Genetic Potential of Newly Developed Maize Hybrids under Different Water-Availability Conditions in an Arid Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Parental Genotypes and Hybridization
2.2. Experimental Design and Agronomic Practices
2.3. Measured Traits
2.4. Statistical Analysis
3. Results
3.1. Analysis of Variance and Mean Performance
3.2. Combining Ability
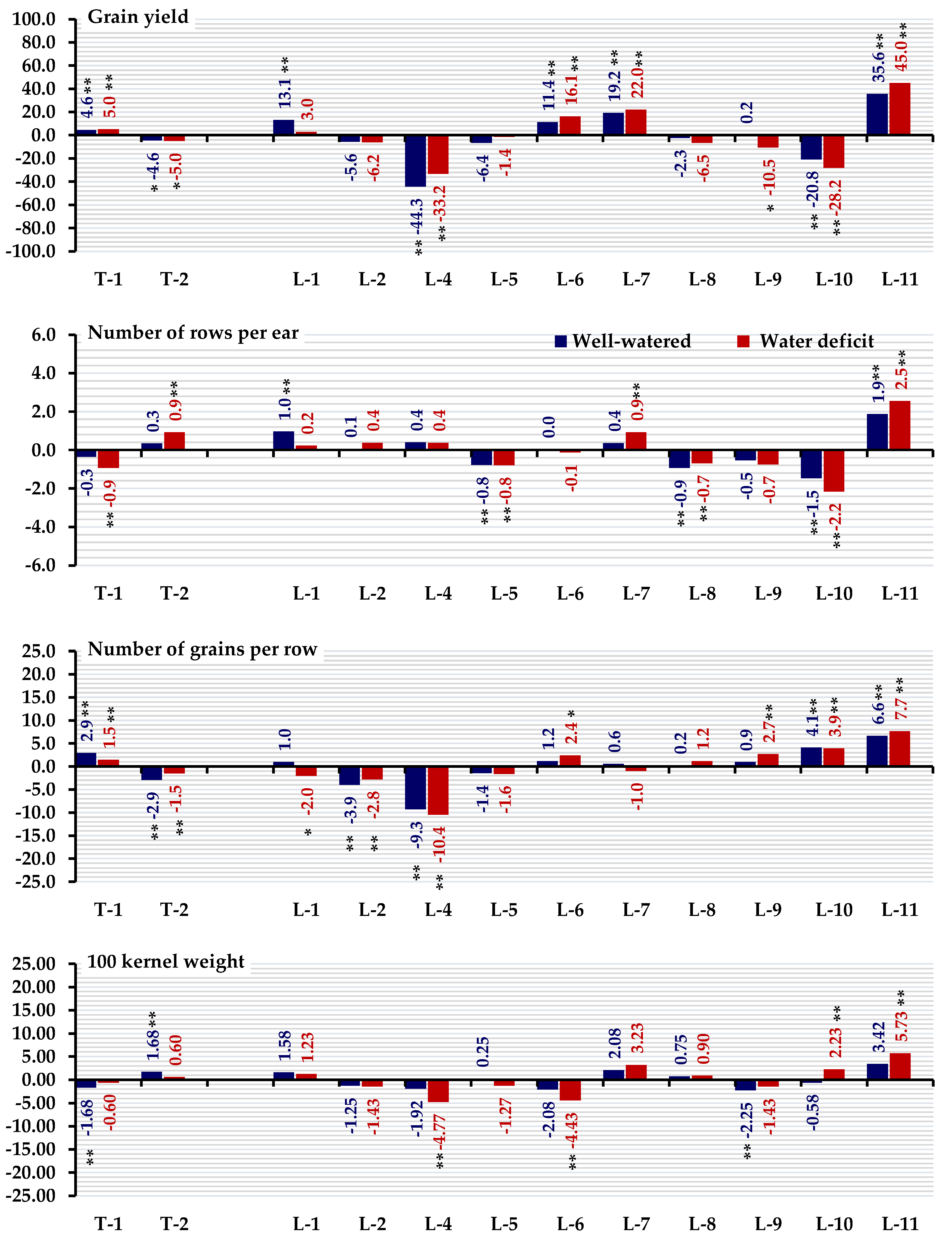
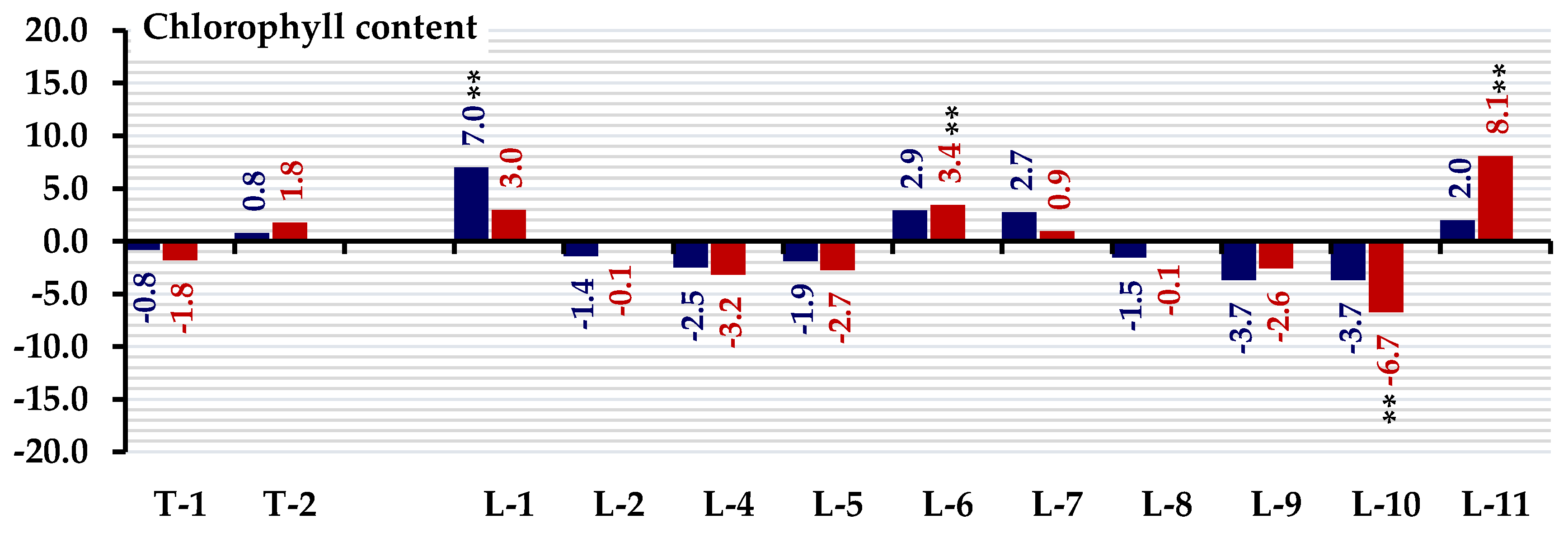
3.3. Standard Heterosis
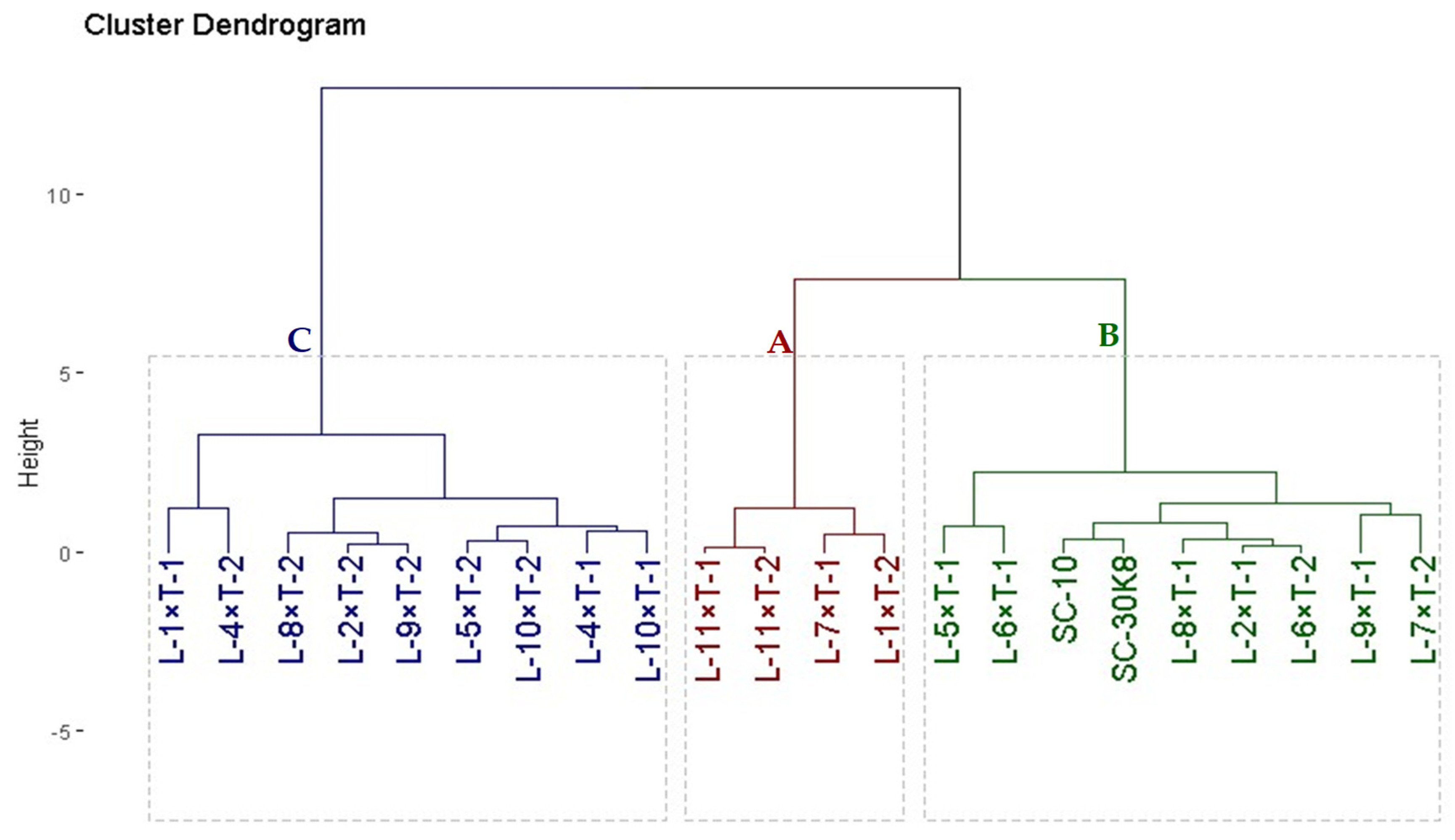
3.4. Genotypic Classification Based on Drought Tolerance
