Exploring the Efficacy of Novel Therapeutic Strategies for Periodontitis: A Literature Review
Abstract
1. Introduction
2. Main Body
2.1. Materials and Methods
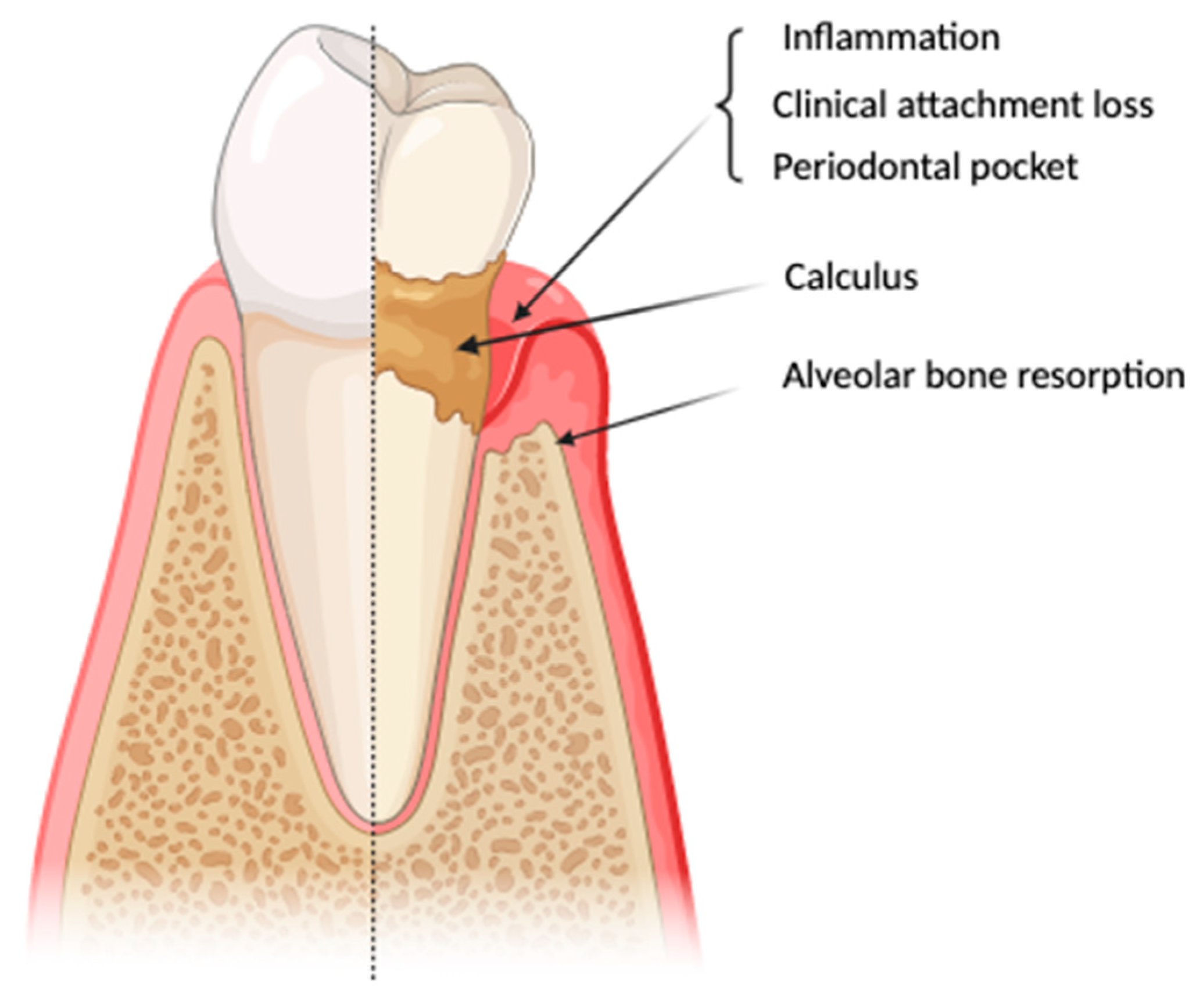
2.2. Periodontitis and Its Pathogenesis
2.3. Treatment Plan
2.4. Novel Therapies
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions—Introduction and Key Changes from the 1999 Classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 2), S162–S170. [Google Scholar] [CrossRef]
- Beck, J.D.; Papapanou, P.N.; Philips, K.H.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral Microbial Biofilms: An Update. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2019, 38, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The Oral Microbiome: Role of Key Organisms and Complex Networks in Oral Health and Disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Ramesh, A.; Varghese, S.S.; Doraiswamy, J.N.; Malaiappan, S. Herbs as an Antioxidant Arsenal for Periodontal Diseases. J. Intercult. Ethnopharmacol. 2016, 5, 92–96. [Google Scholar] [CrossRef]
- Guentsch, A.; Kramesberger, M.; Sroka, A.; Pfister, W.; Potempa, J.; Eick, S. Comparison of Gingival Crevicular Fluid Sampling Methods in Patients with Severe Chronic Periodontitis. J. Periodontol. 2011, 82, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Jepsen, S.; Needleman, I.; Roldán, S. A Systematic Review on the Effect of Systemic Antimicrobials as an Adjunct to Scaling and Root Planing in Periodontitis Patients. J. Clin. Periodontol. 2002, 29, 136–159. [Google Scholar] [CrossRef]
- Kinane, D.F.; Chestnutt, I.G. Smoking and Periodontal Disease. Crit. Rev. Oral Biol. Med. 2000, 11, 356–365. [Google Scholar] [CrossRef]
- Hasturk, H.; Schulte, F.; Martins, M.; Sherzai, H.; Floros, C.; Cugini, M.A.; Chiu, C.J.; Hardt, M.; Van Dyke, T. Safety and Preliminary Efficacy of a Novel Host-Modulatory Therapy for Reducing Gingival Inflammation. Front. Immunol. 2021, 12, 704163. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, D.; Gonçalves, L.S.; Lima-Junior, J.d.C.; Carrouel, F. Editorial: The Oral Microbiome Is a Key Factor in Oral and Systemic Health. Front. Microbiol. 2022, 13, 855668. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Hajishengallis, G. Polymicrobial Synergy and Dysbiosis in Inflammatory Disease. Trends Mol. Med. 2015, 21, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Iqbal, Z.; Ali, J.; Baboota, S.; Talegaonkar, S.; Ahmad, Z.; Sahni, J.K. Treatment Modalities and Evaluation Models for Periodontitis. Int. J. Pharm. Investig. 2012, 2, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Fragkioudakis, I.; Riggio, M.P.; Apatzidou, D.A. Understanding the Microbial Components of Periodontal Diseases and Periodontal Treatment-Induced Microbiological Shifts. J. Med. Microbiol. 2021, 70, 001247. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R. A Review of Factors Influencing the Incidence and Severity of Plaque-Induced Gingivitis. Minerva Stomatol. 2013, 62, 207–234. [Google Scholar]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017, 18, 1748. [Google Scholar] [CrossRef]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How Bacteria Stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A.; Netuveli, G.S. Periodontal Diseases in Europe. Periodontol. 2000 2002, 29, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of Microbial Biofilms in the Maintenance of Oral Health and in the Development of Dental Caries and Periodontal Diseases. Consensus Report of Group 1 of the Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Disease. J. Clin. Periodontol. 2017, 44 (Suppl. 1), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Dannewitz, B.; Holtfreter, B.; Eickholz, P. Periodontitis—Therapy of a Widespread Disease. Bundesgesundheitsblatt-Gesundheitsforsch.-Gesundheitsschutz 2021, 64, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and Surgical Treatment of Periodontitis: How Many Options for One Disease? Periodontol. 2000 2017, 75, 152–188. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M. Lifestyle and Periodontitis: The Emergence of Personalized Periodontics. Periodontol. 2000 2018, 78, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Umeizudike, K.A.; Lähteenmäki, H.; Räisänen, I.T.; Taylor, J.J.; Preshaw, P.M.; Bissett, S.M.; Tervahartiala, T.; Nwhator, S.O.; Pärnänen, P.; Sorsa, T. Ability of Matrix Metalloproteinase-8 Biosensor, IFMA, and ELISA Immunoassays to Differentiate between Periodontal Health, Gingivitis, and Periodontitis. J. Periodontal Res. 2022, 57, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R. Effects of Essential Oils on Central Nervous System: Focus on Mental Health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef]
