison of Cognitive Function between Patients with Restless Legs Syndrome and Healthy Controls. Sleep Med. Res. 2014, 5, 20–24. [Google Scholar] [CrossRef]
- Gamaldo, C.E.; Benbrook, A.R.; Allen, R.P.; Oguntimein, O.; Earley, C.J. A further evaluation of the cognitive deficits associated with restless legs syndrome (RLS). Sleep Med. 2008, 9, 500–505. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Kosmidis, M.H.; Yannakoulia, M.; Hadjigeorgiou, G.M.; Scarmeas, N. The Hellenic Longitudinal Investigation of Aging and Diet (HELIAD): Rationale, Study Design, and Cohort Description. Neuroepidemiology 2014, 43, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Folia, V.; Liampas, I.; Ntanasi, E.; Yannakoulia, M.; Sakka, P.; Hadjigeorgiou, G.; Scarmeas, N.; Dardiotis, E.; Kosmidis, M.H. Longitudinal trajectories and normative language standards in older adults with normal cognitive status. Neuropsychology 2022, 36, 626–639. [Google Scholar] [CrossRef]
- Liampas, I.; Folia, V.; Ntanasi, E.; Yannakoulia, M.; Sakka, P.; Hadjigeorgiou, G.; Scarmeas, N.; Dardiotis, E.; Kosmidis, M.H. Longitudinal episodic memory trajectories in older adults with normal cognition. Clin. Neuropsychol. 2023, 37, 304–321. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939. [Google Scholar] [CrossRef]
- Hachinski, V.; Oveisgharan, S.; Romney, A.K.; Shankle, W.R. Optimizing the Hachinski Ischemic Scale. Arch. Neurol. 2012, 69, 169–175. [Google Scholar] [CrossRef]
- McKeith, I.G.; Galasko, D.; Kosaka, K.; Perry, E.K.; Dickson, D.W.; Hansen, L.A.; Salmon, D.P.; Lowe, J.; Mirra, S.S.; Byrne, E.J.; et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 1996, 47, 1113–1124. [Google Scholar] [CrossRef]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef]
- Liampas, I.; Hatzimanolis, A.; Siokas, V.; Yannakoulia, M.; Kosmidis, M.H.; Sakka, P.; Hadjigeorgiou, G.M.; Scarmeas, N.; Dardiotis, E. Antihypertensive Medication Class and the Risk of Dementia and Cognitive Decline in Older Adults: A Secondary Analysis of the Prospective HELIAD Cohort. J. Alzheimers Dis. 2022, 89, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Siokas, V.; Ntanasi, E.; Kosmidis, M.H.; Yannakoulia, M.; Sakka, P.; Hadjigeorgiou, G.M.; Scarmeas, N.; Dardiotis, E. Cognitive trajectories preluding the imminent onset of Alzheimer’s disease dementia in individuals with normal cognition: Results from the HELIAD cohort. Aging Clin. Exp. Res. 2023, 35, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Siokas, V.; Kyrozis, A.; Sakoutis, G.; Yannakoulia, M.; Kosmidis, M.H.; Sakka, P.; Sakkas, G.K.; Giannaki, C.D.; Stefanidis, I.; et al. Prevalence and Determinants of Restless Legs Syndrome (Willis-Ekbom Disease) in an Older Greek Population. Behav. Sleep Med. 2022, 21, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.; Hening, W.A.; Trenkwalder, C.; Walters, A.S.; Montplaisi, J. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. Sleep Med. 2003, 4, 101–119. [Google Scholar] [CrossRef]
- Aita, S.L.; Beach, J.D.; Taylor, S.E.; Borgogna, N.C.; Harrell, M.N.; Hill, B.D. Executive, language, or both? An examination of the construct validity of verbal fluency measures. Appl. Neuropsychol. Adult 2019, 26, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Amunts, J.; Camilleri, J.A.; Eickhoff, S.B.; Heim, S.; Weis, S. Executive functions predict verbal fluency scores in healthy participants. Sci. Rep. 2020, 10, 11141. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, L.; Hernández-Cabrera, J.A.; Westman, E.; Barroso, J.; Ferreira, D. Cognitive compensatory mechanisms in normal aging: A study on verbal fluency and the contribution of other cognitive functions. Aging 2019, 11, 4090–4106. [Google Scholar] [CrossRef]