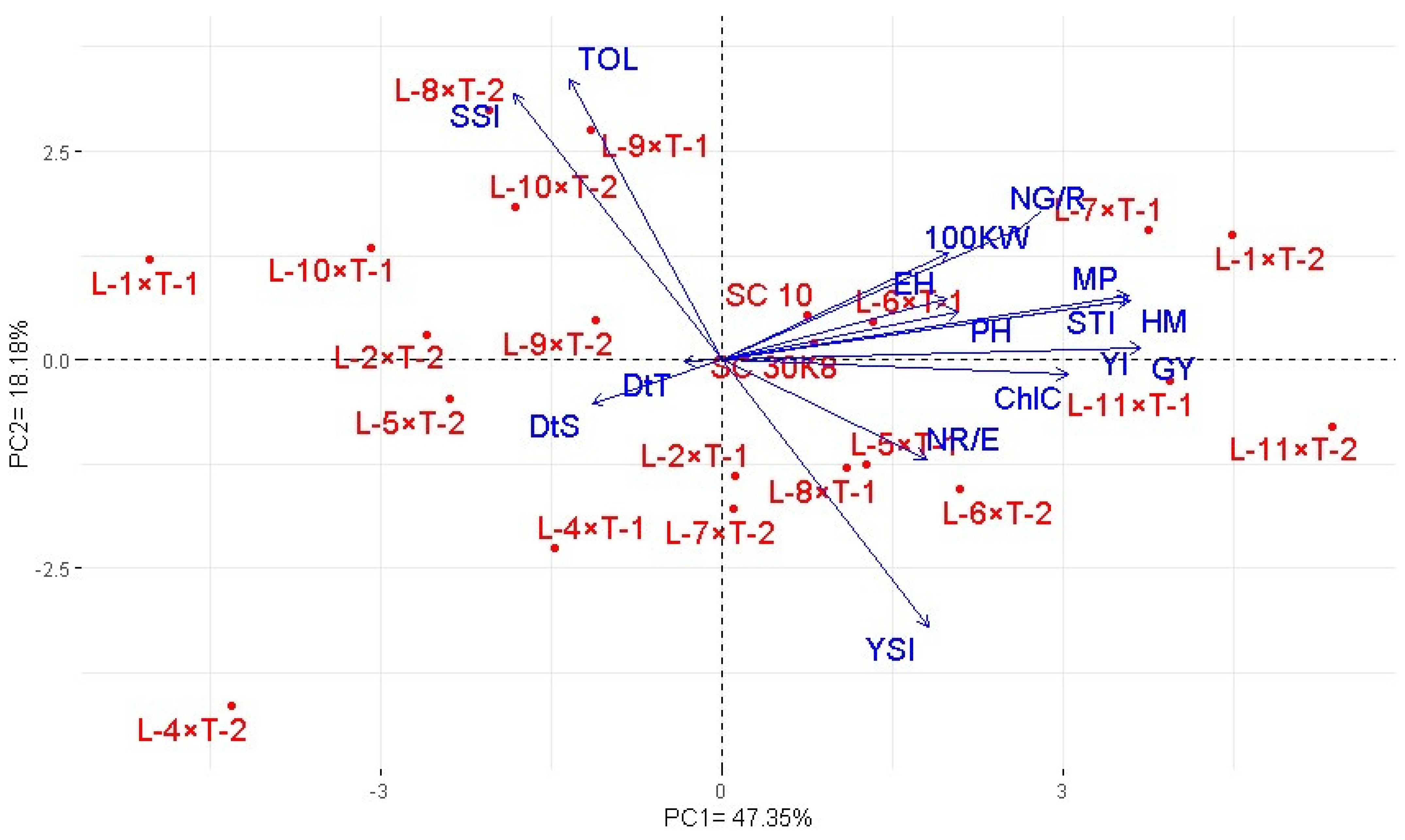
3.5. Association among Assessed Hybrids and Studied Traits under Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albahri, G.; Alyamani, A.A.; Badran, A.; Hijazi, A.; Nasser, M.; Maresca, M.; Baydoun, E. Enhancing essential grains yield for sustainable food security and bio-safe agriculture through latest innovative approaches. Agronomy 2023, 13, 1709. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Statistical Database. 2023. Available online: http://www.fao.org/faostat/en/#data (accessed on 1 October 2023).