- Annisa, Z.U.; Sulijaya, B.; Tadjoedin, E.S.S.; Hutomo, D.I.; Masulili, S.L.C. Effectiveness of Chlorhexidine Gels and Chips in Periodontitis Patients after Scaling and Root Planing: A Systematic Review and Meta-Analysis. BMC Oral Health 2023, 23, 819. [Google Scholar] [CrossRef]
- Thangavelu, A.; Kaspar, S.S.; Kathirvelu, R.P.; Srinivasan, B.; Srinivasan, S.; Sundram, R. Chlorhexidine: An Elixir for Periodontics. J. Pharm. Bioallied Sci. 2020, 12, S57–S59. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of Lifestyle, Behaviour or Systemic Diseases with Dental Caries and Periodontal Diseases: Consensus Report of Group 2 of the Joint EFP/ORCA Workshop on the Boundaries between Caries and Periodontal Diseases. J. Clin. Periodontol. 2017, 44, S39–S51. [Google Scholar] [CrossRef] [PubMed]
- Fi, C.; Wo, W. Periodontal Disease and Systemic Diseases: An Overview on Recent Progresses. J. Biol. Regul. Homeost. Agents 2021, 35, 1–9. [Google Scholar] [PubMed]
- Page, R.C. Gingivitis. J. Clin. Periodontol. 1986, 13, 345–359. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, E.T.; de Araújo, J.S.M.; Pires, A.C.; Lira dos Santos, E.J. Local Delivery Natural Products to Treat Periodontitis: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 4599–4619. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C.; Sturdivant, E.C. Noninflammatory Destructive Periodontal Disease (NDPD). Periodontol. 2000 2002, 30, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.; Floyd, P. Periodontal Examination and Screening. Br. Dent. J. 2023, 235, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A Polymicrobial Disruption of Host Homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, B.; Brandt, B.W.; Cheng, L.; Zhou, X.; Exterkate, R.A.M.; Crielaard, W.; Deng, D.M. Comparison of Red-Complex Bacteria between Saliva and Subgingival Plaque of Periodontitis Patients: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2021, 11, 727732. [Google Scholar] [CrossRef]
- Lafaurie, G.I.; Castillo, D.M.; Iniesta, M.; Sanz, M.; Gómez, L.A.; Castillo, Y.; Pianeta, R.; Delgadillo, N.A.; Neuta, Y.; Diaz-Báez, D.; et al. Differential Analysis of Culturable and Unculturable Subgingival Target Microorganisms According to the Stages of Periodontitis. Clin. Oral Investig. 2023, 27, 3029–3043. [Google Scholar] [CrossRef]
- Van Essche, M.; Quirynen, M.; Sliepen, I.; Loozen, G.; Boon, N.; Van Eldere, J.; Teughels, W. Killing of Anaerobic Pathogens by Predatory Bacteria. Mol. Oral Microbiol. 2011, 26, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, B.; Al Qurashi, Y.M.A.; Chaieb, K. Drug Resistance of Bacterial Dental Biofilm and the Potential Use of Natural Compounds as Alternative for Prevention and Treatment. Microb. Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial Efficacy of Five Essential Oils against Oral Pathogens: An In Vitro Study. Eur. J. Dent. 2013, 7, S071–S077. [Google Scholar] [CrossRef] [PubMed]
- Souto, R.; Colombo, A.P.V. Prevalence of Enterococcus Faecalis in Subgingival Biofilm and Saliva of Subjects with Chronic Periodontal Infection. Arch. Oral Biol. 2008, 53, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.R.; Pushalkar, S.; Gulivindala, D.; Butler, T.; Li, Y.; Annam, K.R.C.; Glodzik, L.; Ballman, K.V.; Corby, P.M.; Blennow, K.; et al. Periodontal Dysbiosis Associates with Reduced Csf Aβ42 in Cognitively Normal Elderly. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12172. [Google Scholar] [CrossRef]
- Ghezzi, C.; Ferrantino, L.; Donghi, C.; Vaghi, S.; Viganò, V.; Costa, D.; Mandaglio, M.; Pispero, A.; Lodi, G. Clinical Audit of Minimally Invasive Nonsurgical Techniques in Active Periodontal Therapy. J. Contemp. Dent. Pract. 2020, 21, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.M.; Yekta-Michael, S.S.; Schittenhelm, F.; Reichert, S.; Kupietz, D.; Dommisch, H.; Kasaj, A.; Wied, S.; Vela, O.C.; Stratul, S.I. Comparison of Three Full-Mouth Concepts for the Non-Surgical Treatment of Stage III and IV Periodontitis: A Randomized Controlled Trial. J. Clin. Periodontol. 2021, 48, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Horz, H.-P.; Conrads, G. Diagnosis and Anti-Infective Therapy of Periodontitis. Expert Rev. Anti-Infect. Ther. 2007, 5, 703–715. [Google Scholar] [CrossRef]