- Higby, E.; Cahana-Amitay, D.; Vogel-Eyny, A.; Spiro, A.; Albert, M.L.; Obler, L.K. The Role of Executive Functions in Object- and Action-Naming among Older Adults. Exp. Aging Res. 2019, 45, 306–330. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R.; Phillips, L.H. Verbal fluency performance in dementia of the Alzheimer’s type: A meta-analysis. Neuropsychologia 2004, 42, 1212–1222. [Google Scholar] [CrossRef]
- Gu, H.J.; Lee, O.S. Effects of Non-Pharmacological Sleep Interventions in Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 3101. [Google Scholar] [CrossRef]
- Currie, K.; Gupta, B.V.; Shivanand, I.; Desai, A.; Bhatt, S.; Tunuguntla, H.S.; Verma, S. Reductions in anxiety, depression and insomnia in health care workers using a non-pharmaceutical intervention. Front. Psychiatry 2022, 13, 983165. [Google Scholar] [CrossRef]
- Purebl, G.; Schnitzspahn, K.; Zsák, É. Overcoming treatment gaps in the management of depression with non-pharmacological adjunctive strategies. Front. Psychiatry 2023, 14, 1268194. [Google Scholar] [CrossRef] [PubMed]
- Alders, R.G.; Ali, S.N.; Ameri, A.A.; Bagnol, B.; Cooper, T.L.; Gozali, A.; Hidayat, M.M.; Rukambile, E.; Wong, J.T.; Catley, A. Participatory Epidemiology: Principles, Practice, Utility, and Lessons Learnt. Front. Vet. Sci. 2020, 7, 532763. [Google Scholar] [CrossRef] [PubMed]
- Gray, L. The importance of post hoc approaches for overcoming non-response and attrition bias in population-sampled studies. Soc. Psychiatry Psychiatr. Epidemiol. 2016, 51, 155–157. [Google Scholar] [CrossRef]
- Liampas, I.; Siokas, V.; Lyketsos, C.G.; Dardiotis, E. The Relationship between Neuropsychiatric Symptoms and Cognitive Performance in Older Adults with Normal Cognition. Medicina 2022, 58, 1586. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B.; et al. Restless legs syndrome/Willis–Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—History, rationale, description, and significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef]
- Walters, A.S. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Mov. Disord. 1995, 10, 634–642. [Google Scholar] [CrossRef]
- Abdulhadi, I.G.; Al-Mahdawi, A.M.; Hamdan, F.B. Electrophysiological findings in patients with restless legs syndrome. Sleep Med. 2021, 87, 151–157. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Ren, R.; Yang, L.; Shi, Y.; Vitiello, M.V.; Sanford, L.D.; Tang, X. Polysomnographic features of idiopathic restless legs syndrome: A systematic review and meta-analysis of 13 sleep parameters and 23 leg movement parameters. J. Clin. Sleep Med. 2022, 18, 2561–2575. [Google Scholar] [CrossRef]
| Parameter | Without RLS/WED (n = 922) | With RLS/WED (n = 81) | p-Value |
|---|---|---|---|
| Age at baseline (N = 1003) | 72.9 ± 5.0 | 73.3 ± 4.1 | 0.496 |
| Sex (women/men) (Ν = 1003) | 534/388 (57.9/42.1%) | 63/18 (77.8/22.2%) | <0.001 |
| Sleep quality (poor/moderate/good) (Ν = 974) | 265/272/358 (29.6/30.4/40.0%) | 30/37/12 (38.0/46.8/15.2%) | <0.001 |
| Anxiety (0–22-point scale) (N = 1003) | 2.3 ± 3.5 | 4.1 ± 4.7 | <0.001 |
| MeDi score (0–55-point scale) (N = 980) | 33.8 ± 4.6 | 33.7 ± 4.4 | 0.972 |
| Daily energy intake (low–low/moderate-moderate/high-high) (N = 973) | 125/355/281/134 (14.0/39.7/31.4/15.0%) | 21/25/22/10 (26.9/32.1/28.2/12.8%) | 0.023 |