- Ekpa, O.; Palacios-Rojas, N.; Kruseman, G.; Fogliano, V.; Linnemann, A.R. Sub-Saharan African maize-based foods: Technological perspectives to increase the food and nutrition security impacts of maize breeding programmes. Glob. Food Secur. 2018, 17, 48–56. [Google Scholar] [CrossRef]
- Alotaibi, B.A.; Baig, M.B.; Najim, M.M.; Shah, A.A.; Alamri, Y.A. Water scarcity management to ensure food scarcity through sustainable water resources management in Saudi Arabia. Sustainability 2023, 15, 10648. [Google Scholar] [CrossRef]
- Jiang, P.; Cai, F.; Zhao, Z.-Q.; Meng, Y.; Gao, L.-Y.; Zhao, T.-H. Physiological and dry matter characteristics of spring maize in northeast China under drought stress. Water 2018, 10, 1561. [Google Scholar] [CrossRef]
- Sah, R.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.; Chakravarty, M.; Narayan, S.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R. Breeding for increased drought tolerance in wheat: A review. Crop Pasture Sci. 2018, 69, 223–241. [Google Scholar] [CrossRef]
- Bocianowski, J.; Nowosad, K.; Bujak, H. Meta-analysis of influence of diversity of parental forms on heterosis and specific combining ability of their hybrids. Appl. Sci. 2023, 13, 8704. [Google Scholar] [CrossRef]
- Shi, R.; Seiler, C.; Knoch, D.; Junker, A.; Altmann, T. Integrated phenotyping of root and shoot growth dynamics in maize reveals specific interaction patterns in inbreds and hybrids and in response to drought. Front. Plant Sci. 2023, 14, 1233553. [Google Scholar] [CrossRef]
- Areche, F.; Gondal, A.; Sumarriva-Bustinza, L.; Zela-Payi, N.; Sumarriva-Hustinza, I.; Oscanoa-León, R.; Calcina-Sotelo, A.; Anguilar MCTTD, L.E.; Julcahuanga-Dominguez, I.; Flores, D. Role of biotechnology in food security: A review. SABRAO J. Breed. Genet. 2023, 55, 1496–1509. [Google Scholar] [CrossRef]
- Shrestha, J.; Gurung, D.B.; Rijal, T.R. Standard heterosis for grain yield in maize hybrids. Farming Manag. 2018, 3, 30–36. [Google Scholar]
- Wolko, J.; Łopatyńska, A.; Wolko, Ł.; Bocianowski, J.; Mikołajczyk, K.; Liersch, A. Identification of SSR markers associated with yield-related traits and heterosis effect in winter oilseed rape (Brassica napus L.). Agronomy 2022, 12, 1544. [Google Scholar] [CrossRef]
- Omar, M.; Rabie, H.A.; Mowafi, S.A.; Othman, H.T.; El-Moneim, D.A.; Alharbi, K.; Mansour, E.; Ali, M. Multivariate analysis of agronomic traits in newly developed maize hybrids grown under different agro-environments. Plants 2022, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Pérez-Rodríguez, P.; San Vicente, F.; Palacios-Rojas, N.; Dhliwayo, T.; Liu, Y.; Cui, Z.; Guan, Y.; Wang, H.; Zheng, H. Genomic prediction of the performance of hybrids and the combining abilities for line by tester trials in maize. Crop J. 2022, 10, 109–116. [Google Scholar] [CrossRef]
- Tesfaye, S.; Zeleke, H.; Abakemal, D. Combining ability of highland adapted maize (Zea mays L.) inbred lines for grain yield and yield related traits under optimum and low nitrogen conditions. Afr. J. Plant Sci. 2019, 13, 125–137. [Google Scholar]
- Kempthorne, O. An Introduction to Genetic Statistics; John Wiley-Sons Inc.: New York, NY, USA, 1957. [Google Scholar]
- Sedhom, S.A.; El-Badawy, M.E.M.; Hosary, A.A.E.; Abd El-Latif, M.S.; Rady, A.M.; Moustafa, M.M.; Mohamed, S.A.; Badr, O.A.; Abo-Marzoka, S.A.; Baiumy, K.A. Molecular markers and GGE biplot analysis for selecting higher-yield and drought-tolerant maize hybrids. Agron. J. 2021, 113, 3871–3885. [Google Scholar] [CrossRef]
- Stepanovic, S.; Rudnick, D.; Kruger, G. Impact of maize hybrid selection on water productivity under deficit irrigation in semiarid western Nebraska. Agric. Water Manag. 2021, 244, 106610. [Google Scholar] [CrossRef]
- Chen, S.; Dang, D.; Liu, Y.; Ji, S.; Zheng, H.; Zhao, C.; Dong, X.; Li, C.; Guan, Y.; Zhang, A. Genome-wide association study presents insights into the genetic architecture of drought tolerance in maize seedlings under field water-deficit conditions. Front. Plant Sci. 2023, 14, 1165582. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Sinana, H.F.; Ravikesavan, R.; Iyanar, K.; Senthil, A. Study of genetic variability and diversity analysis in maize (Zea mays L.) by agglomerative hierarchical clustering and principal component analysis. Electron. J. Plant Breed. 2023, 14, 43–51. [Google Scholar]
- Evamoni, F.Z.; Nulit, R.; Yap, C.K.; Ibrahim, M.H.; Sidek, N.B. Assessment of germination performance and early seedling growth of Malaysian indica rice genotypes under drought conditions for strategic cropping during water scarcity. Chil. J. Agric. Res. 2023, 83, 281–292. [Google Scholar] [CrossRef]