- Liviu, M.; Forna, N.; Agop-forna, D.; Mârţu, I. Clinical and Radiological Assessment of the Periodontal and Peri-Implant Health Status in Patients Treated by Implant-Prosthetic Therapy. Rom. J. Oral Rehabil. 2023, 15, 34–46. [Google Scholar]
- Moore, W.E.; Moore, L.V. The Bacteria of Periodontal Diseases. Periodontol. 2000 1994, 5, 66–77. [Google Scholar] [CrossRef]
- Worthington, H.V.; MacDonald, L.; Poklepovic Pericic, T.; Sambunjak, D.; Johnson, T.M.; Imai, P.; Clarkson, J.E. Home Use of Interdental Cleaning Devices, in Addition to Toothbrushing, for Preventing and Controlling Periodontal Diseases and Dental Caries. Cochrane Database Syst. Rev. 2019, 2020, 4. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental Biofilms: Difficult Therapeutic Targets. Periodontol. 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Bauroth, K.; Charles, C.H.; Mankodi, S.M.; Simmons, K.; Zhao, Q.; Kumar, L.D. The Efficacy of an Essential Oil Antiseptic Mouthrinse vs. Dental Floss in Controlling Interproximal Gingivitis: A Comparative Study. J. Am. Dent. Assoc. 2003, 134, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Benakanakere, M.; Kinane, D.F. Innate Cellular Responses to the Periodontal Biofilm. Front. Oral Biol. 2012, 15, 41–55. [Google Scholar] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Van der Weijden, G.A.; Dekkers, G.J.; Slot, D.E. Success of Non-Surgical Periodontal Therapy in Adult Periodontitis Patients: A Retrospective Analysis. Int. J. Dent. Hyg. 2019, 17, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute Periodontal Lesions (Periodontal Abscesses and Necrotizing Periodontal Diseases) and Endo-Periodontal Lesions. J. Clin. Periodontol. 2018, 45, S78–S94. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbial Colonization of the Periodontal Pocket and Its Significance for Periodontal Therapy. Periodontol. 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Aimetti, M. Nonsurgical Periodontal Treatment. Int. J. Esthet. Dent. 2014, 9, 251–267. [Google Scholar]
- Cuevas-Gonzalez, M.V.; Cuevas-Gonzalez, J.C.; Espinosa-Cristóbal, L.F.; Donohue-Cornejo, A.; Reyes López, S.Y.; Saucedo Acuña, R.A.; García Calderón, A.G.; Guzmán Gastelum, D.A. Use or Abuse of Antibiotics as Prophylactic Therapy in Oral Surgery: A Systematic Review. Medicine 2023, 102, e35011. [Google Scholar] [CrossRef] [PubMed]
- Keestra, J.A.J.; Grosjean, I.; Coucke, W.; Quirynen, M.; Teughels, W. Non-Surgical Periodontal Therapy with Systemic Antibiotics in Patients with Untreated Aggressive Periodontitis: A Systematic Review and Meta-Analysis. J. Periodontal Res. 2014, 50, 294–314. [Google Scholar] [CrossRef]
- Caton, J.G. Treatment with Subantimicrobial Dose Doxycycline Improves the Efficacy of Scaling and Root Planing in Patients with Adult Periodontitis. J. Periodontol. 2000, 71, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Periodontitis: Facts, Fallacies and the Future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Wnukiewicz, W.; Rutowski, R.; Zboromirska-Wnukiewicz, B.; Reichert, P.; Gosk, J. Evaluation of Soft Tissue Reaction to Corundum Ceramic Implants Infiltrated with Colloidal Silver. Adv. Clin. Exp. Med. 2016, 25, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in Novel Therapeutic Approaches for Periodontal Diseases. BMC Oral Health 2022, 22, 492. [Google Scholar] [CrossRef]
- Wang, H.L.; Greenwell, H. Surgical Periodontal Therapy. Periodontol. 2000 2001, 25, 89–99. [Google Scholar] [CrossRef]
- Sculean, A. Biomaterials for Promoting Periodontal Regeneration in Human Intrabony Defects: A Systematic Review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Cafferata, E.A.; Alvarez, C.; Diaz, K.T.; Maureira, M.; Monasterio, G.; González, F.E.; Covarrubias, C.; Vernal, R. Multifunctional Nanocarriers for the Treatment of Periodontitis: Immunomodulatory, Antimicrobial, and Regenerative Strategies. Oral Dis. 2019, 25, 1866–1878. [Google Scholar] [CrossRef]
- Chen, F.-M.; Gao, L.-N.; Tian, B.-M.; Zhang, X.-Y.; Zhang, Y.-J.; Dong, G.-Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of Periodontal Intrabony Defects Using Autologous Periodontal Ligament Stem Cells: A Randomized Clinical Trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Lee, H.-M. Periodontal Therapeutics: Current Host-Modulation Agents and Future Directions. Periodontol. 2000 2020, 82, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef]
- Slamenova, D.; Horvathova, E. Cytotoxic, Anti-Carcinogenic and Antioxidant Properties of the Most Frequent Plant Volatiles. Neoplasma 2013, 60, 343–354. [Google Scholar] [CrossRef]
