Fetal Alcohol Spectrum Disorder: The Honey Bee as a Social Animal Model
Abstract
1. Introduction
2. Materials and Methods
3. Results
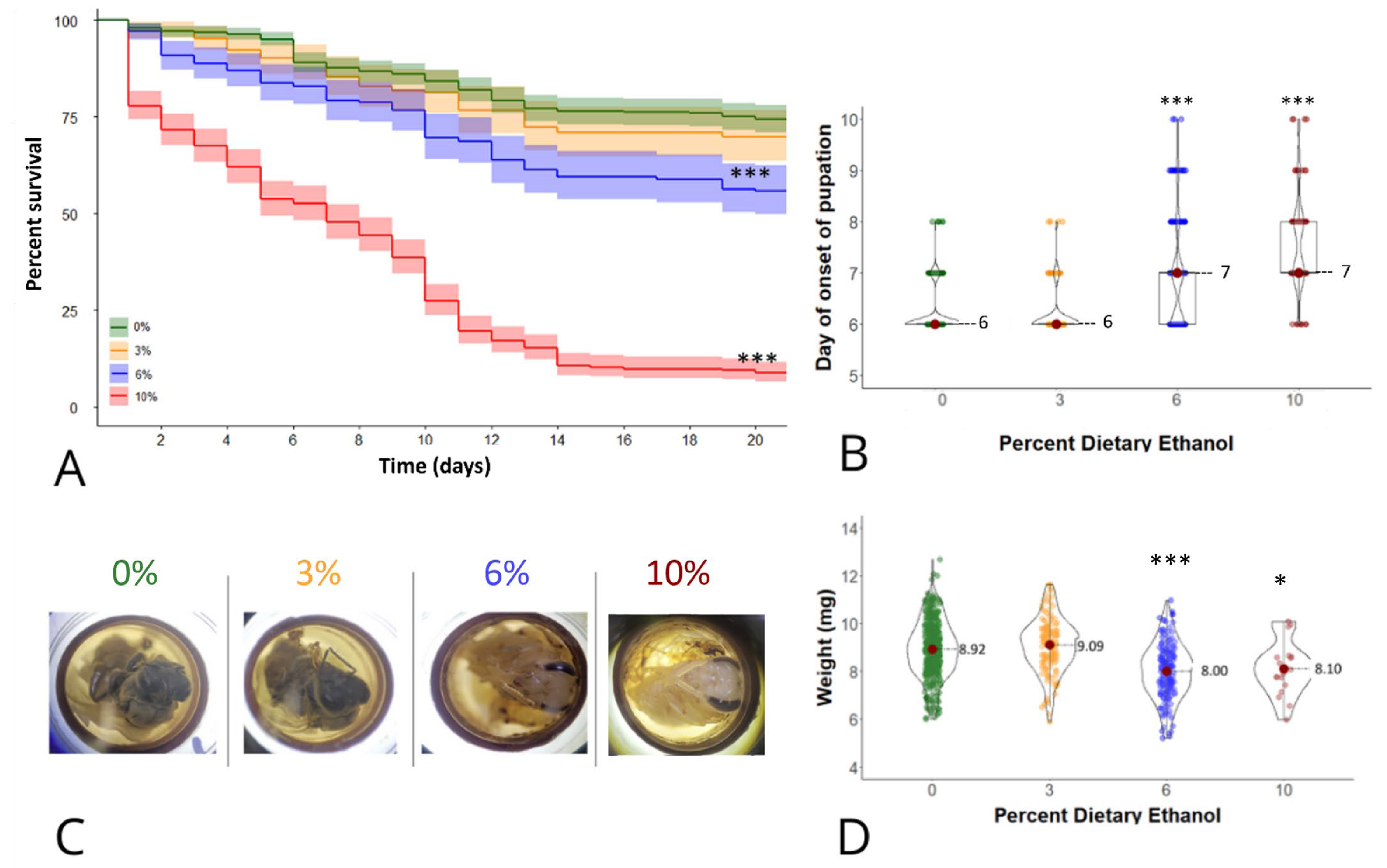
3.1. Survival Analysis
3.2. Developmental Delays
3.3. Weight at Eclosion
3.4. Locomotion Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, S.E.; Cudd, T.A. Focus on: The use of animal models for the study of fetal alcohol spectrum disorders. Alcohol Res. Health 2011, 34, 92. [Google Scholar]
- Murawski, N.J.; Moore, E.M.; Thomas, J.D.; Riley, E.P. Advances in diagnosis and treatment of fetal alcohol spectrum disorders: From animal models to human studies. Alcohol Res. Curr. Rev. 2015, 37, 97. [Google Scholar]
- Patten, A.R.; Fontaine, C.J.; Christie, B.R. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front. Pediatr. 2014, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- McClure, K.D.; French, R.L.; Heberlein, U. A Drosophila model for fetal alcohol syndrome disorders: Role for the insulin pathway. Dis. Models Mech. 2011, 4, 335–346. [Google Scholar] [CrossRef]
- Margret, C.P.; Li, C.X.; Chappell, T.D.; Elberger, A.J.; Matta, S.G.; Waters, R.S. Prenatal alcohol exposure delays the development of the cortical barrel field in neonatal rats. Exp. Brain Res. 2006, 172, 1–13. [Google Scholar] [CrossRef]
- Loucks, E.; Ahlgren, S. Assessing teratogenic changes in a zebrafish model of fetal alcohol exposure. JoVE J. Vis. Exp. 2012, 61, e3704. [Google Scholar]
- Kully-Martens, K.; Pei, J.; Kable, J.; Coles, C.D.; Andrew, G.; Rasmussen, C. Mathematics intervention for children with fetal alcohol spectrum disorder: A replication and extension of the math interactive learning experience (MILE) program. Res. Dev. Disabil. 2018, 78, 55–65. [Google Scholar] [CrossRef]
- Ali, S.; Champagne, D.L.; Spaink, H.P.; Richardson, M.K. Zebrafish embryos and larvae: A new generation of disease models and drug screens. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Fainsod, A.; Kot-Leibovich, H. Xenopus embryos to study fetal alcohol syndrome, a model for environmental teratogenesis. Biochem. Cell Biol. 2018, 96, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sulik, K.K.; Johnston, M.C.; Webb, M.A. Fetal alcohol syndrome: Embryogenesis in a mouse model. Science 1981, 214, 936–938. [Google Scholar] [CrossRef]
- Schneider, M.L.; Moore, C.F.; Adkins, M.M. The effects of prenatal alcohol exposure on behavior: Rodent and primate studies. Neuropsychol. Rev. 2011, 21, 186–203. [Google Scholar] [CrossRef]