- Wang, R.; Peng, W. Principal component analysis and comprehensive evaluation on drought tolerance difference of canola cultivars at germination and emergence stages. Chil. J. Agric. Res. 2021, 81, 557–567. [Google Scholar] [CrossRef]
- Panda, S.; Wali, M.C.; Kachapur, R.; Harlapur, S. Combining ability and heterosis analysis of single cross hybrids of maize (Zea mays L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2608–2618. [Google Scholar] [CrossRef]
- Ruswandi, D.; Supriatna, J.; Makkulawu, A.; Waluyo, B.; Marta, H.; Suryadi, E.; Ruswandi, S. Determination of combining ability and heterosis of grain yield components for maize mutants based on line× tester analysis. Asian J. Crop Sci. 2015, 7, 19–33. [Google Scholar] [CrossRef]
- Amiruzzaman, M.; Islam, M.A.; Hasan, L.; Kadir, M.; Rohman, M.M. Heterosis and combining ability in a diallel among elite inbred lines of maize (Zea mays L.). Emir. J. Food Agric. 2013, 25, 132–137. [Google Scholar] [CrossRef]
- Lekha, R.; Singh, R.; Singh, S.K.; Srivastava, R.P. Heterosis and combining ability studies for quality protein maize. Ekin J. Crop Breed. Genet. 2015, 1, 8–25. [Google Scholar]
- Kahriman, F.; Egesel, C.Ö.; Orhun, G.E.; Alaca, B.; Avci, F. Comparison of graphical analyses for maize genetic experiments: Application of biplots and polar plot to line x tester design. Chil. J. Agric. Res. 2016, 76, 285–293. [Google Scholar] [CrossRef]
- Issa, Z.; Nyadanu, D.; Richard, A.; Sangare, A.; Adejumobi, I.; Ibrahim, D. Inheritance and combining ability study on drought tolerance and grain yield among early maturing inbred lines of maize (Zea mays L.). J. Plant Breed. Crop Sci. 2018, 10, 115–127. [Google Scholar] [CrossRef]
- Adewale, S.A.; Badu-Apraku, B.; Akinwale, R.O. Assessing the suitability of stress tolerant early-maturing maize (Zea mays) inbred lines for hybrid development using combining ability effects and DArTseq markers. Plant Breed. 2023, 142, 223–237. [Google Scholar] [CrossRef]
- Soto-Cerda, B.J.; Westermeyer, F.; Iñiguez-Luy, F.; Muñoz, G.; Montenegro, A.; Cloutier, S. Assessing the agronomic potential of linseed genotypes by multivariate analyses and association mapping of agronomic traits. Euphytica 2014, 196, 35–49. [Google Scholar] [CrossRef]
- Kumar, K.; Anjoy, P.; Sahu, S.; Durgesh, K.; Das, A.; Tribhuvan, K.U.; Sevanthi, A.M.; Joshi, R.; Jain, P.K.; Singh, N.K. Single trait versus principal component based association analysis for flowering related traits in pigeonpea. Sci. Rep. 2022, 12, 10453. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Hameed, A.; Tahir, M.F. Estimation of genetic divergence in wheat genotypes based on agro-morphological traits through agglomerative hierarchical clustering and principal component analysis. Cereal Res. Commun. 2023, 51, 217–224. [Google Scholar] [CrossRef]
- Amegbor, I.K.; van Biljon, A.; Shargie, N.; Tarekegne, A.; Labuschagne, M.T. Heritability and associations among grain yield and quality traits in quality protein maize (QPM) and non-QPM hybrids. Plants 2022, 11, 713. [Google Scholar] [CrossRef] [PubMed]

| Code | Name | Pedigree | Origin | Kernel Type | Kernel Color | Days to Silking | Grain Yield /Plant (g) |
|---|---|---|---|---|---|---|---|
| Lines | |||||||
| L-1 | M5 | Line developed from Cairo-1 | Egypt | Dent corn | White | 66.00 | 63.00 |
| L-2 | M8 | Line developed from Giza-2 | Egypt | Dent corn | White | 64.00 | 68.17 |
| L-4 | CIMMYT-46 | Line developed from La-Posta (dent population) | Mexico | Dent corn | White | 64.83 | 64.50 |
| L-5 | CLM-19 | Line developed from La-Posta (dent population) | Mexico | Dent corn | White | 66.50 | 49.50 |
| L-6 | M26 | Line developed from Giza-2 | Egypt | Dent corn | White | 68.33 | 57.67 |
| L-7 | M29 | Line developed from Cairo-1 | Egypt | Dent corn | White | 67.83 | 75.67 |
| L-8 | M36 | Line developed from Giza-2 | Egypt | Dent corn | White | 70.17 | 50.67 |
| L-9 | M41 | Line developed from Giza-2 | Egypt | Dent corn | White | 69.00 | 64.17 |
| L-10 | M42 | Line developed from Pioneer-514 | Egypt | Dent corn | White | 67.83 | 64.83 |
| L-11 | M47 | Line developed from Pioneer-Fatah | Egypt | Dent corn | White | 66.50 | 57.17 |
| Testers | |||||||
| T-1 | M6 | Line developed from Giza-2 | Egypt | Dent corn | White | 67.50 | 47.17 |
| T-2 | M14 | Line developed from CIMMYT-14 | Mexico | Dent corn | White | 68.00 | 59.83 |