- Rajendhran, J.; Gunasekaran, P. Human Microbiomics. Indian J. Microbiol. 2010, 50, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A. CRC Handbook of Medicinal Herbs. Int. Clin. Psychopharmacol. 1990, 5, 74. [Google Scholar] [CrossRef]
- Sabaoui, Z.; Lakhdar, L. Essential Oils in Periodontics. What Is the Interest? Integr. J. Med. Sci. 2021, 8, 1–3. [Google Scholar] [CrossRef]
- Gorr, S.U.; Abdolhosseini, M. Antimicrobial Peptides and Periodontal Disease. J. Clin. Periodontol. 2011, 38, 126–141. [Google Scholar] [CrossRef]
- Nasiri, K.; Masoumi, S.M.; Amini, S.; Goudarzi, M.; Tafreshi, S.M.; Bagheri, A.; Yasamineh, S.; alwan, M.; Arellano, M.T.C.; Gholizadeh, O. Recent Advances in Metal Nanoparticles to Treat Periodontitis. J. Nanobiotechnol. 2023, 21, 283. [Google Scholar] [CrossRef]
- Bundidpun, P.; Srisuwantha, R.; Laosrisin, N. Clinical Effects of Photodynamic Therapy as an Adjunct to Full-Mouth Ultrasonic Scaling and Root Planing in Treatment of Chronic Periodontitis. Laser Ther. 2018, 27, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.A.; Bakopoulou, A.A.; Kouzi-Koliakou, K.; Karagiannis, V.; Konstantinidis, A. A Tissue-Engineered Biocomplex for Periodontal Reconstruction. A Proof-of-Principle Randomized Clinical Study. J. Clin. Periodontol. 2021, 48, 1111–1125. [Google Scholar] [CrossRef]
- Deandra, F.A.; Ketherin, K.; Rachmasari, R.; Sulijaya, B.; Takahashi, N. Probiotics and Metabolites Regulate the Oral and Gut Microbiome Composition as Host Modulation Agents in Periodontitis: A Narrative Review. Heliyon 2023, 9, e13475. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Gandolfo, A.; Bravo, J.; Carvajal, P.; Silva, N.; Godoy, C.; Garcia-Sesnich, J.; Hoare, A.; Diaz, P.; Gamonal, J. Microbiological and Clinical Effects of Probiotics and Antibiotics on Nonsurgical Treatment of Chronic Periodontitis: A Randomized Placebo- Controlled Trial with 9-Month Follow-Up. J. Appl. Oral Sci. 2018, 26, e20170075. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, Y.; Wang, S. Stem Cell-Based Tooth and Periodontal Regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal Ligament Stem/Progenitor Cells with Protein-Releasing Scaffolds for Cementum Formation and Integration on Dentin Surface. Connect. Tissue Res. 2016, 57, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Singhal, L.; Belludi, S.A.; Pradhan, N.; Manvi, S. A Comparative Evaluation of the Effect of Platelet Rich Fibrin Matrix with and without Peripheral Blood Mesenchymal Stem Cells on Dental Implant Stability: A Randomized Controlled Clinical Trial. J. Tissue Eng. Regen. Med. 2022, 16, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Dhote, R.; Charde, P.; Bhongade, M.; Rao, J. Stem Cells Cultured on Beta Tricalcium Phosphate (β-TCP) in Combination with Recombinant Human Platelet-Derived Growth Factor—BB (Rh-PDGF-BB) for the Treatment of Human Infrabony Defects. J. Stem Cells 2015, 10, 243–254. [Google Scholar]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human Intrabony Defect Regeneration with Micrografts Containing Dental Pulp Stem Cells: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Latuta, N.; Corbella, S.; Taschieri, S.; Diachkova, E.; Tarasenko, S.; Oksentyuk, A.; Trifonova, D.; Admakin, O. Use of an Antiseptic Rinse (NanArgol) for the Oral Hygiene Maintenance of Subjects with Fixed Appliances: A Randomized Clinical Trial. Int. J. Dent. Hyg. 2022, 21, 219–226. [Google Scholar] [CrossRef]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. BioMed Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, V.M.; Daković, D.R.; Bokonjić, D.R. Adjunctive Effect of the Colloidal Silver Ions Solution in the Treatment of Chronic Periodontal Disease: A Preliminary Clinical Study Dopunski Efekat Koloidnog Rastvora Jona Srebra u Lečenju Hronične Parodontopatije: Preliminarna Klinička Studija. Vojn. Pregl 2018, 75, 1216–1221. [Google Scholar] [CrossRef]
- Zarco, M.F.; Vess, T.J.; Ginsburg, G.S. The Oral Microbiome in Health and Disease and the Potential Impact on Personalized Dental Medicine. Oral Dis. 2012, 18, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Mira, A. Oral Microbiome Studies: Potential Diagnostic and Therapeutic Implications. Adv. Dent. Res. 2018, 29, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef] [PubMed]
- Canut-Delgado, N.; Giovannoni, L.; Chimenos-Küstner, E. Probiotics against Periodontal Disease: A Systematic Review: Are Probiotics a Posible Treatment of Periodontitis? pp. 1–15. Available online: https://diposit.ub.edu/dspace/bitstream/2445/182384/1/716127.pdf (accessed on 3 November 2023).