| History of TBI (Yes/No) (N = 986) | 96/810 (10.6/89.4%) | 19/61 (23.4/76.6%) | 0.001 |
| Global cognition (N = 992) | −0.01 ± 0.70 | −0.18 ± 0.72 | 0.040 |
| Memory (N = 984) | 0.03 ± 0.85 | −0.10 ± 0.82 | 0.208 |
| Executive function (N = 989) | −0.02 ± 0.73 | −0.16 ± 0.67 | 0.094 |
| Visuospatial skills (N = 979) | 0.02 ± 0.79 | −0.07 ± 0.90 | 0.315 |
| Language (N = 990) | 0.04 ± 0.81 | −0.24 ± 0.81 | 0.003 |
| Attention (N = 949) | −0.06 ± 1.01 | −0.29 ± 1.19 | 0.072 |
| Parameter | Main Effect of RLS/WED (β, 95% CI, p-Value) | Main Effect of Time (β, 95% CI, p-Value) | Time by RLS/WED Interaction (β, 95% CI, p-Value) |
|---|---|---|---|
| Global cognitive score (unadjusted) | −0.157 (−0.320, 0.002), 0.058 | −0.076 (−0.085, −0.067), <0.001 | 0.017 (−0.007, 0.041), 0.171 |
| Global cognitive score (adjusted) | −0.128 (−0.294, 0.038), 0.130 | −0.077 (−0.087, −0.068), <0.001 | 0.018 (−0.007, 0.045), 0.153 |
| Memory (unadjusted) | −0.118 (−0.302, −0.067), 0.213 | −0.070 (−0.083, −0.057), <0.001 | 0.045 (0.002, 0.088), 0.040 |
| Memory (adjusted) | −0.135 (−0.321, 0.051), 0.156 | −0.068 (−0.081, −0.055), <0.001 | 0.037 (−0.009, 0.082), 0.114 |
| Visuospatial (unadjusted) | −0.080 (−0.277, 0.117), 0.426 | −0.106 (−0.123, −0.089), <0.001 | 0.003 (−0.048, 0.054), 0.907 |
| Visuospatial (adjusted) | −0.033 (−0.235, 0.169), 0.748 | −0.111 (−0.129, −0.094), <0.001 | 0.001 (−0.051, 0.054), 0.958 |
| Executive (unadjusted) | −0.148 (−0.306, 0.010), 0.067 | −0.050 (−0.060, −0.040), <0.001 | −0.008 (−0.045, 0.030), 0.695 |
| Executive (adjusted) | −0.100, (−0.258, 0.059), 0.217 | −0.050 (−0.061, −0.040), <0.001 | −0.010, (−0.049, 0.030), 0.630 |
| Language (unadjusted) | −0.257 (−0.442, −0.073), 0.006 | −0.066 (−0.077, −0.055), <0.001 | 0.054 (0.016, 0.091), 0.005 |
| Language (adjusted) | −0.249 (−0.442, −0.055), 0.012 | −0.069 (−0.080, −0.058), <0.001 | 0.063, (0.024, 0.103), 0.002 |
| Attention (unadjusted) | −0.240 (−0.518, 0.038), 0.091 | −0.065 (−0.084, −0.046), <0.001 | −0.010 (−0.071, 0.051), 0.745 |
| Attention (adjusted) | −0.159 (−0.447, 0.130), 0.281 | −0.067 (−0.087, −0.047), <0.001 | 0.001 (−0.063, 0.065), 0.971 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liampas, I.; Siokas, V.; Kyrozis, A.; Sakoutis, G.; Yannakoulia, M.; Kosmidis, M.H.; Sakka, P.; Scarmeas, N.; Hadjigeorgiou, G.M.; Dardiotis, E. Longitudinal Cognitive Trajectories in Older Adults with Restless Legs Syndrome or Willis–Ekbom Disease. Life 2024, 14, 430. https://doi.org/10.3390/life14040430
Liampas I, Siokas V, Kyrozis A, Sakoutis G, Yannakoulia M, Kosmidis MH, Sakka P, Scarmeas N, Hadjigeorgiou GM, Dardiotis E. Longitudinal Cognitive Trajectories in Older Adults with Restless Legs Syndrome or Willis–Ekbom Disease. Life. 2024; 14(4):430. https://doi.org/10.3390/life14040430
Chicago/Turabian StyleLiampas, Ioannis, Vasileios Siokas, Andreas Kyrozis, George Sakoutis, Mary Yannakoulia, Mary H. Kosmidis, Paraskevi Sakka, Nikolaos Scarmeas, Georgios M. Hadjigeorgiou, and Efthimios Dardiotis. 2024. "Longitudinal Cognitive Trajectories in Older Adults with Restless Legs Syndrome or Willis–Ekbom Disease" Life 14, no. 4: 430. https://doi.org/10.3390/life14040430
APA StyleLiampas, I., Siokas, V., Kyrozis, A., Sakoutis, G., Yannakoulia, M., Kosmidis, M. H., Sakka, P., Scarmeas, N., Hadjigeorgiou, G. M., & Dardiotis, E. (2024). Longitudinal Cognitive Trajectories in Older Adults with Restless Legs Syndrome or Willis–Ekbom Disease. Life, 14(4), 430. https://doi.org/10.3390/life14040430