| Source of Variance | df | Grain Yield (ton/ha) | Number of Rows/Ear | Number of Grains/Row | |||
|---|---|---|---|---|---|---|---|
| W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | ||
| Crosses | 19 | 8.15 ** | 8.96 ** | 4.37 ** | 8.71 ** | 134.4 ** | 138.30 ** |
| Lines | 9 | 9.58 ** | 10.58 ** | 5.74 ** | 9.32 ** | 112.2 ** | 141.91 ** |
| Testers | 1 | 4.11 ** | 4.96 ** | 7.21 ** | 51.34 ** | 510.4 ** | 126.44 ** |
| Line × tester | 9 | 7.17 ** | 7.77 ** | 2.68 ** | 3.37 ** | 114.8 ** | 136.01 ** |
| Error | 38 | 0.26 | 0.39 | 1.22 | 0.53 | 5.25 | 6.29 |
| GCA | 0.022 | 0.03 | 0.04 | 0.12 | 0.44 | 0.05 | |
| SCA | 2.30 | 2.46 | 0.49 | 0.95 | 36.50 | 43.24 | |
| 100-kernel weight (g) | Plant height (cm) | Ear height (cm) | |||||
| Crosses | 19 | 37.36 ** | 40.37 ** | 1331.2 ** | 1312.1 ** | 770.2 ** | 750.7 ** |
| Lines | 9 | 22.75 ** | 65.82 ** | 1145.4 ** | 1200.5 ** | 787.2 ** | 865.4 ** |
| Testers | 1 | 170 ** | 21.6 ** | 1092.3 ** | 1126.7 ** | 1215 ** | 735.0 ** |
| Line × tester | 9 | 37.24 ** | 17.01 ** | 1543.5 ** | 1444.4 ** | 703.7 ** | 637.8 ** |
| Error | 38 | 7.75 | 4.80 | 90.28 | 53.71 | 47.14 | 46.81 |
| GCA | 0.003 | 0.53 | 4.80 | 2.99 | 1.50 | 2.55 | |
| SCA | 9.83 | 4.07 | 484.4 | 463.6 | 218.9 | 197 | |
| Days to tasseling | Days to silking | Chlorophyll content | |||||
| Crosses | 19 | 15.26 ** | 31.01 ** | 17.70 ** | 25.79 ** | 67.28 ** | 86.53 ** |
| Lines | 9 | 20.47 ** | 49.96 ** | 17.55 ** | 22.31 ** | 72.85 ** | 104.04 ** |
| Testers | 1 | 26.67 ** | 41.67 ** | 86.40 ** | 170.02 ** | 36.97 * | 187.97 ** |
| Line × tester | 9 | 8.78 ** | 10.89 ** | 10.21 ** | 13.24 ** | 65.07 ** | 57.75 ** |
| Error | 38 | 5.64 | 3.58 | 2.45 | 2.14 | 8.90 | 6.94 |
| GCA | 0.15 | 0.46 | 0.17 | 0.28 | 0.05 | 0.65 | |
| SCA | 1.05 | 2.44 | 2.59 | 3.70 | 18.72 | 16.93 | |
| Hybrid | Grain Yield (t/ha) | Number of Rows/Ear | Number of Grains/Row | 100 Kernel Weight (g) | Plant Height | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| W- Watered | D- Stress | W- Watered | D- Stress | W- Watered | D- Stress | W- Watered | D- Stress | W- Watered | D- Stress | |
| L-1×T-1 | −2.70 ** | −2.86 ** | −0.72 | −1.84 ** | −5.35 ** | −10.32 ** | −3.65 * | −1.15 | −23.73 ** | −19.07 ** |
| L-2×T-1 | 0.02 | 0.52 | 0.05 | −0.15 | 4.10 ** | 4.08 ** | −1.15 | 0.85 | −1.23 | −6.90 |
| L-4×T-1 | 0.39 | 0.40 | 0.05 | −0.82 | 8.75 ** | 6.75 ** | −2.48 | 0.52 | 33.43 ** | 32.93 ** |
| L-5×T-1 | 0.66 * | 1.02 ** | −1.19 | −0.99 | 1.67 | −0.08 | −0.32 | 1.02 | 16.43 ** | 17.60 ** |
| L-6×T-1 | 0.46 | 0.06 | 0.35 | −0.65 | −1.38 | −1.72 | −2.98 | −0.82 | −12.90 * | −12.40 * |
| L-7×T-1 | 1.46 ** | 0.99 ** | 1.01 | 1.13 | 3.28 ** | 0.62 | 3.52 * | 4.85 ** | 2.27 | 1.77 |
| L-8×T-1 | −0.01 | 0.84 ** | −0.42 | −0.75 | −1.18 | −2.18 | 2.18 | 1.85 | 5.60 | 6.10 |
| L-9×T-1 | 0.45 | 0.06 | 0.18 | −0.77 | −2.62 * | −2.70 * | 2.18 | 1.18 | −10.73 | −10.90 * |
| L-10×T-1 | −0.54 | −0.45 | 0.85 | −0.62 | −3.68 ** | −4.62 ** | 1.85 | 2.18 | −5.73 | −4.57 |
| L-11×T-1 | −0.20 | −0.33 | −0.15 | −0.32 | −3.58 ** | −4.48 ** | 0.85 | 0.35 | −3.40 | −5.23 |
| L-1×T-2 | 2.70 ** | 2.86 ** | 0.72 | 1.84 ** | 5.35 ** | 10.32 ** | 3.65 * | 1.15 | 23.73 ** | 19.07 ** |
| L-2×T-2 | −0.02 | −0.52 | −0.05 | 0.15 | −4.10 ** | −4.08 ** | 1.15 | −0.85 | 1.23 | 6.90 |
| L-4×T-2 | −0.39 | −0.40 | −0.05 | 0.82 | −8.75 ** | −6.75 ** | 2.48 | −0.52 | −33.43 ** | −32.93 ** |
| L-5×T-2 | −0.66 * | −1.02 ** | 1.19 | 0.99 | −1.67 | 0.08 | 0.32 | −1.02 | −16.43 ** | −17.60 ** |
| L-6×T-2 | −0.46 | −0.06 | −0.35 | 0.65 | 1.38 | 1.72 | 2.98 | 0.82 | 12.90 * | 12.40 * |
| L-7×T-2 | −1.46 ** | −0.99 ** | −1.01 | −1.13 | −3.28 * | −0.62 | −3.52 * | −4.85 ** | −2.27 | −1.77 |
| L-8×T-2 | 0.01 | −0.84 ** | 0.42 | 0.75 | 1.18 | 2.18 | −2.18 | −1.85 | −5.60 | −6.10 |
| L-9×T-2 | −0.45 | −0.06 | −0.18 | 0.77 | 2.62 * | 2.70 * | −2.18 | −1.18 | 10.73 | 10.90 * |
| L-10×T-2 | 0.54 | 0.45 | −0.85 | 0.62 | 3.68 ** | 4.62 ** | −1.85 | −2.18 | 5.73 | 4.57 |
| L-11×T-2 | 0.20 | 0.33 | 0.15 | 0.32 | 3.58 ** | 4.48 ** | −0.85 | −0.35 | 3.40 | 5.23 |
| Hybrid | Ear height | Days to tasseling | Days to silking | Chlorophyll content | ||||||
| W- Watered | D- Stress | W- Watered | D- Stress | W- Watered | D- Stress | W- Watered | D- Stress | |||
| L-1×T-1 | −22.83 ** | −13.83 ** | 2.67 * | 1.33 | 1.69 * | 1.67 * | −5.18 ** | −6.93 ** | ||
| L-2×T-1 | 4.67 | 8.50 * | −1.00 | −1.83 | −1.48 | −2.48 ** | −1.88 | −1.03 | ||
| L-4×T-1 | 17.17 ** | 20.00 ** | 0.33 | −0.33 | −0.15 | −0.82 | 2.70 | 0.04 | ||