- Saier, M.H., Jr.; Mansour, N.M. Probiotics and Prebiotics in Human Health. J. Mol. Microbiol. Biotechnol. 2006, 10, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Ballini, A.; De Vito, D.; Abbinante, A.; Altini, V.; Dipalma, G.; Inchingolo, F.; Saini, R. Clinical Results of Improvement in Periodontal Condition by Administration of Oral Probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1329–1334. [Google Scholar] [PubMed]
- Minić, I.; Pejčić, A.; Bradić-Vasić, M. Effect of the Local Probiotics in the Therapy of Periodontitis A Randomized Prospective Study. Int. J. Dent. Hyg. 2022, 20, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and Microbiological Effects of Lactobacillus Reuteri Probiotics in the Treatment of Chronic Periodontitis: A Randomized Placebo-Controlled Study. J. Clin. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef]
- Zhu, Y.; Tao, C.; Goh, C.; Shrestha, A. Innovative Biomaterials for the Treatment of Periodontal Disease. Front. Dent. Med. 2023, 4, 1163562. [Google Scholar] [CrossRef]
- Kaneko, C.; Kobayashi, T.; Ito, S.; Sugita, N.; Murasawa, A.; Ishikawa, H.; Tabeta, K. Association among Periodontitis Severity, Anti-Agalactosyl Immunoglobulin G Titer, and the Disease Activity of Rheumatoid Arthritis. J. Periodontal Res. 2021, 56, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Soh, Y.; Heo, S.-M. Recent Advances of Therapeutic Targets for the Treatment of Periodontal Disease. Biomol. Ther. 2021, 29, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. Teriparatide and Osseous Regeneration in the Oral Cavity. N. Engl. J. Med. 2010, 363, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, U.; Luvizuto, E.R.; Tangl, S.; Watzek, G.; Gruber, R. Short-Term Teriparatide Delivery and Osseointegration: A Clinical Feasibility Study. J. Dent. Res. 2011, 90, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, L.H.; Marcantonio, R.A.C.; Wainwright, M.; Garcia, V.G. Laser No Tratamento Periodontal: É Um Tratamento Efetivo Ou Ficção Científica. Braz. Oral Res. 2021, 35, 1–18. [Google Scholar]
- Lazăr, L.; Dako, T.; Mârțu, M.-A.; Bica, C.-I.; Bud, A.; Suciu, M.; Păcurar, M.; Lazăr, A.-P. Effects of Laser Therapy on Periodontal Status in Adult Patients Undergoing Orthodontic Treatment. Diagnostics 2022, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Polizzi, A.; Cavalcanti, R.; Ronsivalle, V.; Chaurasia, A.; Spagnuolo, G.; Isola, G. Impact of Laser Therapy on Periodontal and Peri-Implant Diseases. Photobiomodul. Photomed. Laser Surg. 2022, 40, 454–462. [Google Scholar] [CrossRef]
- Chiang, C.-P.; Hsieh, O.; Tai, W.-C.; Chen, Y.-J.; Chang, P.-C. Clinical Outcomes of Adjunctive Indocyanine Green-Diode Lasers Therapy for Treating Refractory Periodontitis: A Randomized Controlled Trial with In Vitro Assessment. J. Formos. Med. Assoc. 2020, 119, 652–659. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, R.; Lv, X.; Qu, C. Efficacy of a Combined Er:YAG Laser and Nd:YAG Laser in Non-Surgical Treatment for Severe Periodontitis. Lasers Med. Sci. 2022, 37, 1095–1100. [Google Scholar] [CrossRef]
- Passanezi, E.; Damante, C.A.; de Rezende, M.L.R.; Greghi, S.L.A. Lasers in Periodontal Therapy. Periodontol. 2000 2015, 67, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Meimandi, M.; Talebi Ardakani, M.R.; Esmaeil Nejad, A.; Yousefnejad, P.; Saebi, K.; Tayeed, M.H. The Effect of Photodynamic Therapy in the Treatment of Chronic Periodontitis: A Review of Literature. J. Lasers Med. Sci. 2017, 8, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Bieling, K.; Nuesry, E.; Sculean, A.; Becker, J. Clinical and Histological Healing Pattern of Peri-Implantitis Lesions Following Non-Surgical Treatment with an Er:YAG Laser. Lasers Surg. Med. 2006, 38, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Santonocito, S.; Dalessandri, D.; Migliorati, M.; Indelicato, F. New Frontiers on Adjuvants Drug Strategies and Treatments in Periodontitis. Sci. Pharm. 2021, 89, 46. [Google Scholar] [CrossRef]
- Cappuyns, I.; Cionca, N.; Wick, P.; Giannopoulou, C.; Mombelli, A. Treatment of Residual Pockets with Photodynamic Therapy, Diode Laser, or Deep Scaling. A Randomized, Split-Mouth Controlled Clinical Trial. Lasers Med. Sci. 2012, 27, 979–986. [Google Scholar] [CrossRef]
- Rühling, A.; Fanghänel, J.; Houshmand, M.; Kuhr, A.; Meisel, P.; Schwahn, C.; Kocher, T. Photodynamic Therapy of Persistent Pockets in Maintenance Patients—A Clinical Study. Clin. Oral Investig. 2010, 14, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Hyder, T.; Al-Hamoudi, N.; Binshabaib, M.S.; Alharthi, S.S.; Hanif, A. Efficacy of Photodynamic Therapy versus Antibiotics as an Adjunct to Scaling and Root Planing in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis. Photodiagn. Photodyn. Ther. 2017, 19, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, C.; Cappuyns, I.; Cancela, J.; Cionca, N.; Mombelli, A. Effect of Photodynamic Therapy, Diode Laser, and Deep Scaling on Cytokine and Acute-Phase Protein Levels in Gingival Crevicular Fluid of Residual Periodontal Pockets. J. Periodontol. 2012, 83, 1018–1027. [Google Scholar] [CrossRef]
- Elsadek, M.F.; Farahat, M.F. Effectiveness of Photodynamic Therapy as an Adjunct to Periodontal Scaling for Treating Periodontitis in Geriatric Patients. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1832–1838. [Google Scholar] [CrossRef]
- de Freitas, L.M.; Calixto, G.M.F.; Chorilli, M.; Giusti, J.S.M.; Bagnato, V.S.; Soukos, N.S.; Amiji, M.M.; Fontana, C.R. Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis In Vivo. Int. J. Mol. Sci. 2016, 17, 769. [Google Scholar] [CrossRef]