- Davis, J.R.; Li, Y.; Rankin, C.H. Effects of developmental exposure to ethanol on Caenorhabditis elegans. Alcohol Clin. Exp. Res. 2008, 32, 853–867. [Google Scholar] [CrossRef]
- Morozova, T.V.; Shankar, V.; MacPherson, R.A.; Mackay, T.F.; Anholt, R.R. Modulation of the Drosophila transcriptome by developmental exposure to alcohol. BMC Genom. 2022, 23, 347. [Google Scholar] [CrossRef] [PubMed]
- Mokashi, S.S.; Shankar, V.; MacPherson, R.A.; Hannah, R.C.; Mackay, T.F.; Anholt, R.R. Developmental alcohol exposure in Drosophila: Effects on adult phenotypes and gene expression in the brain. Front. Psychiatry 2021, 12, 699033. [Google Scholar] [CrossRef] [PubMed]
- Schmehl, D.R.; Tomé, H.V.; Mortensen, A.N.; Martins, G.F.; Ellis, J.D. Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. J. Apic. Res. 2016, 55, 113–129. [Google Scholar] [CrossRef]
- Boguslavsky, D.V.; Zakharov, I.S. Role of External Factors in Embryogenesis of Apis mellifera. Russ. J. Dev. Biol. 2021, 52, 422–429. [Google Scholar] [CrossRef]
- Robinson, G.E. Sociogenomics takes flight. Science 2002, 297, 204–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shpigler, H.Y.; Saul, M.C.; Corona, F.; Block, L.; Cash Ahmed, A.; Zhao, S.D.; Robinson, G.E. Deep evolutionary conservation of autism-related genes. Proc. Natl. Acad. Sci. USA 2017, 114, 9653–9658. [Google Scholar] [CrossRef] [PubMed]
- Clarren, S.K.; Bowden, D.M. Fetal alcohol syndrome: A new primate model for binge drinking and its relevance to human ethanol teratogenesis. J. Pediatr. 1982, 101, 819–824. [Google Scholar] [CrossRef]
- Maier, S.E.; West, J.R. Drinking patterns and alcohol-related birth defects. Alcohol Res. Health 2001, 25, 168. [Google Scholar]
- Vázquez, D.E.; Farina, W.M. Differences in pre-imaginal development of the honey bee Apis mellifera between in vitro and in-hive contexts. Apidologie 2020, 51, 861–875. [Google Scholar] [CrossRef]
- Little, R.E. Moderate alcohol use during pregnancy and decreased infant birth weight. Am. J. Public Health 1977, 67, 1154–1156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nwaogu, I.C.; Ihemelandu, E.C. Effects of maternal alcohol consumption on the allometric growth of muscles in fetal and neonatal rats. Cells Tissues Organs 1999, 164, 167–173. [Google Scholar] [CrossRef]
- Sylvain, N.J.; Brewster, D.L.; Ali, D.W. Zebrafish embryos exposed to alcohol undergo abnormal development of motor neurons and muscle fibers. Neurotoxicology Teratol. 2010, 32, 472–480. [Google Scholar] [CrossRef] [PubMed]
- El Shawa, H.; Abbott, C.W.; Huffman, K.J. Prenatal ethanol exposure disrupts intraneocortical circuitry, cortical gene expression, and behavior in a mouse model of FASD. J. Neurosci. 2013, 33, 18893–18905. [Google Scholar] [CrossRef]