| L-5×T-1 | 11.33 ** | 12.17 ** | 0.83 | 1.17 | 2.18 ** | 0.85 | 1.00 | 0.79 | ||
| L-6×T-1 | 0.01 | −0.50 | 0.83 | 1.50 | 1.85 * | 1.52 | −2.56 | −2.25 | ||
| L-7×T-1 | −0.33 | −3.00 | 0.01 | −0.67 | −0.32 | −1.15 | 6.47 ** | 4.45 ** | ||
| L-8×T-1 | −1.50 | −10.00 ** | 0.17 | 1.83 | −0.82 | 0.85 | 1.55 | 1.75 | ||
| L-9×T-1 | −0.83 | 3.33 | −1.34 | −1.32 | −0.65 | −1.82 * | −1.98 | −1.03 | ||
| L-10×T-1 | −9.17 * | −5.83 | −1.33 | −1.33 | 1.35 | 1.35 | −1.50 | −1.88 | ||
| L-11×T-1 | 1.50 | −0.83 | 0.50 | −0.33 | 1.18 | 0.02 | 1.39 | −3.75 * | ||
| L-1×T-2 | 22.83 ** | 13.83 ** | −2.67 * | −1.33 | −1.68 * | −1.68 * | 5.18 ** | 6.93 ** | ||
| L-2×T-2 | −4.67 | −8.50 * | 1.00 | 1.83 | 1.48 | 2.48 ** | 1.88 | 1.03 | ||
| L-4×T-2 | −17.17 ** | −20.00 ** | −0.33 | 0.33 | 0.15 | 0.82 | −2.70 | −0.03 | ||
| L-5×T-2 | −11.33 ** | −12.17 ** | −0.83 | −1.17 | −2.18 ** | −0.85 | −1.00 | −0.78 | ||
| L-6×T-2 | 0.01 | 0.50 | −0.83 | −1.50 | −1.85 * | −1.52 | 2.57 | 2.25 | ||
| L-7×T-2 | 0.33 | 3.00 | 0.01 | 0.67 | 0.32 | 1.15 | −6.47 ** | −4.45 ** | ||
| L-8×T-2 | 1.50 | 10.00 * | −0.17 | −1.83 | 0.82 | −0.85 | −1.55 | −1.75 | ||
| L-9×T-2 | 0.83 | −3.33 | 1.33 | 1.33 | 0.65 | 1.82 * | 1.98 | 1.03 | ||
| L-10×T-2 | 9.17 * | 5.83 | 1.33 | 1.33 | −1.36 | −1.34 | 1.50 | 1.88 | ||
| L-11×T-2 | −1.50 | 0.83 | −0.50 | 0.33 | −1.18 | −0.02 | −1.38 | 3.75 * | ||
| Hybrid | Grain Yield/(t/ha) | Number of Rows/Ear | ||||||
|---|---|---|---|---|---|---|---|---|
| Relative to SC-10 | Relative to SC-30k8 | Relative to SC-10 | Relative to SC-30k8 | |||||
| W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | |
| L-1×T-1 | −19.83 ** | −27.69 ** | −21.17 ** | −29.36 ** | 1.44 | −11.25 * | 2.72 | −11.69 * |
| L-2×T-1 | −5.08 | −0.36 | −6.67 | −2.67 | 0.46 | 0.01 | 1.73 | −0.50 |
| L-4×T-1 | −21.53 ** | −16.21 ** | −22.83 ** | −18.15 ** | −4.41 | −2.50 | −3.21 | −2.99 |
| L-5×T-1 | 0.17 | 7.10 | −1.50 | 4.63 | −19.53 ** | −12.50 ** | −18.52 ** | −12.94 ** |
| L-6×T-1 | 7.46 | 7.47 | 5.67 | 4.98 | −2.46 | −3.50 | −1.23 | −3.98 |
| L-7×T-1 | 20.34 ** | 19.49 ** | 18.33 ** | 16.73 ** | 9.73 | 8.75 | 11.11 | 8.21 |
| L-8×T-1 | −3.73 | 2.55 | −5.33 | 0.18 | −11.73 | −6.50 | −10.62 | −6.97 |
| L-9×T-1 | 1.69 | −7.10 | 0.09 | −9.25 | −7.34 | −10.50 * | −6.17 | −10.95 |
| L-10×T-1 | −17.80 ** | −21.68 ** | −19.17 ** | −23.49 ** | −7.34 | −22.50 ** | −6.17 | −22.89 ** |
| L-11×T-1 | 13.90 ** | 19.49 ** | 12.00 ** | 16.73 ** | 12.17 | 10.00 * | 13.58 * | 9.45 * |
| L-1×T-2 | 23.56 ** | 22.04 ** | 21.50 ** | 19.22 ** | 13.14 | 19.00 ** | 14.57 * | 18.41 ** |
| L-2×T-2 | −10.17 * | −15.30 ** | −11.67 ** | −17.26 ** | −0.02 | 5.00 | 1.23 | 4.48 |
| L-4×T-2 | −33.05 ** | −28.96 ** | −34.17 ** | −30.60 ** | 4.85 | 20.00 ** | 6.17 | 19.40 ** |
| L-5×T-2 | −16.27 ** | −17.49 ** | −17.67 ** | −19.40 ** | 7.78 | 2.50 | 9.14 | 1.99 |
| L-6×T-2 | −5.42 | 1.28 | −7.00 | −1.07 | −1.49 | 5.00 | −0.25 | 4.48 |
| L-7×T-2 | −10.34 * | −4.37 | −11.83 ** | −6.58 | −0.02 | −0.50 | 1.23 | −1.00 |
| L-8×T-2 | −8.14 * | −18.58 ** | −9.67 * | −20.46 ** | 0.95 | 1.50 | 2.22 | 1.00 |
| L-9×T-2 | −11.02 ** | −13.30 ** | −12.50 ** | −15.30 ** | −6.61 | 1.25 | −5.43 | 0.75 |
| L-10×T-2 | −12.88 ** | −18.03 ** | −14.33 ** | −19.93 ** | −8.32 | −10.50 * | −7.16 | −10.95 * |
| L-11×T-2 | 12.71 ** | 20.77 ** | 10.83 ** | 17.97 ** | 4.85 | 22.50 ** | 6.17 | 21.89 ** |
| Hybrid | Number of grains/rows | 100-kernel weight | ||||||
| Relative to SC-10 | Relative to SC-30k8 | Relative to SC-10 | Relative to SC-30k8 | |||||
| W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | |
| L-1×T-1 | −5.19 | −31.31 ** | −6.10 | −32.81 ** | −10.99 | −13.73 * | −15.24 * | −15.38 ** |
| L-2×T-1 | 7.79 | 10.10 | 6.76 | 7.71 | −11.99 | −15.69 ** | −16.19 * | −17.31 ** |
| L-4×T-1 | 5.77 | −5.05 | 4.76 | −7.11 | −17.99 * | −26.47 ** | −21.90 ** | −27.88 ** |
| L-5×T-1 | 7.98 | 1.01 | 6.95 | −1.19 | −4.99 | −14.71 ** | −9.52 | −16.35 ** |
| L-6×T-1 | 6.73 | 8.28 | 5.71 | 5.93 | −19.99 ** | −29.41 ** | −23.81 ** | −30.77 ** |
| L-7×T-1 | 18.46 ** | 5.05 | 17.33 ** | 2.77 | 12.01 | 9.80 | 6.67 | 7.69 |
| L-8×T-1 | 4.62 | 3.03 | 3.62 | 0.79 | 4.01 | −5.88 | −0.95 | −7.69 |
| L-9×T-1 | 2.50 | 6.06 | 1.52 | 3.75 | −4.99 | −14.71 ** | −9.52 | −16.35 ** |
| L-10×T-1 | 8.46 | 4.04 | 7.43 | 1.78 | −0.99 | −0.98 | −5.71 | −2.88 |
| L-11×T-1 | 16.15 ** | 15.76 * | 15.05 ** | 13.24 * | 8.01 | 3.92 | 2.86 | 1.92 |
| L-1×T-2 | 8.85 | 13.54 * | 7.81 | 11.07 | 21.01 | 2.94 | 15.24 ** | 0.96 |
| L-2×T-2 | −32.69 ** | −32.32 ** | −33.33 ** | −33.79 ** | 5.01 | −10.78 * | 0.01 | −12.50 * |