- Coelho, T.d.R.C.; Pinto Filho, J.M.; Ribeiro Caponi, L.S.F.E.; Soares, J.d.M.; Dos Santos, J.N.; Cury, P.R. Photodynamic Therapy as an Adjunctive Treatment for Grade C Periodontitis in Molar Teeth: A Preliminary Trial. Quintessence Int. 2023, 54, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of Aligned PCL-PEG Nanofibers into Porous Chitosan Scaffolds Improved the Orientation of Collagen Fibers in Regenerated Periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Staples, R.J.; Ivanovski, S.; Vaquette, C. Fibre Guiding Scaffolds for Periodontal Tissue Engineering. J. Periodontal Res. 2020, 55, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Shanbhag, S.; Mustafa, K. Scaffolds in Periodontal Regenerative Treatment. Dent. Clin. N. Am. 2022, 66, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G. 3D-Printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef] [PubMed]
- Tumer, H.; Berberoglu, A.; Caygur, A.; Yilmaz, H.G. Clinical Evaluation of Chlorhexidine and Essential Oils’Adjunctive Effects in Subgingival Ultrasonic Instrumentation on Periodontal Parameters and Halitosis. J. Essent. Oil Bear. Plants 2019, 22, 169–175. [Google Scholar] [CrossRef]
- Sahebnasagh, M.; Aksi, V.; Eslami, F.; Lashkardoost, H.; Kasaian, J.; Golmohammadzadeh, S.; Parkam, B.; Negarandeh, R.; Saghafi, F.; Sahebnasagh, A. Prevention of Radiotherapy-Related Oral Mucositis with Zinc and Polyherbal Mouthwash: A Double-Blind, Randomized Clinical Trial. Eur. J. Med. Res. 2023, 28, 109. [Google Scholar] [CrossRef] [PubMed]
- Goes, P.; Dutra, C.S.; Lisboa, M.R.P.; Gondim, D.V.; Leitão, R.; Brito, G.A.C.; Rego, R.O. Clinical Efficacy of a 1% Matricaria chamomile L. Mouthwash and 0.12% Chlorhexidine for Gingivitis Control in Patients Undergoing Orthodontic Treatment with Fixed Appliances. J. Oral Sci. 2016, 58, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.A.L.; Casana, S.T.V.; Gómez, P.A.M. Effectiveness of Chlorhexidine and Essential Oils Associated with Scaling and Root Planing in the Treatment of Chronic Periodontitis. Rev. Cienc. Salud 2020, 18, 30–40. [Google Scholar] [CrossRef]
- Alshehri, M.; Alshail, F.; Aldosary, K.M.; Alamri, A.A. Comparison of an Essential-Oil-Based Oral Rinse and Chlorhexidine as Adjuncts to Scaling and Root Planing in the Treatment of Periodontal Inflammation. Interv. Med. Appl. Sci. 2015, 7, 78–84. [Google Scholar] [CrossRef]
- Ripari, F.; Cera, A.; Freda, M.; Zumbo, G.; Zara, F.; Vozza, I. Tea Tree Oil versus Chlorhexidine Mouthwash in Treatment of Gingivitis: A Pilot Randomized, Double Blinded Clinical Trial. Eur. J. Dent. 2020, 14, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mansour Ali, A.; Alsenaid, A.I.; Al Mosilhi, A.H.; Al Mofeez, F.I.; Yousef, A.; Sulaiman, A.; Al Yousef, H.M. Evaluation of Anti-Caries Effect of Essential Oil Mouthwash. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 47–50. [Google Scholar] [CrossRef]
- Cortelli, S.C.; Cortelli, J.R.; Shang, H.; Costa, R.; Charles, C.A. Gingival Health Benefits of Essential-Oil and Cetylpyridinium Chloride Mouthrinses: A 6-Month Randomized Clinical Study. Am. J. Dent. 2014, 27, 119–126. [Google Scholar] [PubMed]
- Alshehri, M.; Alshail, F.; Alshehri, F.A. Effect of Scaling and Root Planing with and without Adjunctive Use of an Essential-Oil-Based Oral Rinse in the Treatment of Periodontal Inflammation in Type-2 Diabetic Patients. J. Investig. Clin. Dent. 2017, 8, e12188. [Google Scholar] [CrossRef] [PubMed]
- Hugar, S.S.; Patil, S.; Metgud, R.; Nanjwade, B.; Hugar, S.M. Influence of Application of Chlorhexidine Gel and Curcumin Gel as an Adjunct to Scaling and Root Planing: A Interventional Study. J. Nat. Sci. Biol. Med. 2016, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Charugundla, B.R.; Anjum, S.; Mocherla, M. Comparative Effect of Fluoride, Essential Oil and Chlorhexidine Mouth Rinses on Dental Plaque and Gingivitis in Patients with and without Dental Caries: A Randomized Controlled Trial. Int. J. Dent. Hyg. 2015, 13, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, J. Comparison of Chrysopogon Zizanioides Mouthwash with Chlorhexidine Mouthwash in Chronic Periodontitis Patients—An Clinical Trial. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 1–7. [Google Scholar] [CrossRef]
- Kaplan, V.; Hasanoglu Erbasar, G.N.; Cigerim, L.; Altay Turgut, H.; Cerit, A. Effect of St. John’s Wort Oil and Olive Oil on the Postoperative Complications after Third Molar Surgery: Randomized, Double-Blind Clinical Trial. Clin. Oral Investig. 2021, 25, 2429–2438. [Google Scholar] [CrossRef]
- Saliasi, I.; Llodra, J.C.; Bravo, M.; Tramini, P.; Dussart, C.; Viennot, S.; Carrouel, F. Effect of a Toothpaste/Mouthwash Containing Carica Papaya Leaf Extract on Interdental Gingival Bleeding: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2018, 15, 2660. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, Medicinal and Toxicological Significance of Eucalyptus Leaf Essential Oil: A Review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Neagu, O.M.; Ghitea, T.; Marian, E.; Vlase, L.; Vlase, A.-M.; Ciavoi, G.; Fehér, P.; Pallag, A.; Bácskay, I.; Nemes, D.; et al. Formulation and Characterization of Mucoadhesive Polymeric Films Containing Extracts of Taraxaci Folium and Matricariae Flos. Molecules 2023, 28, 4002. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.D.S.; Rosalen, P.L.; Benso, B.; de Cássia Orlandi Sardi, J.; Denny, C.; Alves de Sousa, S.; Queiroga Sarmento Guerra, F.; de Oliveira Lima, E.; Almeida Freires, I.; Dias de Castro, R. The Use of Essential Oils and Their Isolated Compounds for the Treatment of Oral Candidiasis: A Literature Review. Evid. Based. Complement. Altern. Med. 2021, 2021, 1059274. [Google Scholar] [CrossRef] [PubMed]