- Driscoll, C.D.; Streissguth, A.P.; Riley, E.P. Prenatal alcohol exposure: Comparability of effects in humans and animal models. Neurotoxicology Teratol. 1990, 12, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Cadena, P.G.; Cadena, M.R.; Sarmah, S.; Marrs, J.A. Folic acid reduces the ethanol-induced morphological and behavioral defects in embryonic and larval zebrafish (Danio rerio) as a model for fetal alcohol spectrum disorder (FASD). Reprod. Toxicol. 2020, 96, 249–257. [Google Scholar] [CrossRef]
- Kully-Martens, K.; Denys, K.; Treit, S.; Tamana, S.; Rasmussen, C. A review of social skills deficits in individuals with fetal alcohol spectrum disorders and prenatal alcohol exposure: Profiles, mechanisms, and interventions. Alcohol Clin. Exp. Res. 2012, 36, 568–576. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987; ISBN 978-0-674-07409-5. [Google Scholar]
- Breed, M.D. Nestmate Recognition in Honey Bees. Anim. Behav. 1983, 31, 86–91. [Google Scholar] [CrossRef]

| % EtOH in Diet | Diet eA (9.1 mL) | Diet eB (9.1 mL) | Diet eC (45.4 mL) |
|---|---|---|---|
| 3 | 0.29 | 0.29 | 1.44 |
| 6 | 0.57 | 0.57 | 2.89 |
| 10 | 0.87 | 0.87 | 4.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camilli, M.P.; Simko, O.M.; Bevelander, B.; Thebeau, J.M.; Masood, F.; da Silva, M.C.B.; Raza, M.F.; Markova, S.; Obshta, O.; Jose, M.S.; et al. Fetal Alcohol Spectrum Disorder: The Honey Bee as a Social Animal Model. Life 2024, 14, 434. https://doi.org/10.3390/life14040434
Camilli MP, Simko OM, Bevelander B, Thebeau JM, Masood F, da Silva MCB, Raza MF, Markova S, Obshta O, Jose MS, et al. Fetal Alcohol Spectrum Disorder: The Honey Bee as a Social Animal Model. Life. 2024; 14(4):434. https://doi.org/10.3390/life14040434
Chicago/Turabian StyleCamilli, Marcelo P., Olena M. Simko, Breanne Bevelander, Jenna M. Thebeau, Fatima Masood, Marina C. Bezerra da Silva, Muhammad Fahim Raza, Sofiia Markova, Oleksii Obshta, Midhun S. Jose, and et al. 2024. "Fetal Alcohol Spectrum Disorder: The Honey Bee as a Social Animal Model" Life 14, no. 4: 434. https://doi.org/10.3390/life14040434
APA StyleCamilli, M. P., Simko, O. M., Bevelander, B., Thebeau, J. M., Masood, F., da Silva, M. C. B., Raza, M. F., Markova, S., Obshta, O., Jose, M. S., Biganski, S., Kozii, I. V., Zabrodski, M. W., Moshynskyy, I., Simko, E., & Wood, S. C. (2024). Fetal Alcohol Spectrum Disorder: The Honey Bee as a Social Animal Model. Life, 14(4), 434. https://doi.org/10.3390/life14040434