| L-4×T-2 | −61.54 ** | −63.64 ** | −61.90 ** | −64.43 ** | 7.01 | −19.61 ** | 1.90 | −21.15 ** |
| L-5×T-2 | −18.46 ** | −16.16 ** | −19.24 ** | −17.98 ** | 7.01 | −10.78 * | 1.90 | −12.50 * |
| L-6×T-2 | −2.12 | 1.01 | −3.05 | −1.19 | 8.01 | −14.71 ** | 2.86 | −16.35 ** |
| L-7×T-2 | −17.31 ** | −16.36 * | −18.10 ** | −18.18 ** | 1.01 | −8.82 | −3.81 | −10.58 |
| L-8×T-2 | −5.38 | −1.41 | −6.29 | −3.56 | 1.01 | −6.86 | −3.81 | −8.65 |
| L-9×T-2 | 0.77 | 4.75 | −0.19 | 2.47 | −7.99 | −11.76 * | −12.38 | −13.46 ** |
| L-10×T-2 | 12.88 | 14.34 * | 11.81 * | 11.86 | −1.99 | −3.92 | −6.67 | −5.77 |
| L-11×T-2 | 20.00 ** | 25.25 ** | 18.86 ** | 22.53 ** | 18.00 | 12.35 * | 12.37 | 10.19 |
| Hybrid | Plant Height (cm) | Ear Height (cm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Relative to SC-10 | Relative to SC-30k8 | Relative to SC-10 | Relative to SC-30k8 | |||||
| W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | |
| L-1×T-1 | −20.97 ** | −19.69 ** | −8.44 * | −9.20 ** | −36.84 ** | −33.84 ** | −23.48 ** | −23.53 ** |
| L-2×T-1 | −8.95 ** | −13.65 ** | 5.48 | −2.37 | −24.40 | −20.1 | −8.41 | −7.65 |
| L-4×T-1 | −2.69 | −1.18 | 12.74 ** | 11.72 ** | −7.66 * | −3.56 | 11.88 * | 11.47 * |
| L-5×T-1 | −1.79 | −0.26 | 13.78 | 12.76 | −12.20 ** | −7.38 | 6.38 | 7.06 |
| L-6×T-1 | −4.09 | −2.89 | 11.11 ** | 9.79 ** | −3.11 | 1.27 | 17.39 ** | 17.06 ** |
| L-7×T-1 | −3.07 | −2.23 | 12.30 | 10.53 ** | −7.89 * | −7.12 | 11.59 * | 7.35 |
| L-8×T-1 | −1.53 | −0.92 | 14.07 ** | 12.02 ** | −6.22 | −13.49 ** | 13.62 ** | 0.01 |
| L-9×T-1 | −3.32 | −3.02 | 12.00 ** | 9.64 ** | −7.66 * | −3.31 | 11.88 * | 11.76 * |
| L-10×T-1 | −11.25 ** | −10.10 ** | 2.81 | 1.63 | −19.62 ** | −17.56 ** | −2.61 | −4.71 |
| L-11×T-1 | −4.86 | −4.86 | 10.22 ** | 7.57 ** | −8.61 * | −6.87 | 10.72 | 7.65 |
| L-1×T-2 | 0.51 | −1.31 | 16.44 ** | 11.57 ** | 2.39 | −5.85 | 24.06 | 8.82 |
| L-2×T-2 | −4.73 | −4.86 | 10.37 ** | 7.57 ** | −24.64 ** | −26.21 ** | −8.70 | −14.71 ** |
| L-4×T-2 | −25.06 ** | −23.75 ** | −3.19 ** | −13.80 ** | −25.84 ** | −27.23 ** | −10.14 * | −15.88 ** |
| L-5×T-2 | −11.13 ** | −10.76 ** | 2.96 | 0.89 | −22.01 ** | −19.08 ** | −5.51 | −6.47 |
| L-6×T-2 | 9.08 ** | 10.24 ** | 26.37 ** | 24.63 ** | 3.35 | 8.91 * | 25.22 ** | 25.88 ** |
| L-7×T-2 | −1.53 | −0.26 | 14.07 ** | 12.76 ** | −0.96 | 4.33 | 20.00 ** | 20.59 ** |
| L-8×T-2 | −2.56 | −2.36 | 12.89 ** | 10.39 ** | 2.39 | 8.65 * | 24.06 ** | 25.59 ** |
| L-9×T-2 | 8.18 ** | 8.92 ** | 25.33 ** | 23.15 ** | 0.01 | −1.53 | 21.16 ** | 13.82 ** |
| L-10×T-2 | −3.58 | −3.15 | 11.70 ** | 9.50 ** | 0.01 | −1.78 | 21.16 ** | 13.53 ** |
| L-11×T-2 | 1.02 | 2.62 | 17.04 ** | 16.02 ** | −4.31 | 1.27 | 15.94 ** | 17.06 ** |
| Hybrid | Days to tasseling | Days to silking | ||||||
| Relative to SC-10 | Relative to SC-30k8 | Relative to SC-10 | Relative to SC-30k8 | |||||
| W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | W-Watered | D-Stress | |
| L-1×T-1 | 2.22 | 3.98 | 3.95 | 8.28 * | −2.05 | −1.57 | −0.52 | 1.62 |
| L-2×T-1 | 1.11 | −3.98 | 2.82 | 0.01 | −2.56 | −4.71 * | −1.04 | −1.62 |
| L-4×T-1 | −6.11 * | −4.55 | −4.52 | −0.59 | −4.10 | −4.71 * | −2.60 | −1.62 |
| L-5×T-1 | −1.11 | −1.70 | 0.56 | 2.37 | −2.05 | −1.57 | −0.52 | 1.62 |
| L-6×T-1 | −6.11 * | −6.82 * | −4.52 | −2.96 | −4.62 * | −6.28 ** | −3.13 | −3.24 |
| L-7×T-1 | −5.56 * | −4.55 | −3.95 | −0.59 | −7.18 ** | −6.28 ** | −5.73 ** | −3.24 |
| L-8×T-1 | 4.44 | −6.25 * | 6.21 * | −2.37 | −3.08 | −5.76 ** | −1.56 | −2.70 |
| L-9×T-1 | −1.67 | −5.11 | 0.01 | −1.18 | −1.54 | −3.14 | 0.01 | 0.01 |
| L-10×T-1 | −13.89 ** | −13.64 ** | −12.43 ** | −10.06 ** | −5.13 ** | −8.38 ** | −3.65 | −5.41 * |
| L-11×T-1 | −4.44 | −3.41 | −2.82 | 0.59 | −5.13 ** | −4.71 * | −3.65 | −1.62 |
| L-1×T-2 | 0.56 | −2.27 | 2.26 | 1.78 | −2.05 | −1.57 | −0.52 | 1.62 |
| L-2×T-2 | 10.00 ** | 2.27 | 11.86 ** | 6.51 * | 10.26 ** | 5.24 ** | 11.98 ** | 8.65 ** |
| L-4×T-2 | −2.22 | −2.84 | −0.56 | 1.18 | 3.59 | 1.05 | 5.21 ** | 4.32 * |
| L-5×T-2 | −2.22 | −1.70 | −0.56 | 2.37 | 0.51 | −3.14 | 2.08 | 0.01 |
| L-6×T-2 | −8.33 ** | −6.82 * | −6.78 * | −2.96 | −4.10 * | −6.81 ** | −2.60 | −3.78 |
| L-7×T-2 | −0.56 | −1.70 | 1.13 | 2.37 | 1.54 | 0.01 | 3.13 | 3.24 |
| L-8×T-2 | 1.11 | −3.98 | 2.82 | 0.01 | −0.51 | 2.09 | 1.04 | 5.41 * |
| L-9×T-2 | 5.56 * | 2.27 | 7.34 ** | 6.51 * | 9.23 ** | 4.19 * | 10.94 ** | 7.57 ** |
| L-10×T-2 | −6.67 * | −6.25 * | −5.08 | −2.37 | −4.10 * | −7.33 ** | −2.60 | −4.32 * |