- Subha, D.S.; Pradeep, T. Periodontal Therapy with 0.25%Lemongrass Oil Mouthwash in Reducing Risk of Cardiovascular Diseases: A 3-Arm Prospective Parallel Experimental Study. Ethiop. J. Health Sci. 2017, 27, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Kaur, P.; Kaushal, U.; Kaur, V.; Shekhar, N. Essential Oils in Treatment and Management of Dental Diseases. Biointerf. Res. Appl. Chem. 2022, 12, 7267–7286. [Google Scholar] [CrossRef]
- Alexa, V.T.; Szuhanek, C.; Cozma, A.; Galuscan, A.; Borcan, F.; Obistioiu, D.; Dehelean, C.A.; Jumanca, D. Natural Preparations Based on Orange, Bergamot and Clove Essential Oils and Their Chemical Compounds as Antimicrobial Agents. Molecules 2020, 25, 5502. [Google Scholar] [CrossRef] [PubMed]
- Máximo, P.d.M.; Cortelli, S.C.; Aquino, D.R.; de Miranda, T.B.; Costa, F.O.; Cortelli, J.R. Preoperative Mouthwash in Subjects with Different Periodontal Status: A Randomised Controlled Clinical Trial. Oral Health Prev. Dent. 2020, 18, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Quintas, V.; Prada-López, I.; Prados-Frutos, J.C.; Tomás, I. In Situ Antimicrobial Activity on Oral Biofilm: Essential Oils vs. 0.2% Chlorhexidine. Clin. Oral Investig. 2015, 19, 97–107. [Google Scholar] [CrossRef]
- Radu, C.-M.; Radu, C.C.; Bochiș, S.-A.; Arbănași, E.M.; Lucan, A.I.; Murvai, V.R.; Zaha, D.C. Revisiting the Therapeutic Effects of Essential Oils on the Oral Microbiome. Pharmacy 2023, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Antonescu Mintas, A.-I.; Miere Groza, F.; Fritea, L.; Ganea, M.; Zdrinca, M.; Dobjanschi, L.; Antonescu, A.; Vicas, S.I.; Bodog, F.; Sindhu, R.K.; et al. Perspectives on the Combined Effects of Ocimum Basilicum and Trifolium Pratense Extracts in Terms of Phytochemical Profile and Pharmacological Effects. Plants 2021, 10, 1390. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam Tumpa, M.A.; Zehravi, M.; Sarker, M.T.; Yamin, M.; Islam, M.R.; Harun-Or-Rashid, M.; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An Overview of Antimicrobial Stewardship Optimization: The Use of Antibiotics in Humans and Animals to Prevent Resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- Ciavoi, G.; Dobjanschi, L.; Jurca, T.; Osser, G.; Scrobota, I.; Pallag, A.; Muresan, M.E.; Vicaș, L.G.; Marian, E.; Bechir, F.; et al. Comparative Effectiveness of a Commercial Mouthwash and an Herbal Infusion in Oral Health Care. Appl. Sci. 2021, 11, 3008. [Google Scholar] [CrossRef]
- Rogers, K.R.; Henson, T.E.; Navratilova, J.; Surette, M.; Hughes, M.F.; Bradham, K.D.; Stefaniak, A.B.; Knepp, A.K.; Bowers, L. In Vitro Intestinal Toxicity of Commercially Available Spray Disinfectant Products Advertised to Contain Colloidal Silver. Sci. Total Environ. 2020, 728, 138611. [Google Scholar] [CrossRef] [PubMed]
- Skrinjar, I.; Vucicevic Boras, V.; Bakale, I.; Andabak Rogulj, A.; Brailo, V.; Vidovic Juras, D.; Alajbeg, I.; Vrdoljak, D.V. Comparison between Three Different Saliva Substitutes in Patients with Hyposalivation. Clin. Oral Investig. 2015, 19, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Shaheena, S.; Chintagunta, A.D.; Dirisala, V.R.; Sampath Kumar, N.S. Extraction of Bioactive Compounds from Psidium Guajava and Their Application in Dentistry. AMB Express 2019, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Karadağlıoğlu, Ö.İ.; Ulusoy, N.; Başer, K.H.C.; Hanoğlu, A.; Şık, İ. Antibacterial Activities of Herbal Toothpastes Combined with Essential Oils against Streptococcus Mutans. Pathogens 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Taalab, M.R.; Mahmoud, S.A.; El Moslemany, R.M.; Abdelaziz, D.M. Intrapocket Application of Tea Tree Oil Gel in the Treatment of Stage 2 Periodontitis. BMC Oral Health 2021, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Angaji, M.; Gelskey, S.; Nogueira-Filho, G.; Brothwell, D. A Systematic Review of Clinical Efficacy of Adjunctive Antibiotics in the Treatment of Smokers with Periodontitis. J. Periodontol. 2010, 81, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Noreikaitė, A.; Ayupova, R.; Satbayeva, E.; Seitaliyeva, A.; Amirkulova, M.; Pichkhadze, G.; Datkhayev, U.; Stankevičius, E. General Toxicity and Antifungal Activity of a New Dental Gel with Essential Oil from Abies sibirica L. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Amin, A.; Farid, A.; Selim, S.; Rashid, S.A.; Aziz, M.I.; Kamran, S.H.; Khan, M.A.; Rahim Khan, N.; Mashal, S.; et al. Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections. Gels 2023, 9, 252. [Google Scholar] [CrossRef]
- Alven, S.; Ubanako, P.; Adeyemi, S.A.; Ndinteh, D.T.; Choonara, Y.E.; Aderibigbe, B.A. Carboxymethyl Cellulose/Poloxamer Gels Enriched with Essential Oil and Ag Nanoparticles: Promising Wound Dressings. Ther. Deliv. 2023, 14, 139–156. [Google Scholar] [CrossRef]
- Muresan, S.M.C.; Dreanca, A.; Repciuc, C.; Dejescu, C.; Rotar, O.; Pop, R.A.; Pantea, S.; Pall, E.; Ciotlaus, I.; Sarosi, C.; et al. Dental Hydrogels with Essential Oils with Potential Activity in Periodontitis. Appl. Sci. 2023, 13, 1787. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA) 2021 National Registers of Authorised Medicines. 2021. Available online: https://www.ema.europa.eu/en/medicines/national-registers-authorised-medicines (accessed on 2 October 2023).
| Novel Treatments | Advantages | Disadvantages | Therapeutic Properties | Administration | Reference |
|---|---|---|---|---|---|
| Stem cell-based tissue engineering |
|
|
|
| [71,72,85,86,87,88,89] |
| Colloidal silver nanoparticles |
|
|
|
| [90,91,92] |
| Pre- and Probiotics |
|
|
|
| [84,93,94,95,96,97,98,99,100] |
| Immunomodulatory therapy |
|
|
|
| [66,101,102,103] |
| Anabolic agents (teriparatide, sclerostin antibody) |
|
|
|
| [104,105,106] |
| Lasers |
|
|
|
| [107,108,109,110,111,112,113,114,115] |
| Photodynamic therapy |
|
|
|
| [116,117,118,119,120,121,122] |
| Scaffolds |
|
|
|
| [72,82,86,123,124,125,126] |
| Essential Oil | Reference | Administration | No. of Participants | Duration | Results |
|---|---|---|---|---|---|
| Peppermint oil | [127] | mouthwash (oral rinse) | 90 | 30 days |
|
| [128] | mouthwash (oral rinse) | 67 | 6 weeks |
| |
| Chamomile oil | [129] | oral gel (intra-sulcular application) | 30 | 4 weeks |
|
| Eucalyptus oil | [130] | mouthwash (oral rinse) | 42 | 5 months |
|
| [131] | mouthwash (oral rinse) | 90 | 30 days |
| |
| Tea tree oil | [132] | mouthwash (oral rinse) | 30 | 14 days |
|
| [133] | mouthwash | 30 | 20 days |
| |
| Salvia officinalis oil | [134] | mouthwash (oral rinse) | 338 | 6 months |
|
| Thyme oil | [135] | mouthwash (oral rinse) | 60 | 3 months |
|
| Curcumin oil | [136] | oral gel (intra-sulcular application) | 30 | 45 days |
|
| Rosmarinus officinalis oil | [137] | mouthwash (oral rinse) | 36 | 8 weeks |
|
| Vetiver grass oil | [138] | mouthwash (oral rinse) | 20 | 14 days |
|
| Olive oil and St. John’s wort oil | [139] | mouthwash (oral rinse) | 90 | 6 months |
|
| Carica papaya leaf extract | [140] | mouthwash (oral rinse) | 138 | 4 weeks |
|
| Oral Product | Essential Oils | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Mouthwashes | Chamomile oil Lemongrass oil |
|
| |
| Zingiber officinalis oil Citrus oil Zataria multiflora oil Peppermint oil Curcumin oil Calendula officinalis oil |
|
| [140,141,142,143,144,145,146,147,148,149,150,151,152] | |
| Sprays | Thyme oil Ginger oil Citrus oil Salvia officinalis oil Rosmarinus officinalis oil Peppermint oil |
|
| [153,154] |
| Toothpastes | Lavender oil |
|
| |
| Thyme oil |
|
| [140,155,156] | |
| Coconut oil |
|
| ||
| Eucalyptus oil Tea tree oil Cinnamon oil |
| |||
| Gels | Menthol Oregano oil Lavender oil Peppermint oil |
|
| |
| Thyme oil Clove oil Cinnamon oil Tea tree oil Thieves oil Lemongrass oil Citrus oil Eucalyptus oil |
|
| [78,157,158,159,160,161,162] |
| Product Name | Essential Oils | Topical Pharmaceutical Form | Administration | Indications |
|---|---|---|---|---|
| Dentosept® (PlantExtrakt, Cluj, Romania) | Peppermint oil Salvia officinalis extract Eucalyptus oil Thyme oil Castor oil | oral spray |
|
|
| Kamistad® (Stada Arzneimittel AG, Bad Vilbel, Germany) | Chamomile oil Cinnamon oil | oral gel |
|
|
| Mucosit® (Herbapol S.A., Poznan, Poland) | Salvia officinalis oil Chamomile oil Thyme oil | oral gel |
|
|
| Septosan® (Herbapol S.A., Poznan, Poland) | Calendula officinalis oil Thyme oil Chamomile oil | sachets infusion |
|
|
| Aperisan® (Dentinox Lenk & Schuppan KG, Berlin, Germany) | Salvia officinalis oil | oral gel |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, C.-M.; Radu, C.C.; Arbănaşi, E.-M.; Hogea, T.; Murvai, V.R.; Chiș, I.-A.; Zaha, D.C. Exploring the Efficacy of Novel Therapeutic Strategies for Periodontitis: A Literature Review. Life 2024, 14, 468. https://doi.org/10.3390/life14040468
Radu C-M, Radu CC, Arbănaşi E-M, Hogea T, Murvai VR, Chiș I-A, Zaha DC. Exploring the Efficacy of Novel Therapeutic Strategies for Periodontitis: A Literature Review. Life. 2024; 14(4):468. https://doi.org/10.3390/life14040468
Chicago/Turabian StyleRadu, Casandra-Maria, Carmen Corina Radu, Emil-Marian Arbănaşi, Timur Hogea, Viorela Romina Murvai, Ioana-Andreea Chiș, and Dana Carmen Zaha. 2024. "Exploring the Efficacy of Novel Therapeutic Strategies for Periodontitis: A Literature Review" Life 14, no. 4: 468. https://doi.org/10.3390/life14040468
APA StyleRadu, C.-M., Radu, C. C., Arbănaşi, E.-M., Hogea, T., Murvai, V. R., Chiș, I.-A., & Zaha, D. C. (2024). Exploring the Efficacy of Novel Therapeutic Strategies for Periodontitis: A Literature Review. Life, 14(4), 468. https://doi.org/10.3390/life14040468