| L-11×T-2 | −0.56 | −2.27 | 1.13 | 1.78 | 0.01 | −3.14 | 1.56 | 0.01 |
| Hybrid | Chlorophyll Content | |||
|---|---|---|---|---|
| Relative to SC-10 | Relative to SC-30k8 | |||
| W-Watered | D-Stress | W-Watered | D-Stress | |
| L-1×T-1 | 0.95 | −8.69 | −1.84 | −12.98 * |
| L-2×T-1 | −9.34 * | −2.50 | −11.84 ** | −7.09 |
| L-4×T-1 | −2.23 | −6.85 | −4.93 | −11.23 * |
| L-5×T-1 | −4.40 | −4.27 | −7.04 | −8.77 |
| L-6×T-1 | −1.96 | 2.65 | −4.67 | −2.18 |
| L-7×T-1 | 16.04 ** | 11.93 * | 12.83 ** | 6.67 |
| L-8×T-1 | −2.64 | 3.76 | −5.33 | −1.12 |
| L-9×T-1 | −14.07 ** | −7.95 | −16.45 ** | −12.28 * |
| L-10×T-1 | −13.19 ** | −19.00 ** | −15.59 ** | −22.81 ** |
| L-11×T-1 | 4.19 | 9.57 | 1.32 | 4.42 |
| L-1×T-2 | 25.17 ** | 25.41 ** | 21.71 ** | 19.51 ** |
| L-2×T-2 | 1.49 | 5.52 | −1.32 | 0.56 |
| L-4×T-2 | −10.01 * | −3.53 | −12.50 ** | −8.07 |
| L-5×T-2 | −5.28 | −4.27 | −7.89 | −8.77 |
| L-6×T-2 | 11.64 * | 16.05 ** | 8.55 | 10.60 * |
| L-7×T-2 | −7.04 | −4.27 | −9.61 * | −8.77 |
| L-8×T-2 | −5.75 | −0.52 | −8.36 | −5.19 |
| L-9×T-2 | −2.84 | 0.07 | −5.53 | −4.63 |
| L-10×T-2 | −3.92 | −7.22 | −6.58 | −11.58 * |
| L-11×T-2 | 1.76 | 29.60 ** | −1.05 | 23.51 ** |
| Hybrid | Yp | Ys | MP | TOL | HM | SSI | STI | YI | YSI | GMP | YRR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L-1×T-1 | 9.0 | 7.6 | 8.30 | 1.40 | 8.24 | 2.37 | 0.59 | 0.76 | 0.84 | 8.27 | 15.56 |
| L-2×T-1 | 10.7 | 10.4 | 10.55 | 0.30 | 10.55 | 0.43 | 0.96 | 1.03 | 0.97 | 10.55 | 2.80 |
| L-4×T-1 | 8.8 | 8.8 | 8.80 | 0.00 | 8.80 | 0.00 | 0.67 | 0.88 | 1.00 | 8.80 | 0.00 |
| L-5×T-1 | 11.3 | 11.2 | 11.25 | 0.10 | 11.25 | 0.14 | 1.09 | 1.11 | 0.99 | 11.25 | 0.88 |
| L-6×T-1 | 12.1 | 11.2 | 11.65 | 0.90 | 11.63 | 1.14 | 1.17 | 1.11 | 0.93 | 11.64 | 7.44 |
| L-7×T-1 | 13.5 | 12.5 | 13.00 | 1.00 | 12.98 | 1.13 | 1.46 | 1.24 | 0.93 | 12.99 | 7.41 |
| L-8×T-1 | 10.8 | 10.7 | 10.75 | 0.10 | 10.75 | 0.14 | 1.00 | 1.06 | 0.99 | 10.75 | 0.93 |
| L-9×T-1 | 11.4 | 9.7 | 10.55 | 1.70 | 10.48 | 2.28 | 0.96 | 0.97 | 0.85 | 10.52 | 14.91 |
| L-10×T-1 | 9.2 | 8.2 | 8.70 | 1.00 | 8.67 | 1.66 | 0.65 | 0.82 | 0.89 | 8.69 | 10.87 |
| L-11×T-1 | 12.8 | 12.5 | 12.65 | 0.30 | 12.65 | 0.36 | 1.38 | 1.24 | 0.98 | 12.65 | 2.34 |
| L-1×T-2 | 13.9 | 12.8 | 13.35 | 1.10 | 13.33 | 1.21 | 1.54 | 1.27 | 0.92 | 13.34 | 7.91 |
| L-2×T-2 | 10.1 | 8.9 | 9.50 | 1.20 | 9.46 | 1.81 | 0.78 | 0.89 | 0.88 | 9.48 | 11.88 |
| L-4×T-2 | 7.5 | 7.4 | 7.45 | 0.10 | 7.45 | 0.20 | 0.48 | 0.74 | 0.99 | 7.45 | 1.33 |
| L-5×T-2 | 9.4 | 8.6 | 9.00 | 0.80 | 8.98 | 1.30 | 0.70 | 0.86 | 0.91 | 8.99 | 8.51 |
| L-6×T-2 | 10.6 | 10.6 | 10.60 | 0.00 | 10.60 | 0.00 | 0.97 | 1.05 | 1.00 | 10.60 | 0.00 |
| L-7×T-2 | 10.1 | 10.0 | 10.05 | 0.10 | 10.05 | 0.15 | 0.87 | 1.00 | 0.99 | 10.05 | 0.99 |
| L-8×T-2 | 10.3 | 8.5 | 9.40 | 1.80 | 9.31 | 2.67 | 0.76 | 0.85 | 0.83 | 9.36 | 17.48 |
| L-9×T-2 | 10.0 | 9.1 | 9.55 | 0.90 | 9.53 | 1.37 | 0.79 | 0.91 | 0.91 | 9.54 | 9.00 |
| L-10×T-2 | 9.8 | 8.6 | 9.20 | 1.20 | 9.16 | 1.87 | 0.73 | 0.86 | 0.88 | 9.18 | 12.24 |
| L-11×T-2 | 12.7 | 12.6 | 12.65 | 0.10 | 12.65 | 0.12 | 1.38 | 1.25 | 0.99 | 12.65 | 0.79 |
| S.C.10 | 11.2 | 10.5 | 10.85 | 0.70 | 10.84 | 0.95 | 1.02 | 1.04 | 0.94 | 10.84 | 6.25 |
| S.C. 30K8 | 11.4 | 10.7 | 11.05 | 0.70 | 11.04 | 0.94 | 1.05 | 1.06 | 0.94 | 11.04 | 6.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedhom, Y.S.A.; Rabie, H.A.; Awaad, H.A.; Alomran, M.M.; ALshamrani, S.M.; Mansour, E.; Ali, M.M.A. Genetic Potential of Newly Developed Maize Hybrids under Different Water-Availability Conditions in an Arid Environment. Life 2024, 14, 453. https://doi.org/10.3390/life14040453
Sedhom YSA, Rabie HA, Awaad HA, Alomran MM, ALshamrani SM, Mansour E, Ali MMA. Genetic Potential of Newly Developed Maize Hybrids under Different Water-Availability Conditions in an Arid Environment. Life. 2024; 14(4):453. https://doi.org/10.3390/life14040453
Chicago/Turabian StyleSedhom, Youstina S. A., Hassan A. Rabie, Hassan A. Awaad, Maryam M. Alomran, Salha M. ALshamrani, Elsayed Mansour, and Mohamed M. A. Ali. 2024. "Genetic Potential of Newly Developed Maize Hybrids under Different Water-Availability Conditions in an Arid Environment" Life 14, no. 4: 453. https://doi.org/10.3390/life14040453
APA StyleSedhom, Y. S. A., Rabie, H. A., Awaad, H. A., Alomran, M. M., ALshamrani, S. M., Mansour, E., & Ali, M. M. A. (2024). Genetic Potential of Newly Developed Maize Hybrids under Different Water-Availability Conditions in an Arid Environment. Life, 14(4), 453. https://doi.org/10.3390/life14040453








